Abstract
Phenazine solid crystals have been attached to gold electrodes and investigated by cyclic and potential step electrochemical quartz crystal microbalance (ECQM) measurements in the presence of aqueous acidic media. The freshly deposited phenazine layers exhibit a break-in phenomenon. The number of potential cycles required for the layer to be fully electroactive depends on its thickness and also on the nature and concentration of the supporting electrolyte as well as on the scan rate. After the break-in, a considerable amount of solvent molecules remains embedded in the surface layer. The protonated and unprotonated forms of phenazine, whose relative amounts depend on the pH of the contacting solutions, are reduced at different potentials; however, the stable product of the first electron transfer is the respective phenazylium salt. During the second reduction step 5,10-dihydrophenazine and charge-transfer complexes of different compositions are formed. Both the current and microgravimetric responses supplied evidences for the structural rearrangements of the solid phases that accompany the redox reactions. The large separation of the reduction and oxidation peaks relates to the additional energy needed to create the solid/solid interface between the reduced and unreduced or partially reduced forms. The chronoamperometric response shows the characteristics of nucleation and growth kinetics. The phase transformation proceeds with the release of hydration water, and the EQCM response is affected by the strain that develops as a consequence of the phase transformation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenazine and its derivatives have been extensively studied owing to the interesting chemical, biochemical, electrochemical and photochemical reactions of these compounds [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. Phenazine is practically insoluble in water; however, it can be dissolved in organic solvents or in concentrated aqueous solutions of acids. Roullier et al. [9] have studied thick crystalline deposits of phenazine on platinum and glassy carbon surfaces by cyclic voltammetry. They found that when the deposit is thick enough, only a fraction of it is electroactive, and the loss of material by dissolution is small at pH values of 2 or higher. According to their model only the layer near the electrode plays a role in the redox processes, while the outer part of the deposit remains inactive and protects the active zone from dissolution into the bulk solution. In contrast with the mechanism generally considered in the case of redox or conducting polymer films, where the electron transfer is accompanied by the transport of ions (usually counterions) and solvent molecules while the polymer remains attached to the surface, Roullier et al. [9] proposed a mechanism which involves the dissolution of the oxidized form of phenazine; the electroreduction takes place in a solution layer near the electrode surface and the reverse processes, i.e. electrooxidation and recrystallization, occur during reoxidation. The crystallization of the reduced form is also considered. The mechanism proposed was based on visual observations and on the fact that no difference was found on changing the size of counterions. This study can be regarded as a forerunner of the new technique, introduced by Scholz et al. [13, 14, 15] and based on the perception that microparticles can be stably attached to an electrode surface. In this way, very small amounts of substances can be studied by electrochemical methods. The immobilization of microparticles has become one of the most popular methods, owing to the ease of the electrode preparation and the surprisingly good reproducibility of the measurements [15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46]. The theory of the voltammetry at the three-phase junction, including the charge and mass transports [15, 19, 22, 25, 40] and phase transitions [15, 18, 19, 23, 27, 28, 38] has also been developed. Although even the early studies attested that solid-state voltammetry is a powerful tool for studying water-insoluble organic compounds, this technique is still less used in this area [21, 24, 27, 28, 29, 30, 31, 32, 36, 37, 38, 39, 43, 44, 45, 46], especially in comparison with the number of similar investigations of inorganic or metalloorganic compounds [15, 16, 27, 33, 34, 35]. It has been shown that not only solid particles but also microdroplets can be attached to metal or graphite surfaces [39, 40, 41, 42, 43], which further widened the opportunities. Combined methods have been also used [15, 16] to gain a deeper understanding of the processes occurring on the surface. In this respect the application of the piezoelectric microgravimetry at an electrochemical quartz crystal microbalance (EQCM) has been proved very useful, as in the case of other modified electrodes [16, 27, 30, 32, 34, 37, 40, 44].
The EQCM investigation of phenazine microcrystals immobilized on a gold surface is of importance because it can contribute to our knowledge regarding the redox behaviour of this compound in solid state, and such experiments can supply evidence in respect of the mechanism. Phenazine is also an excellent model system for studying such complex events as the formation of charge-transfer complexes or the break-in phenomenon. The understanding of the break-in effect, i.e. how a steady-state electrochemical response develops during potential cycling or at a given potential is a crucial question in the electrochemistry of solid compounds. The simultaneous measurements of the electrochemical and microgravimetric responses reveal the role of the ingress of ions and solvent molecules from the electrolyte solution into the surface layer.
In this paper, we present results of EQCM investigations on the sorption/desorption processes accompanying the redox transformations of phenazine microcrystals attached to a gold surface. It will be shown that the results are more consistent with the model where the uptake and expulsion of counterions and water molecules as well as solid–solid phase transformations accompany the electron transfer reactions in the crystals.
Materials and methods
Phenazine (Aldrich), HClO4, NaClO4, HCl, NaCl, H2SO4 and Na2SO4 (Merck, analytical grade) were used as received. Doubly distilled water was used. An AT-cut crystal (10 MHz) coated with gold or platinum was used in the EQCM measurements. A detailed description of the apparatus and its calibration has been published in our previous papers [12, 44, 46]. The geometrical and piezoelectrically active area of the working electrode was 0.4 cm2. The roughness factor, which was determined from the charge associated with the gold oxide formation [12], was 2.01. A Pt wire was used as a counter electrode. The reference electrode was a saturated sodium calomel electrode (SCE). All potentials are referred to the SCE. The electrodes were prepared in two different ways. The microcrystals were attached to the gold surface by wiping the electrode with a cotton swab or filter paper containing the material [44, 45]. Alternatively, the electrodes were covered by phenazine by using an evaporation technique; i.e. phenazine crystals were dissolved in tetrahydrofuran (THF) and some drops of the solution were placed on the electrode surface; the layer formed in this way may be at least partially amorphous, although no difference has been observed between the crystalline phenazine and the layer formed by the evaporation technique in respect of their electrochemical behaviours. The electrodes were used after evaporation of the solvent and rinsing with the supporting electrolyte. The evaporation technique was used in EQCM experiments in order to obtain a more uniform layer; however, the “dry” method—according to a visual inspection—also results in a rather uniform coating. The Sauerbrey equation [47] was therefore used for the calculation of the surface mass changes (Δm) from the frequency changes (Δf), with an integral sensitivity (Cf=(2.05±0.2)×108 Hz cm2 g–1) that was determined in separate experiments [12, 46]. The amount of phenazine immobilized on the surface was estimated by measuring the crystal frequency before and after the deposition in dry state. A 10 kHz frequency decrease is equivalent to 4.88×10–5 g cm–2, which corresponds to an average layer thickness of circa 50 μm, given a density of 1 g cm–3. The surface mass determined was in direct proportion to the amount of phenazine content of the drops applied in the case of the evaporation technique. Usually a single drop was placed in the middle of the active crystal surface. The gold surfaces wetted well, i.e. the drops spread; however, care was taken in covering only the piezoelectrically active area. An immersion of the crystal into 1 mol dm–3 HClO4/NaClO4 (pH 2) solution caused a frequency decrease circa 4,500 Hz, which is in good agreement with the expected value taking into account the density and viscosity of the contacting solution. This frequency decrease was observed both for uncoated and coated electrodes, attesting that there is neither dissolution of the material attached to the electrode surface, nor swelling of the virgin organic layer. We mostly used solutions of pH 2 in order to avoid dissolution of the microcrystals; however, the thick surface deposit is quite durable even in solutions of 1 mol dm–3 strong acids.
All solutions were purged with oxygen-free argon and an inert gas blanket was maintained throughout the experiments.
An Elektroflex 453 potentiostat and an Universal Frequency Counter TR-5288 connected with an IBM personal computer were used for the control of the measurements and for the acquisition of the data.
Results and discussion
Break-in phenomena
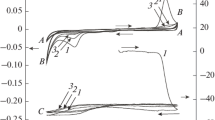
In the case of freshly deposited, thick organic layers [28] or polymer films [48] on the metal surface, several potential sweeps are required for the films to be fully electroactive and reach a steady-state voltammogram as well as EQCM frequency–potential curves. This phenomenon has been referred to as the break-in effect. The number of cycles required for this process depends on the thickness of the surface layer, on the nature and concentration of the supporting electrolyte and on the scan rate. In Fig. 1 and Fig. 2 are shown the break-in effects observed for phenazine microcrystals attached to gold in contact with two different electrolytes (HCl/NaCl and HClO4/NaClO4, pH 2) at v=50 mV s–1. As seen, substantial amounts of electrolyte are gradually incorporated into the surface layer together with increasing charge consumed (Q) during subsequent cycles. The mass increase that can be assigned to sorption of ions and solvent molecules starts simultaneously with the first reduction step (peak I) and continues—albeit at a smaller rate—in the region of peak II, and even during reoxidation (peak III). The mass decrease begins at the second oxidation wave (peak IV); however, at the end of the cycles a considerable amount of electrolyte remains embedded in the surface layer. The difference between the initial and final mass (frequency) values decreases with the number of cycles and eventually reaches a more or less constant value (Fig. 3). It should be mentioned that the oxidation is not completed after the end of the cycle, Qred>Qox, and a small current still flows that is accompanied by a slight frequency increase for some minutes. Consequently, the next cycle starts at a somewhat higher frequency value depending on the waiting time, although no dissolution of microcrystals occurs. It is clearly seen by comparing the final frequency value after the first cycle in Fig. 1a and the starting frequency of the second cycle (Fig. 1b) after 1 min waiting time at 0.5 V. It should be mentioned that in some cases a slight dissolution was observed during the break-in phenomenon at 0.5 V; however, it can be clearly distinguished from the desorption of sorbed species. The desorption (mass decrease) occurs until I>0 and the whole change is usually minor. The dissolution of the microcrystals causes a somewhat higher frequency increase in an abrupt process even at a currentless condition. During continuous cycling the positions of the peaks do not change substantially. In chloride-containing solutions peak I outgrows peak II (Fig. 1), while in perchlorate solutions peak II remains dominant. In both solutions a prepeak (Ia) appears during reduction. Its counterpart can be seen in the course of oxidation (peak IVa), especially in the early period of break-in (Fig. 2); however, in the later phase peaks IV and IVa merge. The number and the variation of the peaks indicate a complex mechanism, the formation and transformation of several reduction intermediate products and the reoxidation of those species.
Consecutive cyclic voltammograms (solid line) and the simultaneously detected electrochemical quartz crystal microbalance (EQCM) frequency responses (dotted line) for phenazine microcrystals attached to a gold electrode in the presence of 1 mol dm–3 HCl/NaCl (pH 2) solution. Scan rate: 50 mV s–1. a First cycle for the freshly prepared layer. Dry thickness is ca. 50 μm. b Cycles 2—6; the second cycle was taken after 1 min waiting time at 0.5 V. SCE Saturated sodium calomel electrode
The mass changes versus number of cycles calculated from the differences between the initial and final frequency values at each cycle by using the data shown in Fig. 1
The swelling of the phenazine layer
The mass changes accompanying the redox reactions help to elucidate the mechanism. It follows from the mass changes observed during break-in that besides the incorporation of counterions, a substantial amount of water molecules enter the surface layer. Even after the completion of the swelling the sorption/desorption of both ionic species and solvent molecules can be assumed. It is possible to separate the contributions of the mobile species to the total mass change by calculating the mass change associated with the sorption of ions that maintains the electroneutrality conditions within the surface layer (Δmion):
where Q is the charge consumed, Mion is the molar mass of the counterions, n is the charge number of the electrode reaction and F is the Faraday constant. Then the sorbed amount of water molecules \({\left( {\Delta m_{{{\text{H}}_{{\text{2}}} {\text{O}}}} } \right)}\) can be derived from the total mass change measured (Δm):
The calculation of the apparent molar mass of the incorporated species (Mapp) is also instructive regarding the ratio of mol ion/mol water (x):
It is evident that in the case of a complex mechanism a hypothesis concerning the electrochemical/chemical reactions is needed. It is known [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] that phenazine exhibits two successive one-electron reduction steps in acidic media.
The phenazine at pH 2—since pKa=1.23—exists in both unprotonated (P) and protonated forms (PH+) at equilibrium. Therefore, the first electron transfer can be described as follows:
The apparent molar masses calculated from the measurements (Fig. 1 ) are shown in Fig. 4. It can be seen that in the beginning, rather high Mapp values were derived for the mobile species, which were exchanged in the potential regions of peak I and peak IV, respectively, while the variation of Mapp was much less in the region of peaks II and III. However, in all cases Mapp was much higher than Mion=35.5 g mol–1. First, we will deal with the mass changes at peaks I and IV since the permanent change (the break-in) mostly occurs at peak I, and the amount of embedded species depends on the difference between the mass changes during these two processes. The mass increase is almost linear with charge. From the charge and mass change obtained for the first reduction step of the virgin phenazine layer, a ratio as high as 59 mol H2O/1 mol Cl– can be derived. This value gradually decreases and eventually the ratio becomes 4 mol H2O/1 mol Cl– (Fig. 4), which is in good accordance with the hydration number of Cl– ions. The possibility that the high excess of mass is not entirely owing to the sorption of water molecules, but also to electrolyte, i.e. ions in neutral combination also entering the surface layer, cannot be excluded.
The apparent molar mass values and the number of water molecules moving together with one chloride ion calculated from the data presented in Fig. 1 as a function of the number of cycles. The respective values are displayed at different potential regions corresponding to peak I (square), peak II (circle), peak III (triangle) and peak IV (cross). Note that at peaks I, II and III mass increase, while at peak IV mass decrease occur. Mapp Apparent molar mass
It is instructive to compare the total amount of phenazine on the surface with the amount of sorbed water molecules. The deposition of the phenazine caused a frequency change Δf=−10,200 Hz in the dry state. It is equivalent to 2.19×10–7 mol phenazine on the gold surface. No swelling or dissolution was observed upon immersing the electrode into water or acid solutions since Δf=–(4,800±400) Hz frequency decrease was observed for both the uncoated and the coated gold crystals. It follows that during the initial cycle at peak I only 8% of phenazine was reduced, since 1.6×10–8 mol Cl– entered the layer; however, 4.2 mol H2O/1 mol phenazine sorbed in the layer. The latter ratio is 54 if we consider only the “active” part of phenazine crystals. The amount of embedded water at the end of the cycle is 7.59×10–7 mol H2O (or H2O equivalent electrolyte), or taking into account the desorption (relaxation) at +0.5 V, this value becomes 6.72×10–7 mol H2O, i.e. the permanent residue after the first cycle is circa 3 mol H2O/1 mol phenazine. At the end of the six cycles the total swelling is circa 7 mol H2O/1 mol phenazine, while 12% of the phenazine became active.
The question of dissolution–crystallization and phase transformation
Because the character of the Δf versus E curves does not change during subsequent cycles, the dissolution–recrystallization scheme outlined by Roullier et al. [9] is not supported by the EQCM data. If it were true, the transition from the elastic to viscoelastic or even viscous behaviour would have been manifested. According to the model expounded in [9] only the layer near the metal is active and the outer, inert layer protects the inner zone. We could not see the electrochemically induced dissolution of the solid since the frequency practically did not change until the first reduction step—excepting a rather small increase just before the reduction—when a mass increase has been detected. Therefore, we have to conclude that even if some kind of solution-like zone is formed near the metal surface, the whole system maintains its elastic behaviour. This solution is very concentrated: even considering 55 mol water/1 mol phenazine, the concentration of the solution is 1 mol dm–3, which is about three orders of magnitude higher than the solubility of phenazine in aqueous acid solution (pH 2), i.e. the solution\( \rightleftarrows \)crystal equilibrium is shifted entirely to the formation of crystals. Nevertheless, we can envisage highly swollen parts of the layer; however, the layer still moves together with the quartz crystal. We will see later that dissolution can indeed occur, i.e. the idea of Roullier et al. cannot be entirely rejected, although instead of a simple active, inner zone (solution)–inactive outer zone (solid) structure we have to use model in which the inner zone is rather a crystalline phase kept together with attractive forces and containing embedded water molecules (like crystal water) and ions. The dissolution does not occur just as a consequence of the interaction between phenazine and water molecules, but is a rather complex event in that the phase transitions accompanying the redox transformations also play a role.
The break-in phenomenon is similar in different electrolytes (Fig. 1 and Fig. 2). Neither the peak potentials nor the character of the frequency–potential curves change substantially when chloride-containing solutions are replaced by perchlorate-containing electrolyte. The nature of electrolyte affects only peak I inasmuch as peak Ia and peak II are more developed in HClO4/NaClO4 solutions. It can be stated that at a given scan rate (v=50 mV s–1) during consecutive cycling the electrochemical activity of the surface layer increases and eventually steady-state current and mass responses can be obtained. The rather complex variation of the f versus E curves and the wide separation of the cathodic and anodic peaks indicate that the electron transfer steps are accompanied by follow-up chemical reactions [12] and phase transformations [15, 18, 19, 23, 27, 28]. It is known that phenazine and 5,10-dihydro-phenazine (PH2, fully reduced form) at relatively high concentrations can form charge-transfer complexes of different compositions [1, 12]. The formation of charge transfer complexes during electroreduction has been demonstrated in the case of other compounds such as quinone–hydroquinone (quinhydrone) [49] or methylene blue–leucomethylene blue [50]. The wide peak separations are characteristic of solid–solid phase transformations under rate control by nucleation and growth [28]. In this case, an overpotential (energy) is needed to create the new solid–solid interface between the reduced and oxidized forms. It should be mentioned that besides the formation of the new interface in the crystal bulk, the orientation of the molecules at the gold/organic crystal interface may also change. For instance, it has been shown that quinone molecules have a flat orientation while the formation of quinhydrone leads to a vertical face-to-face orientation [49]. In order to gain a deeper understanding of these rather complicated transformations, we investigated the influence of layer thickness, the scan rate dependence and the effect of potentiostatic reduction at different potential ranges.
Effect of the layer thickness
Figure 5 shows the cyclic voltammograms and the simultaneously obtained EQCM responses of phenazine microcrystals as a function of the layer thickness at v=50 mV s–1. It can be seen that the characteristics of the current and mass responses do not depend on the amount of phenazine attached to a gold surface in a rather wide thickness range. The mass changes in the whole potential interval are proportional to the charge consumed. In these experiments the negative potential limit was extended to −0.4 V. A new feature of the frequency–potential curves that can be seen in Fig. 5b is the mass decrease in the region of peak II. This characteristic is also independent of the thickness. It is of importance that the mass loss is regained during reoxidation, i.e. it is not a consequence of the dissolution of the fully reduced phenazine.
The effect of the layer thickness (amount of phenazine) on the cyclic voltammetric curves (a) and the simultaneously detected EQCM responses (b). Au|phenazine electrodes were in contact with 1 mol dm–3 HClO4/NaClO4 (pH 2). Scan rate: 50 mV s–1. The peak currents are getting higher and higher with the layer thickness (a), while the gradually lower initial frequencies and the increasing frequency changes during cycling correspond to thicker layers (b). The highest and lowest average thicknesses in the dry state are about 40 μm and 5 μm, respectively
The effects of scan rate, potential range and pH
Figure 6 shows the cyclic voltammograms and the simultaneous EQCM responses obtained at different scan rates for a Au|phenazine electrode in contact with 1 mol dm–3 H2SO4. The surface layer remained stable—except a small dissolution at low scan rates—even in this solution (pH 0), although phenazine is more soluble in strong acidic solutions. The peak potentials shifted in the direction of the more positive potentials that relate to the participation of protons in the electrode reactions. The peak currents are proportional to v1/2, which indicates a diffusion-limited process. The voltammograms resembled those obtained in perchlorate-containing solutions (Fig. 5a), especially regarding the splitting of peak I.
The potential differences between the peaks belonging to the first and second electron transfer reactions, respectively, are larger at pH 0, which is a consequence of the different pH dependences of these redox reactions. The shifts of the peak potentials belonging to the first redox processes are rather high: ΔEpc (Ia)=−(110±10) mV/pH, ΔEpc (I)=−(90±10) mV/pH, ΔEpa (IV)=−(125±10) mV/pH attesting to a more complex mechanism with participation of two protons/electron. In the case of the second redox reaction these values are ΔEpc (II)=−(35±10) mV/pH and ΔEpa (III)=−(35±10) mV/pH which correspond to a process with one proton exchanged per two electrons transferred (the position of the peak potentials depends on the scan rate, however: the shift with the pH is more or less insensitive to it). The fundamental characteristics of the EQCM curves are rather similar in the two electrolytes. At slow sweep rates, a small but interesting effect appears (Fig. 6b), i.e. there is a frequency increase at the beginning of peak III before the frequency decreases again. Although this effect is minor, it is an indication that non-equilibrium processes exist and produce unstable species, which later decay to thermodynamically stable ones.
Effect of waiting-time at different potentials, potential step experiments, and evidence for nucleation
If we hold the potential at −0.15 V the colour of the layer changes from yellow to green and eventually to dark greenish blue. Figure 7a shows that by executing a potential cycle between −0.15 V and −0.4 V a mass decrease can be detected at E<−0.3 V. It means that at peak II species (Mapp=210±10) leave the surface layer. If we hold the potential at + 0.5 V for some minutes the yellow colour of the crystals appears again, and the regular voltammograms and EQCM responses will be regained. However, if the scan is started without a waiting time at potentials more positive than 0.1 V, rather surprising cyclic voltammograms and microgravimetric responses can be obtained. Figure 7b shows the results obtained at 10 mV s–1, when the scan was started from 0.5 V. Although, the four waves characteristic to the phenazine reduction/reoxidation are present and the voltammograms show diffusion-limited behaviour, the frequency change is rather different from that observed for phenazine. The mass decrease starts at peak I (just the opposite to the “regular” behaviour), it continues in the region of peak II, with practically no mass change until peak III, where the mass begins to increase, eventually reaching the starting value. At slow scan rates the “fine structure” of the changes, which cannot be seen at higher scan rates, comes to light. There are two distinct mass decrease intervals at peaks I and II, respectively. During reoxidation the mass increase also splits into two well-defined portions at peaks III and IV, respectively. However, even more interesting, the mass-increase sections are preceded by temporary mass decreases. These experiments reveal that at −0.15 V, i.e. in the region of peak Ia and peak I, the thermodynamically stable species are \({\text{PH}}^{{^{{\text{ + }}}_{\raise0.145em\hbox{${\scriptscriptstyle \bullet}$}} }}_{{\text{2}}} {\text{X}}^{ - } \) and probably protonated dimers. The mass decrease during further reduction indicates an anion loss. Somewhat surprisingly the Mapp values calculated show a rather strong sweep rate dependence. Mapp values are almost doubled at 10 mV s–1 in comparison with those determined at 50 mV s–1 (Mapp=280±10). These values are much higher than can be assigned to an ion exchange process. Consequently, we have to assume that chloride ions, together with 13–24 water molecules, leave the layer during reduction and enter the film again during reoxidation in the region of peaks III and IV. The results of slow-sweep-rate experiments demonstrate that the actual processes proceed in a more complex way, although the eventual frequency changes are practically identical (ca. 4,000 Hz).
The cyclic voltammetric (solid line) and the simultaneously detected EQCM (dotted line) responses after holding the potential at −0.15 V for 1 min. Starting potentials: −0.15 V (a), 0.5 V (b). Scan rates: 50 mV/s (a) and 10 mV s–1 (b). The Au|phenazine electrode was in contact with 1 mol dm–3 HCl/NaCl (pH 2) electrolyte
The results of potential step–potentiostatic experiments (Fig. 8) reveal that beside the potential-induced fast and large mass changes in connection with electron transfer reactions accompanied with the sorption/desorption of ions and water molecules, there are also slow mass changes which are almost independent of the potential, and continue for a long time while only a very small or no current flows. It indicates a slow diffusion inside the layer and chemical reactions. There are dominant mass changes: mass increase during reduction in the potential interval from 0 V to −0.1 V (peak I on the cyclic voltammogram) and mass decrease with the same magnitude that starts at circa 0.1 V which corresponds to peak IV.
Another important feature appears when the potential was stepped from −0.1 V to −0.2 V (Fig. 9). After the vertical spike, which is connected with the rapid onset and decay of the capacitive charging current, a peaked current–time transient develops. The latter is a proof for the existence of nucleation and growth kinetics. With this observation we have all the diagnostic criteria for solid–solid phase transformation under the rate control of nucleation and growth, since the other characteristic features (large separation of the reduction and oxidation peaks, narrow peak widths, and the typical scan rate dependence when the peaks at lower scan rates emerge from the rising portion of the voltammograms) [28] can be seen on the cyclic voltammograms. However, we can add a new characteristic to those theoretically established in the literature. A closer inspection of the frequency response presented in Fig. 9 reveals that in the region of phase transformation a characteristic pattern, frequency increase/decrease, can be seen. Such frequency variation can be seen also on the f versus E curves obtained simultaneously with the cyclic voltammograms. This effect is especially striking in Fig. 6b: a small frequency increase before the large frequency decrease in the potential region between −0.2 V and −0.25 V during reduction, and a well-developed frequency maximum at circa −0.03 V during reoxidation. When the layer was fully reduced at −0.15 V such effects could be seen at all voltammetric peaks (Fig. 7b). This phenomenon is better seen at slower scan rates.
Successive potential steps from 0 V to −0.1 V and then to −0.2 V. Experimental conditions are described in Fig. 8. Note the peaked current–time transient after stepping from −0.1 V to −0.2 V. Solid line current, dotted line frequency
The apparent molar mass that can be calculated from these data is unusually high: although it is generally in the order of magnitude of some hundreds of hertz, for the change occurring at peak III (Fig. 7b) Mapp was as high as 2,800! It should be mentioned that an anomalously large mass increase was observed during the reduction of methylene blue on gold electrodes in the presence of fluoride ions. It was explained by a 2D phase transition, and it was postulated that the phase transition proceeds with the release of hydration water [50]. Anomalously large frequency transients were found during the initial reduction step after electrogeneration of the tetracyanoquinodimethane (TCNQ) surface state from [9-aminoacridinium (TCNQ)2], which was explained by extensive hydration and/or surface structural changes [32]. It is exactly what we have observed for phenazine microcrystals. The current–potential responses supply further evidences for the hypothesis based on EQCM data [50], that indeed a phase transformation occurs during the transition of phenazine to its cation salt. The occurrence of strain at sites of lattice mismatch strongly influences the EQCM response. The results of X-ray diffraction [28] and atomic force microscopy (AFM) [38] measurements have also confirmed in the case of TCNQ that the neutral molecules and the anionic salts have different crystal structures, and morphological changes at micrometer and nanometer scales occur after extensive redox cycling of the microcrystals. Based on the in situ AFM observations for TCNQ microcrystals, a model was proposed [38] which seems to be operative for phenazine deposits, too. According to this model the transformation of the initial structure takes place in several steps involving an electrochemical dissolution and formation of a new crystalline solid which remains stable during further redox cycling [38]. After a period of redox cycling the cyclic voltammetric response shows the characteristics of a simple nucleation-growth mechanism, where reduced and oxidized phases can be interconverted without the involvement of dissolution [38]. The only difference regarding phenazine microcrystals is that no macroscopic dissolution causing mass loss and decreasing current was observed. However, the loss of material from the surface depends on the solubility and the adhesion to the metal of the species involved as well as on the rate of the diffusion and crystallization, which are different for the two systems.
Mechanistic considerations
In the potential region of peak I, the reactions shown in Eq. 5, Eq. 6, Eq. 7, and Eq. 8, take place. First, at peak Ia the reduction of the protonated phenazine (PH+) occurs (Eq. 6). However, PH· species immediately react further, forming \({\text{PH}}^{{^{{\text{ + }}}_{\raise0.145em\hbox{${\scriptscriptstyle \bullet}$}} }}_{{\text{2}}} {\text{X}}^{ - } \) (Eq. 7). At peak I the reduction of unprotonated phenazine (P) takes place according to the reactions Eq. 5 and Eq. 8. During this process anions together with water molecules enter the film. In the case of chloride-containing solutions at steady state Mapp=107±10 which indicate the ingress of one Cl– ion+four H2O molecules per electron transferred. Perchlorate ions enter the surface layer practically in unhydrated form since Mapp=116±10 which indicates 1 mol H2O/1 mol ClO4– (in both cases the contribution of H+ ions to the mass change is neglected, and for the sake of simplicity the hydration is taken into account only for anions). This difference can be explained by the higher hydration number of Cl– ions, since according to the literature data the enthalpy of hydration of Cl– ions is 1.5 times higher than that of ClO4– ions [51], but also by a stronger interaction between \({\text{PH}}^{{^{{\text{+}}}_{\raise0.145em\hbox{${\scriptscriptstyle \bullet}$}} }}_{{\text{2}}} \) and ClO4–. The comparison of the results of HClO4/NaClO4 (pH 2) and 1 mol dm–3 H2SO4 (pH 0) experiments are also instructive. The pH dependence of Epc (Ia) and Epc (I) supports the assumption of Eq. 5, Eq. 6, Eq. 7, and Eq. 8. Furthermore, it can be seen in Fig. 6 that the mass increase that starts at peak Ia not only continues in the potential region of peak I—as in the solutions of pH 2—but the rate of the mass increase is even higher. It is an expected difference between the more and the less acidic solutions inasmuch as at pH 0 there are more species in a protonated form (PH+X–), and therefore a more intensive incorporation of anions prevails when the reduction of the unprotonated P starts at peak I. It should be mentioned that HSO4– enter the surface layer together with one H2O molecule since Mapp=125±10.
The events at peak II are more complicated. We assume the reduction of \({\text{PH}}^{{^{{\text{+}}}_{\raise0.145em\hbox{${\scriptscriptstyle \bullet}$}} }}_{{\text{2}}} \) and the formation of 5,10-dihydrophenazine (PH2) which may also be protonated in strong acid solutions (PH3+):
The reaction shown by Eq. 9 is most likely at pH 2, and it is in accordance with the small frequency increase, or at least the substantial decrease of the slope of frequency-potential curves in this potential region. Because H+ ions do not participate in Eq. 9 and Eq. 10 proceeds only to a certain extent in very acidic media, the much smaller pH-dependence of the peak potential becomes understandable. However, unreacted phenazine is still present in high concentration in the adjoining layer. Therefore, the formation of phenazinehydrine charge-transfer complex is preferred:
The blue colour of this complex can be seen. The composition of the charge-transfer complex may be varied as was suggested in the case of methylene blue [50]. Therefore, the formulas of the complexes can be given as follows: \( {\left( {\text{P}} \right)}_{x} {\left( {{\text{PH}}_{2} } \right)}_{y} {\left( {{\text{H}}_{2} {\text{O}}} \right)}_{z} ,\;{\left( {\text{P}} \right)}_{x} {\left( {{\text{PH}}^{{^{ + }_{ \bullet } }}_{{\text{2}}} } \right)}_{q} {\left( {{\text{X}}^{ - } } \right)}_{q} {\left( {{\text{H}}_{2} {\text{O}}} \right)}_{z} \) or \( {\left( {{\text{PH}}^{{^{ + }_{ \bullet } }}_{{\text{2}}} } \right)}_{q} {\left( {{\text{PH}}_{2} } \right)}_{y} {\left( {{\text{X}}^{ - } } \right)}_{q} {\left( {{\text{H}}_{2} {\text{O}}} \right)}_{z} \). The charged complexes can be formed by the reaction between P and \( {\text{PH}}^{{^{{\text{ + }}}_{ \bullet } }}_{{\text{2}}} {\text{X}}^{ - } \) or in the course of further reduction of P-PH2, i.e.
It explains the further mass increase after the sweep reversal until I<0. However, all these changes depend on the experimental time scale. Equation 11 is a chemical reaction the rate of which may be limited either by the rate of the chemical reaction, or by the diffusion. It is the very reason for the potential-independent and/or currentless mass changes observed during potentiostatic experiments. The most surprising effect is that after a slight mass decrease at the foot of peak III, a rather large mass increase can be observed. The uptake of further anions is connected with the oxidation of the reduced phenazine. Two types of species are present: the charge-transfer (CT) complexes of different compositions and 5,10-dihydro-phenazine. Equation 9 proceeds in the reverse direction in this potential region and the oxidation of CT complexes can be described as follows:
It explains the mass increase observed, since these reactions result in the formation of additional, positively charged ions and the charge compensation involves the sorption of anions. The maximum mass increase is achieved in the region of peak IV, which may be related to the formation of unstable PH22+(X–)2 species during further oxidation:
Equation 15 has been suggested by Haas et al. [52] to explain the similar frequency minimum prior to the frequency increase observed for poly(1-hydroxyphenazine) electrodes. It was assumed that a local shift in the pH of the film to lower values occurs, and that it causes the formation of the unstable dication. The decay of the dication to the stable PH+X– or P species follows Eq. 15 which involves the expulsion of one proton and one anion (or two protons and two anions).
The sorption of additional anions and the expulsion of two anions together with two protons and some water molecules are supported by the molar mass data calculated from the results of cyclic EQCM experiments, since the following Mapp values were derived for the mass decrease during reoxidation: Mapp=159±10 (HCl/NaCl), Mapp (HClO4)=192±10 and Mapp (H2SO4)=247±10. Similar Mapp values were obtained from the potential step experiment. For instance, from the data shown in Fig. 8 Mapp=140±10 (−0.1 V to 0 V) and Mapp=203±10 (0 V to 0.1 V), i.e. at peak III an additional ClO4– enters, and at peak IV two perchlorate ions leave the surface layer. It is also evident that the formation of the unstable intermediate is a fast process while the expulsion of anions, hydrogen ions and water molecules, which is driven by thermodynamic constraints and/or diffusion and chemical reaction, is relatively slow. It has been mentioned that at \( - 0.15{\text{V}}{\text{,}}\;{\text{PH}}^{{^{{\text{ + }}}_{ \bullet } }}_{{\text{2}}} {\text{X}}^{ - } \) and stable charged dimers are formed. At peaks Ia and I the primary product is \( {\text{PH}}^{{^{{\text{ + }}}_{ \bullet } }}_{{\text{2}}} \). It forms a green stable salt [1, 12]. The dimerization of the phenazylium radical cations results in the formation of greenish-blue dimers according to the reaction
Such a dimer can be formed only when \( {\text{PH}}^{{^{{\text{ + }}}_{ \bullet } }}_{{\text{2}}} \) is present in a high concentration; it is not therefore surprising that it has not been described in the organic or electrochemical literature [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12], because such a system has never been investigated in the solid state (high enough concentration) before. The further reaction leads to the formation of PH2 (colourless) accompanied by the release of anions:
Equation 17 by itself does not explain the Mapp values observed since Mapp>200 indicates a −2 X–/1 e– process except if we assume a two-electron reaction for the reduction of each phenazine unit. However, this contradicts the experimental observations. The most reasonable assumption is that the charged dimer is highly hydrated while the neutral PH2 is not. The Mapp values derived indicate the desorption of 13–24 H2O molecules/1 Cl– ion, assuming a one-electron reaction. It follows that the most likely reaction is:
Conclusions
It has been demonstrated that phenazine microcrystals can be attached to gold surfaces and can be investigated in aqueous acidic solutions by electrochemical techniques without the dissolution of the immobilized samples. The results of the cyclic voltammetric and potential step quartz crystal microbalance experiments attest that the electroreduction of phenazine microcrystals in the presence of aqueous acidic solutions takes place via two successive one-electron steps. The first electron-transfer step consists of the reduction of protonated (PH+) and unprotonated phenazine (P), respectively, resulting in the salt of phenazylium radical cation \( {\left( {{\text{PH}}^{{^{{\text{ + }}}_{ \bullet } }}_{{\text{2}}} {\text{X}}^{ - } } \right)} \). In the second reduction step 5,10-dihydrophenazine (PH2) is formed which reacts with the unreduced or partially reduced species. The product of this reaction is phenazinehydrine charge-transfer complex (P-PH2). Charged CT complexes can be formed by the further reduction or oxidation of phenazinehydrine. During reoxidation the processes take place in the reverse direction.
Several cycles are needed for the virgin phenazine layer to become fully electroactive. In the course of this process, a substantial amount of water molecules and ions enters the layer and remains accommodated during further cycling. The electron-transfer reactions are accompanied by the sorption/desorption of counterions and water molecules. Because of the different crystal structures of phenazine and its reduction products \( {\left( {{\text{PH}}^{{^{{\text{ + }}}_{ \bullet } }}_{{\text{2}}} {\text{X}}^{ - } ,\;{\text{PH}}_{2} ,\;{\left( {\text{P}} \right)}_{x} {\left( {{\text{PH}}_{2} } \right)}_{y} {\left( {{\text{PH}}^{{^{{\text{ + }}}_{ \bullet } }}_{{\text{2}}} } \right)}_{q} {\left( {{\text{X}}^{ - } } \right)}_{q} {\left( {{\text{H}}_{2} {\text{O}}} \right)}_{z} } \right)} \) solid–solid phase transformations occur during the reduction and the subsequent reoxidation. The rates of these processes are determined by nucleation/growth kinetics. Evidence for the nucleation mechanism has been found in response functions of both the cyclic voltammetric and potential step experiments; however, it also affects the EQCM frequency response due to the strain in the surface layer. It has also been demonstrated that the phase transition proceeds with the release of hydration water. Reasonable molar mass values were calculated for the species exchanged between the layer and the contacting liquid phase. Based on these data an adequate mechanism can be established, although the strain effect influences the actual values. The amount of sorbed/desorbed solvent molecules strongly depends on the time-scale of the experiments (scan rates).
References
Ramage GR,Lundquist JK(1959) Compounds containing a six membered ring with two hetero atoms.The diazines. In: Rodd EH (ed.) Chemistry of carbon compounds, vol. IVB. Heterocyclic compounds. Elsevier, Amsterdam, pp.1374,1386
Müller OH, Baumberger JP (1937) Trans Electrochem Soc 71:181
Kaye RC, Stonehill HJ (1952) J Chem Soc (London) 3240
Bailey DN, Hercules DM, Roe DK (1969) J Electrochem Soc 116:190
Bailey DN, Roe DK, Hercules DM (1968) J Am Chem Soc 90:6291
Klatt LN, Rouseff RL (1972) J Am Chem Soc 94:7295
Volke J, Beran S (1975) Coll Czechoslov Chem Commun 40:2232
Laviron E, Roullier L (1983) J Electroanal Chem 157:7
Roullier L, Waldner E, Laviron E (1985) J Electrochem Soc 132:1121
Baumgärtel H, Retzlav K-J (1984) In: Bard AJ, Lund H (eds) Encyclopedia of electrochemistry of elements, vol. XV, Dekker, New York, pp 241–265
Takahashi M, Goto M, Ito M (1989) J Electroanal Chem 51:177
Inzelt G, Puskás Z (2004) Electrochim Acta 49:1969
Scholz F, Nitschke L, Henrion G (1989) Naturwissenschaften 76:71
Scholz F, Nitschke L, Henrion G, Damaschun F (1989) Naturwissenschaften 76:167
Scholz F, Meyer B (1998) Voltammetry of solid microparticles immobilized on electrode surfaces. In: Bard AJ, Rubinstein I (eds) Electroanalytical chemistry, vol. 20. Dekker, New York, pp 1–86
Fiedler DA, Scholz F (2002) Electrochemical studies of solid compounds and materials. In: Scholz F (ed) Electroanalytical methods chap. II Springer, Berlin Heidelberg New York, pp 201–222
Komorsky-Lovric S (1997) J Solid State Electrochem 1:94
Scholz F, Lovric M, Stojek Z (1997) J Solid State Electrochem 1:134
Lovric M, Hermes M, Scholz F (1998) J Solid State Electrochem 2:401
Komorsky-Lovric S, Mirceski V, Scholz F (1999) Microchim Acta 132:67
Zhuang QK, Scholz F, Pragst F (1999) Electrochem Commun 1:406
Lovric M, Scholz F (1999) J Solid State Electrochem 3:172
Lovric M, Hermes M, Scholz F (2000) J Solid State Electrochem 4:394
Komorsky-Lovric S, Lovric M, Scholz F (2001) J Electroanal Chem 508:129
Schröder U, Oldham KB, Myland JC, Mahon PJ, Scholz F (2000) J Solid State Electrochem 4:314
Bond AM, Marken F (1994) J Electroanal Chem 372:125
Shaw SJ, Marken F, Bond AM (1996) J Electroanal Chem 404:227
Bond AM, Fletcher S, Marken F, Shaw SJ, Symons PG (1996) J Chem Soc Faraday Trans 92:3925
Bond AM, Marken F, Hill E, Compton RG, Hugel H (1997) J Chem Soc Perkin Trans 2:1735
Wooster TJ, Bond AM, Honeychurch MJ (2001) Electrochem Commun 3:746
Keyes TE, Foster RJ, Bond AM, Miao W (2001) J Am Chem Soc 123:2877
Evans CD, Chambers JQ (1994) Chem Mater 6:454
Kulesza PJ, Jedral T, Galus Z (1989) Electrochim Acta 34:851
Zadronecki M, Wrona PK, Galus Z (1999) J Electrochem Soc 146:620
Zadronecki M, Linek IA, Stroka J, Wrona PK, Galus Z (2001) J Electrochem Soc 148:E348
Mounts RD, Widlund K, Gunadi H, Perez J, Pech B, Chambers JQ (1992) J Electroanal Chem 340:227
Scaboo KM, Grover WH, Chambers JQ (1999) Anal Chim Acta 380:47
Suárez MF, Bond AM, Compton RG (1999) J Solid State Electrochem 4:24
Marken F, Compton RG, Goeting CH, Foord JS, Bull SD, Davies SG (1998) Electroanalysis 10:821
Schröder U, Compton RG, Marken F, Bull SD, Davies SG, Gilmour S (2001) J Phys Chem B 105:1344
Wadhawan JD, Evans RG, Compton RG (2002) J Electroanal Chem 533:71
Banks CE, Davies TJ, Evans RG, Hignett G, Wain AJ, Lawrence NS, Wadhawan JD, Marken F, Compton RG (2003) Phys Chem Chem Phys 5:4053
Gergely A, Inzelt G (2001) Electrochem Commun 3:753
Fehér K, Inzelt G (2002) Electrochim Acta 47:3551
Inzelt G (2002) J Solid State Electrochem 6:265
Inzelt G (2003) J Solid State Electrochem 7:503
Sauerbrey G (1959) Z Phys 155:206
Inzelt G (1994) Mechanism of charge transport in polymer-modified electrodes. In: Bard AJ (ed) Electroanalytical chemistry, vol. 18. Dekker, New York, pp 89–241
Kim YG, Soriaga MP (2001) J Colloid Interface Sci 236:197
Hepel M, Janusz W (2000) Electrochim Acta 45:3785
Erdey-Grúz T (1974) Transport phenomena in aqueous solutions. Hilger, London
Miras MC, Barbero C, Kötz R, Haas O, Schmidt VM (1992) J Electroanal Chem 338:279
Acknowledgements
Financial support by the National Scientific Research Fund (OTKA T031762) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Zbigniew Galus on the occasion of his 70th birthday in recognition of his outstanding contributions to electrochemistry
Rights and permissions
About this article
Cite this article
Puskás, Z., Inzelt, G. Electrochemical microgravimetric study on microcrystalline particles of phenazine attached to gold electrodes. J Solid State Electrochem 8, 828–841 (2004). https://doi.org/10.1007/s10008-004-0551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-004-0551-8