Abstract
The best treatment modality for the management of painful temporomandibular disorders of muscular origin (M-TMD) with predictable outcomes based on solid evidence is still not well defined. Thus, the aim of this network meta-analysis (NMA) was to identify the best treatment for adult patients with M-TMD. An electronic search was undertaken from the inception of each database to August 2018, to identify randomized clinical trials (RCTs), which are comparing two or more of the following treatment modalities in patients with M-TMD: counseling therapy; occlusal appliances; manual therapy; laser therapy; dry needling; intramuscular injection of local anesthesia (LA) or botulinum toxin-A (BTX-A); muscle relaxants; hypnosis/relaxation therapy; oxidative ozone therapy; and placebo or no treatment. Primary outcome variables were the reduction of pain and mechanical sensitivity. The secondary outcome was the maximal mouth opening (MMO). The quality of evidence was rated according to Cochrane’s tool for assessing risk of bias. Standardized mean difference was used to analyze via frequentist network meta-analysis (NMA), using STATA software. 52 RCTs were included in this NMA. At the most follow up moments, manual therapy, counseling therapy, occlusal splints therapy, and needling using BTX-A or LA as well as dry needling significantly decreased post-treatment pain intensity in M-TMDs, when compared to placebo. At short term (≤5 months), the four highest-ranked treatments for post-treatment pain reduction were manual therapy (83.5%, low quality evidence), ozone therapy (75.7%, very low quality evidence),counseling therapy (71.2%, moderate quality), and occlusal appliances (71.7%,moderate quality evidence). When intermediate term (≥6 months)was considered, BTX-A (85.8%, very low quality evidence) , counseling therapy(80%, low quality evidence), occlusal appliances (62.8%, low quality evidence) and hypnosis (50.6%, very low quality evidence) were the four highest-ranked treatments. This NMA reveals that manual therapy can be considered the most effective treatment for M-TMD, followed by counseling treatment, intramuscular injection of LA, and occlusal appliances . However, considering the limitations of the studies included, and the scarce of strong evidence, the present findings should be interpreted cautiously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temporomandibular disorders (TMDs) include conditions such as muscular and temporomandibular joint (TMJ) pain, impaired jaw function, and TMJ joint sounds [1, 2]. Next to chronic back pain, painful TMDs are the second most common musculoskeletal disorder [3, 4], affecting 5–25% of the adult population [5,6,7].
Painful TMD of muscular origin (i.e., TMD-myalgia [M-TMD]) is a musculoskeletal disorder of the masticatory system [8]. It is often described as a dull, pressing muscle pain of moderate intensity, which can become a more intense and sharper pain when provoked [9, 10]. M-TMD is often reported to occur while chewing, and to result in mouth opening difficulties as well as head pain; it has also been shown to be associated with depression and anxiety [11,12,13]. The involved masticatory muscles in M-TMD are usually associated with tender points and/or areas upon palpation [8]. Patients with M-TMD seek treatment to a greater extent than patients with painful TMJ pain [7], and these patients are mainly women aged 20–45 years old [14]. M-TMD has been shown to negatively affect quality of life [3] and, therefore, this chronic pain of moderate to severe intensity requires specific care and treatment [3, 15].
Several different treatments have been reported as successful for the treatment of M-TMD [7]. However, there is still no consensus regarding the most effective treatment for patients with this condition. Suggested treatments for M-TMD include behavioral-cognitive therapy or counseling [16]; physiotherapy or postural therapy [17, 18]; jaw exercises [19, 20]; behavioral medicine [22, 23]; manual or physical treatment such as acupuncture, dry needling, and wet needling therapies [24, 25]; transcutaneous electrical nerve stimulation (TENS) [26]; heat [27] and cold [28]; occlusal appliances [29, 30]; and pharmacological treatments [31], among others.
Many clinical studies have investigated the efficacy of various treatment modalities for the management of M-TMD. However, the best treatment modality with predictable outcomes based on solid evidence is still unknown. Conventional direct meta-analysis is designed to compare only head-to-head studies, which results in comparisons limited to these direct clinical trials [32]. Network meta-analysis (NMA) has emerged as a suitable tool to conduct a collective assessment of various interventions in a single study [33] and not only to compare two interventions that have not been compared directly in a head-to-head clinical trial [32]. An NMA of randomized controlled trials (RCTs) could, therefore, be appropriate to assess different treatments for M-TMD. The null hypothesis for this study was that there would be no differences in pain reduction and maximal mouth opening between the different treatment options for M-TMD. The specific aims of this NMA were to challenge this hypothesis and to identify the best treatment for adult patients with M-TMD.
Materials and methods
Protocol and registration
A NMA of randomized controlled clinical trials (RCTs) was conducted according to the Preferred Reporting Items for the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions (the PRISMA-P checklist) (Appendix A) [34]. This NMA was also registered in the International prospective register of systematic reviews (PROSPERO) with no. CRD42018103671 [35].
Search strategy
Relevant RCTs, in any language and with any publication date, were retrieved by a systematic search from the inception of each database to August 2018 of the following major databases: MEDLINE, EMBASE, CINAHL, the Cochrane Central Registry of Controlled Trials (CENTRAL), and SCOPUS (Appendix B).
Selection criteria
The following inclusion criteria were adopted based on the PICOTS process:
-
(P) Patients: (1) Adult patients with pain due to TMD of myogenous origin (Ia and Ib) based on the research diagnostic criteria for TMD protocol [36] or pain due to myalgia or myofascial pain based on the diagnostic criteria for TMD protocol [5]; (2) adult patients with a clear clinical diagnosis confirmed by the presence of signs and symptoms of TMD of muscular origin as follows: (a) patients with symptoms for at least three months; and (b) patients with two or more areas tender to palpation in the masticatory muscles on one side, namely, the temporalis, masseter, and/or pterygoid muscles.
-
(I) Intervention: RCTs comparing two or more of the following treatment modalities for M-TMD: (1) counseling therapy (including cognitive-behavioral therapy, behavior therapy and education, and self-care and home exercises); (2) occlusal appliances (including full hard/soft flat maxillary or mandibular stabilization splints and an anterior midline stop device); (3) manual therapy (including joint mobilization, manipulation, or treatment of the soft tissues and therapeutic exercises performed by a physiotherapist); (4) intramuscular injections of botulinum toxin-A (BTX-A) into the masticatory muscles regardless of type and dosage; (5) low-level laser therapy (LLT) (the application of soft laser with a wavelength ranging between 630 and 1300 nm on painful masticatory muscles); (6) dry needling (referring to direct needling with a thin monofilament needle without any chemical agent injected directly (superficially or deeply) into the masticatory muscles, provided that it does not conform to the principles of traditional Chinese medicine); (7) local anesthesia (including intramuscular injection of plain lidocaine); (8) muscle relaxants (including oral muscle relaxant regardless of dosage, such as benzodiazepines or cyclobenzaprine); (9) hypnosis/relaxation therapy; (10) oxidative ozone therapy (a gas mixture of medical oxygen and ozone that is produced from pure oxygen and that is passed through a high-voltage gradient (5–13 mV) in a medical generator).
-
(C) Comparator: Only RCTs with a placebo (such as a non-occluding splint, sham needling without any skin penetration, and sham laser therapy) or a control group (patients who did not receive any treatment or those on a waiting list for treatment) were included.
-
(O) Outcomes: Primary outcomes were pain reduction measured with a visual analogue scale (VAS) or pressure pain thresholds (PPT) (i.e., mechanical sensitivity) measured using an algometer. The secondary outcome was maximal mouth opening (MMO).
-
(T) Time: The follow-up time of the included studies was either short term (≤ 5 months), or intermediate term (≥ 6 months).
-
(S) Study design: Only RCTs that reported the outcomes of interest were included.
The following exclusion criteria were applied: (1) studies with missing data required to perform a meta-analysis, such as the post-treatment mean and standard deviation for the outcomes of interest; (2) RCTs that assessed articular or mixed TMDs; (3) non-randomized clinical trials, case series, and cohort studies; (4) review articles; and (5) publications using duplicated data.
Data extraction
A data extraction form was developed for this review and pilot-tested independently on two randomly selected studies by two of the authors (KA and AE) working independently to ensure consistency in extraction. The extraction form was refined accordingly. Data were extracted in duplicate. Any disagreement was resolved by discussion with a third author taking the role of judge (EA). The extracted information included the characteristics of the studies and the participants, including the authors, study design, subgroup diagnosis/criteria used, age of patients, male-female ratio, number of treatments groups, duration/frequency of treatments, and outcome measures.
Assessment of risk of bias and publication bias
The risk of bias of the included trials was assessed independently by two of the authors (AA and KA) using Cochrane’s tool for assessing risk of bias [37, 38].
A comparison-adjusted funnel plot was conducted to assess network-wide publication bias [39, 40].
Certainty of the evidence
To identify the certainty of meta-analysis effect estimates for all outcomes of interest, the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach to meta-analysis was used independently by two of the authors (AA and KA) [38, 41].
Data synthesis
The network geometry was reported with a network plot, used to identify whether the different treatments were connected [42]. The post-treatment value of the outcomes of interest was used to calculate the mean difference (MD) or standardized mean difference (SMD). Results from the NMA were presented as a summary of relative effect sizes for each possible pair of treatments. The statistical unit was the number of patients. NMA was preformed using STATA (StataCorp. 2011, Stata Statistical Software: release 14, College Station, TX, USA) [43], using the mvmeta command [44].
The loop-specific approach using the ifplot command in the Stata program and “design-by-treatment” model using the mvmeta command was taken to evaluate the assumption of consistency at local and global levels [41, 44, 45].
The ranking probabilities for all treatments at each possible rank were investigated using the surface under the cumulative ranking (SUCRA) curve and mean ranks [46]. A rank-heat plot was conducted to visualize and present the treatment hierarchy across the multiple outcomes of interest [46, 47].
To identify the possible sources of inconsistency, the patients were classified into the subgroup follow-up time (i.e., short term and long term). To assess whether the duration of follow-up influenced the outcomes of interest, a meta-regression analysis of the mean of pain reduction based on VAS and the increase of MMO and follow-up time was performed.
Results
Study selection
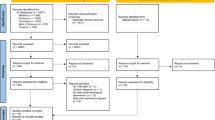
Figure 1 illustrates the PRISMA flow diagram, including the process of evaluating articles for inclusion in the review and NMA. The literature search in all databases resulted in a total of 1200 articles, while 20 additional articles were identified from other sources. Of the 1220 hits in the literature search, 580 articles were duplicates and were removed. Of the 640 remaining articles, 220 were excluded after reading the titles and abstracts. Finally, after reading the remaining 420 full-text articles, 368 were excluded since they did not meet the criteria, resulting in a total of 52 RCTs included and processed in this NMA [10, 40, 48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97].
Presentation of network geometry
Twelve interventions (control, placebo, counseling therapy, occlusal appliances, manual therapy, BTX-A, laser therapy, dry needling, local anesthesia, muscle relaxant, hypnosis/relaxation therapy, and ozone therapy) were included in the network diagrams for the outcome of post-treatment pain intensity via VAS, as shown in Fig. 2. Ten interventions (control, placebo, counseling therapy, occlusal appliances, manual therapy, BTX-A, laser therapy, dry needling, local anesthesia, and ozone therapy) were included in the network diagrams for the outcome of mechanical sensitivity via PPT.
Presentation of the network geometry. This figure shows the network geometry for the outcome of overall post-treatment pain intensity regarding the twelve included interventions: control, placebo, counseling therapy, occlusal appliances, manual therapy, BTX-A, laser, dry needling, local anesthesia, muscle relaxant, hypnosis/relaxation therapy, and oxidative ozone therapy
Study characteristics, individual data, and confidence of evidence
The characteristics of the included RCTs are summarized in Appendix C. Twenty-five of the included studies showed a low risk of bias, thirteen an unclear risk of bias, and fourteen a high risk of bias, as shown in Appendix D. The quality of evidence of direct, indirect, and NMA estimates for all comparisons ranged from high to very low. In various comparisons, the evidence was downgraded because of study limitations, imprecision, or incoherence. More details about the quality of evidence for all outcomes based on the GRADE system are indicated in the figures by different colors on the bar indicating the confidence interval.
Results of individual studies
The individual results of every included RCT, including means, standard deviations, and sample size for overall post-treatment pain intensity, short-term (≤ 5 months), intermediate term (≥ 6 months), MMO, and PPT, are reported in the five tables in Appendix E.
Synthesis of results
Overall pain reduction via VAS
Forty-two RCTs (n = 1989 participants) reported pain reduction using the VAS after treatment of patients with myogenous TMDs with twelve different interventions (Appendix E1). The follow-up times ranged from two weeks to 12 months post-treatment.
This NMA revealed a significant decrease in pain following the use of occlusal appliances (moderate-quality evidence) and counseling therapy (low-quality evidence) when compared with the control or no treatment. Further, this NMA showed a significant reduction in pain after the use of occlusal appliances (moderate-quality evidence), counseling (low-quality evidence), manual therapy (low-quality evidence), BTX-A (very low-quality evidence), dry needling (very low-quality evidence), local anesthesia (very low-quality evidence), ozone therapy (very low-quality evidence), or control (very low-quality evidence) when compared with a placebo, as shown in Fig. 3.
Forest plot of network meta-analysis. This forest plot shows the overall post-treatment pain intensity, in myogenous temporomandibular disorders. The quality of evidence for all outcomes based on the GRADE system is illustrated by different colors on the bar indicating the confidence interval. Green indicates high quality of evidence, blue moderate quality of evidence, black low high quality of evidence, and red very low quality of evidence. SMD, standardized mean difference; CI, confidence interval; BTX-A, botulinum toxin-A; LA, local anesthesia
However, there were no statistically significant differences between the following modalities: occlusal appliances, counseling therapy, manual therapy, BTX-A, dry needling, local anesthesia, and ozone therapy as well as control and placebo when compared with laser treatment, muscle relaxants, and hypnosis.
Overall mechanical sensitivity of masticatory muscles via PPT
Twenty RCTs (n = 828 participants) reported the degree of mechanical sensitivity of the masticatory muscles using algometer-assessed PPT for ten different interventions, as shown in Appendix E2. The follow-up times ranged from two weeks to 6.5 months following treatment. Since treatment RCTs using muscle relaxants and/or hypnosis did not report PPT figures, these two groups were excluded from the network analysis for this variable.
The NMA showed that there were no statistically significant differences between any of the ten different treatments or placebo (all very low-quality evidence), as illustrated in Fig. 4.
Forest plot of network meta-analysis. This forest plot shows the overall change in mechanical sensitization (PPT), in myogenous temporomandibular disorders. The quality of evidence for all outcomes based on the GRADE system is illustrated by different colors on the bar indicating the confidence interval. Green indicates high quality of evidence, blue moderate quality of evidence, black low high quality of evidence, and red very low quality of evidence. SMD, standardized mean difference; CI, confidence interval; PPT, pressure pain threshold; BTX-A, botulinum toxin-A; LA, local anesthesia
Overall change in maximum mouth opening (MMO)
Twenty-one RCTs (n = 807 participants) reported changes in MMO for twelve different interventions, as shown in Appendix E3. The follow-up times ranged from two weeks to 12 months post-treatment.
There was a significant increase in MMO after treatment with manual therapy (low-quality evidence) and local anesthesia (very low-quality evidence) when compared with a control group. Manual therapy also showed a significantly greater increase in MMO when compared with counseling therapy, occlusal appliances, dry needling, control, and/or muscle relaxants (all low-quality evidence). Further, manual therapy, counseling therapy, and hypnosis resulted in a significant greater MMO than occlusal appliances (all low-quality evidence). This NMA could show that the increase in MMO was significantly higher after treatment with muscle relaxants than counseling therapy (moderate-quality evidence), occlusal appliances (low-quality evidence), dry needling (low-quality evidence), and/or BTX-A and laser (both low-quality evidence), as shown in Fig. 5.
Forest plot of network meta-analysis. This forest plot shows the post-treatment maximum mouth opening (MMO), in myogenous temporomandibular disorders. The quality of evidence for all outcomes based on the GRADE system is illustrated by different colors on the bar indicating the confidence interval. Green indicates high quality of evidence, blue moderate quality of evidence, black low high quality of evidence, and red very low quality of evidence. SMD, standardized mean difference; CI, confidence interval; BTX-A, botulinum toxin-A; LA, local anesthesia
Instead, there was a significant decrease in MMO after dry needling when compared with counseling therapy, hypnosis, manual therapy, and/or local anesthesia (all very low-quality evidence). There was also a significant decrease in MMO after dry needling and muscle relaxants when compared with local anesthesia (all very low-quality evidence). Finally, there was a significant decrease after hypnosis when compared with occlusal appliances, muscle relaxants, and dry needling (all very low-quality evidence). No statistically significant differences between ozone therapy and other treatments were found.
Results of additional analyses
This NMA conducted two subgroup analyses based on the duration of the follow-up time, one short-term and one intermediate term.
RCTs with short-term (≤ 5 months) follow-up on pain intensity via VAS
Forty-two RCTs (n = 1525 participants) reported pain intensity via VAS after twelve different interventions, as shown in Appendix E4. The follow-up times ranged from two weeks to three months post-treatment.
There was a significant decrease in pain intensity after all treatments except for hypnosis when compared with a placebo. Further, there was a significant decrease in pain intensity after treatment with manual therapy when compared with the control group (low-quality evidence). Instead, there was a significant increase in pain intensity in the control group when compared with a placebo (very low-quality evidence). Finally, the decrease in pain intensity was significantly greater after treatment with manual therapy when compared with a placebo (moderate-quality evidence), control (moderate-quality evidence), BTX-A, muscle relaxants, dry needling, and/or laser therapy (all low-quality evidence), as shown in Fig. 6.
Forest plot of network meta-analysis. This forest plot shows the overall post-treatment pain intensity in the short term (≤ 5 months), in myogenous temporomandibular disorders. The quality of evidence for all outcomes based on the GRADE system is illustrated by different colors on the bar indicating the confidence interval. Green indicates high quality of evidence, blue moderate quality of evidence, black low high quality of evidence, and red very low quality of evidence. SMD, standardized mean difference; CI, confidence interval; BTX-A, botulinum toxin-A; LA, local anesthesia
RCTs with intermediate-term (≥ 6 months) follow-up on pain intensity via VAS
Nine RCTs (n = 897 participants) reported pain intensity via VAS after seven different interventions, as shown in Appendix E5. The follow-up times ranged from 6 to 12 months after treatment. Since the RCTs on laser therapy, ozone therapy, local anesthesia, and muscle relaxants did not report the presence of pain at the intermediate-term follow-up, these groups were excluded from the network analysis.
There was a significant decrease in pain intensity scores after manual therapy, occlusal appliances, and counseling therapy when compared with a placebo (all low-quality evidence). Further, BTX-A showed a significant decrease in pain intensity when compared with manual therapy (very low-quality evidence). Also, pain intensity was significantly lower after manual therapy when compared with counseling therapy and occlusal appliances (all very low-quality evidence), as seen in Fig. 7.
Forest plot of network meta-analysis. This forest plot shows the overall post-treatment pain intensity in the intermediate term (≥ 6 months), in myogenous temporomandibular disorders. The quality of evidence for all outcomes based on the GRADE system is illustrated by different colors on the bar indicating the confidence interval. Green indicates high quality of evidence, blue moderate quality of evidence, black low high quality of evidence, and red very low quality of evidence. SMD, standardized mean difference; CI, confidence interval; BTX-A, botulinum toxin-A; LA, local anesthesia
Treatment rankings
Overall pain reduction via VAS
The most effective option to reduce pain intensity in the overall follow-up of patients with myogenous TMD was manual therapy (83%, low quality evidence), followed by ozone therapy (79.8%, very low quality evidence), occlusal appliances (73%, moderate quality evidence), counseling therapy (71.2%, low quality evidence), local anesthesia (54.1%), BTX-A (51.5%), dry needling (48%), hypnosis (48 %), control (28.7%), muscle relaxants (27.7%), and placebo (2%) (all very low quality evidence), as illustrated in Fig. 8 and Appendix F.
Rank-heat plot. This rank-heat plot shows the hierarchy of the twelve included treatments regarding the overall post-treatment pain intensity as well as the short-term (≤ 5 months) and intermediate-term (≥ 6 months) post-treatment pain intensities, the maximum mouth opening (MMO), and the mechanical sensitization (PPT), in myogenous temporomandibular disorders. BTX-A, botulinum toxin-A; PPT, pressure pain threshold
Overall mechanical sensitivity of masticatory muscles via PPT
The most effective technique to reduce the mechanical sensitivity of the masticatory muscles in the overall follow-up of patients with myogenous TMD was manual therapy (74.9%), counseling therapy (73%), local anesthesia (72.8%), laser (69.3%), occlusal appliances (49.8%), placebo (44.9%), dry needling (37.1%), BTX-A (29.6%), ozone therapy (26.3%), and control (22.3%) (all very low quality evidence), as shown in Fig. 8 and Appendix F.
Overall change in MMO
According to the SCURA value, the most effective treatments to increase the MMO for patients with myogenous TMD at follow-up times ranging from 1 to 12 months were hypnosis (88%, very low quality evidence), followed by local anesthesia (85%, very low quality evidence), manual therapy (84%, low quality evidence), counseling (58%, low quality evidence), BTX-A (55%), low laser therapy (50%), ozone therapy (47%), control (33.5%), occlusal appliances (22%), dry needling (13.1%), and muscle relaxants (5%) (all very low quality evidence), as shown in Fig. 8 and Appendix F.
RCTs with short- term (≤ 5 months) follow-up on pain intensity via VAS
The most effective treatments to reduce pain intensity in the short term for patients with myogenous TMD were manual therapy (95.5%, low quality evidence), followed by counseling (95.5%, moderate quality evidence), ozone therapy (75.7%, very low quality evidence), occlusal appliances (71.2%, moderate quality evidence), BTX-A (47.6%), local anesthesia (45.6%), laser therapy (42.6%), dry needling (42.2%), control (34.4%), hypnosis (32%), muscle relaxants (29.9%), and placebo (34.4%) (all very low quality evidence), as shown in Fig. 8 and Appendix F.
RCTs with intermediate-term (≥ 6 months) follow-up on pain intensity via VAS
The most effective treatments to reduce pain intensity in the intermediate term were BTX-A (85.5%), followed by counseling (80%), occlusal appliances (62.8%), hypnosis (50.6%), control (41.1%), manual therapy (17.4%), and placebo (12.6%), as shown in Fig. 8 and Appendix F.
Additional analysis
Meta-regression analysis between follow-up time and pain
Meta-regression analysis showed that there was a negative, not statistically significant relationship between pooled means of post-treatment pain intensity and follow-up time in the included studies (coefficient = −0.35, CI: −0.13,0.11, P = 0.870).
Funnel plot and publication bias
The funnel plot for outcomes of overall pain intensity is shown in (Fig. 9). Scatters in the funnel plot were relatively symmetrical, indicating the absence of small-size effect and publication bias.
Funnel plot, publication bias, and overall post-treatment pain intensity. A, control; B, placebo; C, counseling therapy; D, occlusal appliances; E, manual therapy; F, BTX-A; G, laser; I, dry needling; J, local anesthesia; K, muscle relaxant; L, hypnosis/relaxation therapy; M, oxidative ozone therapy
Exploration for inconsistency
For the outcome of overall pain, loop-specific tests to assess local inconsistency did not detect any statistical inconsistency between direct and indirect evidence. All confidence intervals were truncated from zero. There were small inconsistencies (they did not reach statistical significance) in the loops: control – manual-BTX-A therapy; control – placebo – manual therapy; and placebo – occlusal appliances – manual therapy. These insignificant statistical inconsistencies were due to variations in follow-up times. Thus, after subgroup analysis based on follow-up time, the number of formed triangular loops was decreased significantly.
Based on the design-by-treatment interaction model, used to test a global inconsistency in the network, no significant inconsistency between direct and indirect evidence was identified within the evidence network as a whole (I = 23.14; P = 0.39). Therefore, both inconsistency and consistency models were fitted for all analysis (overall pain intensity via VAS, mechanical sensitization of the muscles via PPT, MMO, and the subgroup analyses based on follow-up time) according to global, local, and node-splitting models. The ifplots for all outcomes and subgroup analyses are presented in Appendix G.
Discussion
Currently, there is no consensus regarding the most effective treatment strategies for M-TMD, although several treatments have been presented as successful in the management of TMD [7], which makes it very hard to provide clear therapeutic recommendations. With this in mind, the results from this NMA can help to increase knowledge regarding treatment modalities of M-TMD by providing data regarding both treatment outcomes and treatment rankings.
The main findings of this NMA regarding pain reduction indicate that manual therapy, counseling therapy, occlusal appliances, and BTX-A have a superior treatment effect, both in the short term and intermediate term. This NMA also highlights that local anesthetics and ozone therapy have a superior effect when compared to dry needling, hypnosis, laser therapy, and muscle relaxants in the short term. With regard to maximum mouth opening capacity, manual therapy, local anesthesia, hypnosis, counseling therapy, and BTX-A were superior to the other treatments. Finally, with regard to mechanical sensitivity, no differences in pressure pain threshold were detected, but in terms of treatment rankings, manual therapy, counseling therapy, local anesthesia, laser therapy, and occlusal appliances were superior to the other five treatment modalities.
Altogether, this NMA shows manual therapy to be the most effective treatment for M-TMD, followed by counseling treatment, local anesthesia, and occlusal appliances. These results are not surprising and agree with previous studies. Manual therapy obtains positive results not just in the orofacial region [86, 87] but also in other parts of the body [98, 99]. With regard to counseling therapy, previous studies [10, 100, 101] as well as the Swedish national guidelines for dentistry have stressed the importance of always including or even starting the treatment of TMD with this modality [16]. The superiority of local anesthesia when compared to other needling therapies, as shown in this NMA, was also reported in a recently published NMA showing that local anesthesia is superior to treatment with BTX-A or dry needling, both in the short term and intermediate term [25]. Finally, it is not surprising that the use of occlusal appliances appears in the top-ranked treatments for M-TMD as several studies have reported good treatment outcomes with this modality [48, 102,103,104,105,106]. In addition, a recent NMA reported that occlusal appliances had a real pain-reducing effect at follow-ups, beyond the placebo effect [107].
Except for mechanical sensitivity, placebo was ranked the lowest among all the treatment modalities, indicating that the effect of the included treatment modalities in this NMA is real, beyond the placebo effect only. From one perspective, this could be considered an unexpected finding, since previous studies have detected a significant placebo effect in at least half of the participants in clinical studies [108]. Moreover, this positive placebo effect is even greater in studies investigating pain-reducing treatment modalities where the control group is treated with a placebo [109]. The different results for reported pain (VAS) and mechanical sensitivity (PPT) are expected. The patients’ experience and perception of improvement is usually the first and most sensitive parameter to be noted. Alterations in the PPT, expressed by local changes (peripheral sensitization) and central factors such as central sensitization and impaired inhibitory modulation, seem to need more time to occur and to be expressed in the algometry. Less surprising was the fact that untreated controls were ranked in the lower third of the treatment modalities, oscillating between the third or fourth position from the end. The superiority of untreated controls compared to some of the included treatment modalities, such as muscle relaxants and hypnosis, and the superiority of MMO over occlusal appliances and dry needling may have several different explanations. One is that M-TMD symptoms are usually mild to moderate, fluctuating and self-limiting, and tend not to be progressive [110]; therefore, the passage of time usually has a beneficial outcome on pain outcomes regardless of the treatment strategy. Another explanation is that most outcomes are related only to pain reduction on a single-dimension scale (for this NMA, the VAS), which has the shortcoming of not assessing and identifying all treatment-related changes, such as such satisfaction with the treatment, behavioral improvements, and improved quality of life, among others. It has also been shown that treatment success in M-TMD is poorly correlated with reduction in pain intensity [111, 112]. Hence, by using other variables (in this NMA, the MMO, e.g., physical functioning), more dimensions of the patients’ experiences are taken into consideration. The use of a single-dimension scale can therefore explain why treatment modalities such as occlusal appliances and dry needling were ranked among the top five treatments in this NMA but ranked lower than the untreated controls in relation to physical functioning. Therefore, future studies not only should include changes in pain intensity as a single variable but should also consider pain as a multidimensional experience [113]. This would result in the inclusion of several outcome variables, such as physical functioning (including jaw function), psychosocial and behavioral aspects, and emotional status (stress, depression, anxiety, somatization, pain catastrophizing, etc.) [111, 112, 114,115,116]. Improvements in psychological variables as such anxiety and depression were reported in patients after counseling and the use of occlusal appliances, while decreased pain catastrophizing levels were detected in those receiving only counseling [81]. These findings highlight the urgent need to expand the parameters of judging treatment efficacy beyond the single pain report, which is usually done usually in a biased fashion with the use of single-dimension scales.
The current NMA has the following limitations: (1) since all the included RCTs used different criteria in the recruitment of patients with respect to the severity and chronicity of M-TMD at baseline, selection bias may be present in the original RCTs. Thus, a minimum of three months of signs and symptom of M-TMD was used in the present study as an inclusion criteria; (2) heterogeneity was also present in the treatment modalities with regard to medication dosage, number of treatment sessions/injections, etc., which may also have affected the outcomes.
The present study has the following strengths: (1) to the best of the authors’ knowledge, this is the first NMA including 52 RCTs that assesses the hierarchy of twelve different treatment modalities versus placebo for patients with M-TMD; (2) only RCTs that assessed M-TMD were included; (3) the GRADE system to assess the certainty of the evidence for all outcomes was used to avoid under- and overestimation of the effect size measure; (4) subgroup analyses were conducted based on follow-up times (e.g., short term and intermediate term) to identify the impact of effect modifiers such as the follow-up time; (5) all evidence and analyses were derived from consistency assumptions, since the presence of transitivity and the absence of incoherence were checked using global, local, and node split statistical tests, which all indicate insignificant inconsistencies; (6) transitivity and consistency assumptions were upheld in the current study since it showed an insignificant correlation between the follow-up times and the changes in post-treatment pain intensity; and (7) the reference group (common comparator) was the placebo group. Although these results are not surprising and reflect those of previous studies, it is interesting to note that all reversible treatments considered in the actual NMA showed some degree of pain and MMO improvement over time. The time effect (short term or intermediate term) and the variables considered did not strongly affect these favorable outcomes. Even the placebo and control groups showed improvements at a certain level, which reinforces the benign progression of M-TMD. Therefore, there is a need to expand the way the response of patients to treatments is judged. The actual findings suggest the inclusion of other psychological and behavioral outcomes in future studies designed to investigate the efficacy of different treatment modalities not only for M-TMD but, ideally, for all clinical studies dealing with a complex phenomenon such as pain, especially in chronic conditions. These complementary outcome variables could be patient satisfaction or global improvement; physical functioning, including jaw function; psychosocial and behavioral measurements; and emotional status, including stress, depression, anxiety, and somatization.
In conclusion, this NMA showed that manual therapy is the most effective treatment for M-TMD, followed by counseling, local anesthesia, and occlusal appliances. However, considering the limitations of the studies included, and the scarce of strong evidence, the present findings should be interpreted cautiously. For future studies, the authors suggest the inclusion of behavioral and psychosocial variables when judging the efficacy of pain therapies.
Change history
05 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10006-021-01019-w
References
Kraaijenga S, Molen LVD, Tinteren HV, Hilgers F, Smeele L (2014) Treatment of myogenic temporomandibular disorder : a prospective randomized clinical trial, comparing a mechanical stretching device ( TheraBite H ) with standard physical therapy exercise. Cranio 32(3):208–16
Miller VJ, Karic VV, Myers SL, Exner HV (2000) Myogenous temporomandibular disorder patients and the temporomandibular opening index. J Oral Rehabil 27(8):720–722
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB et al (2015) A classification of chronic pain for ICD-11. Pain 156(6):1003–1007
Chisnoiu AM, Picos AM, Popa S, Chisnoiu PD, Lascu L, Picos A, Chisnoiu R (2015) Factors involved in the etiology of temporomandibular disorders - a literature review. Clujul Med 88(4):473–478
Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F et al (2014) Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache 28(1):6–27
Lovgren A, Haggman-Henrikson B, Visscher CM, Lobbezoo F, Marklund S, Wanman A (2016) Temporomandibular pain and jaw dysfunction at different ages covering the lifespan–a population based study. Eur J Pain 20(4):532–540
Kuttila M, Niemi PM, Kuttila S, Alanen P, Le Bell Y (1998) TMD treatment need in relation to age, gender, stress, and diagnostic subgroup. J Orofac Pain 12(1):67–74
Fricton J, Look JO, Wright E, Alencar FG Jr, Chen H, Lang M, Ouyang W, Velly AM (2010) Systematic review and meta-analysis of randomized controlled trials evaluating intraoral orthopedic appliances for temporomandibular disorders. J Orofac Pain 24(3):237–254
Wright EF, North SL (2009) Management and treatment of temporomandibular disorders: a clinical perspective. J Man Manip Ther 17(4):247–254
Craane B, Dijkstra PU, Stappaerts K, De Laat A (2012) One-year evaluation of the effect of physical therapy for masticatory muscle pain: a randomized controlled trial. Eur J Pain 16(5):737–747
Dao TT, LeResche L: Gender differences in pain. J Orofac Pain 2000, 14(3):169-184; discussion 184-195.
Unell L, Johansson A, Carlsson GE, Halling A, Soderfeldt B (2006) Changes in reported orofacial symptoms over a ten-year period as reflected in two cohorts of fifty-year-old subjects. Acta Odontol Scand 64(4):202–208
Wadhwa S, Kapila S (2008) TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ 72(8):930–947
Gauer RL, Semidey MJ (2015) Diagnosis and treatment of temporomandibular disorders. Am Fam Physician 91(6):378–386
Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D (2006) Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 10(4):287–333
Socialstyrelsen: Nationella riktlinjer för vuxentandvård. Västerås: Edita Västra Aros; 2011.
Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH (2017) Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev 4:CD011279
Naugle KM, Fillingim RB, Riley JL 3rd (2012) A meta-analytic review of the hypoalgesic effects of exercise. J Pain 13(12):1139–1150
Michelotti A, de Wijer A, Steenks M, Farella M (2005) Home-exercise regimes for the management of non-specific temporomandibular disorders. J Oral Rehabil 32(11):779–785
Medlicott MS, Harris SR (2006) A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Phys Ther 86(7):955–973
McMahon FG, Arndt WF Jr, Newton JJ, Montgomery PA, Perhach JL (1987) Clinical experience with flupirtine in the US. Postgrad Med J 63(3 Suppl):81–85
Main CJ, Keefe FJ, Jensen MP, Vlaeyen JW, Vowles KE: Fordyce’s behavioral methods for chronic pain and illness: republished with invited commentaries: Wolters Kluwer Health; 2015.
Meichenbaum D: The evolution of cognitive behavior therapy: a personal and professional journey with Don Meichenbaum: Taylor & Francis; 2017.
Shermer M, Linse P: The skeptic encyclopedia of pseudoscience: ABC-CLIO; 2002.
Al-Moraissi EA, Alradom J, Aladashi O, Goddard G (2020) Christidis N Needling therapies in the management of myofascial pain of the masticatory muscles: a network meta-analysis of randomised clinical trials. J Oral Rehabil 47(7):910–922
Long DM, Hagfors N (1975) Electrical stimulation in the nervous system: the current status of electrical stimulation of the nervous system for relief of pain. Pain 1(2):109–123
Okeson JP: Management of temporomandibular disorders and occlusion-E-book: Elsevier Health Sciences; 2014.
Travell J (1952) Ethyl chloride spray for painful muscle spasm. Arch Phys Med Rehabil 33(5):291–298
List T, Axelsson S (2010) Management of TMD: evidence from systematic reviews and meta-analyses. J Oral Rehabil 37(6):430–451
Kurita H, Kurashina K, Kotani A (1997) Clinical effect of full coverage occlusal splint therapy for specific temporomandibular disorder conditions and symptoms. J Prosthet Dent 78(5):506–510
Haggman-Henrikson B, Alstergren P, Davidson T, Hogestatt ED, Ostlund P, Tranaeus S, Vitols S, List T (2017) Pharmacological treatment of oro-facial pain - health technology assessment including a systematic review with network meta-analysis. J Oral Rehabil 44(10):800–826
Rouse B, Chaimani A, Li T (2017) Network meta-analysis: an introduction for clinicians. Intern Emerg Med 12(1):103–111
Kanters SFN, Druyts E, Thorlund K, Mills EJ, Bansback N (2016) Use of network meta-analysis in clinical guidelines. Bull World Health Organ 94(10):782–784
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784
Al-Moraissi EA: Hierarchy of different treatments for temporomandibular myogenous disorders: a network meta-analysis of randomized controlled clinical trials. In.: PROSPERO; 2018.
Dworkin SF, LeResche L (1992) Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord: facial & oral pain 6(4):301–355
Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors): Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane, 2019.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336(7650):924–926
Macaskill P, Walter SD, Irwig L (2001) A comparison of methods to detect publication bias in meta-analysis. Stat Med 20(4):641–654
Nixdorf DR, Heo G, Major PW (2002) Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain 99:465–473
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G (2013) Graphical tools for network meta-analysis in STATA. PLoS One 8(10):e76654
Salanti G, Kavvoura FK, Ioannidis JP (2008) Exploring the geometry of treatment networks. Ann Intern Med 148(7):544–553
StataCorp: Stata statistical software: release 13. In: College Station, TX: StataCorp; 2013.
White IR (2015) Network meta-analysis. Stata Journal 15(4):951–985
Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR (2012) Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 3(2):98–110
Salanti G, Ades AE, Ioannidis JP (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64(2):163–171
Veroniki AA, Straus SE, Fyraridis A, Tricco AC (2016) The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J Clin Epidemiol 76:193–199
Turk DC, Zaki HS, Rudy TE (1993) Effects of intraoral appliance and biofeedback/stress management alone and in combination in treating pain and depression in patients with temporomandibular disorders. J Prosthet Dent 70(2):158–164
Herman C, Schiffman E, Look J, Rindal D (2002) The effectiveness of adding pharmacologic treatment with clonazepam or cyclobenzaprine to patient education and self-care for the treatment of jaw pain upon awakening: a randomized clinical trial. J Orofac Pain 16:64–70
Winocur E, Gavish A, Emodi-Perlman A, Halachmi M, Eli I (2002) Hypnorelaxation as treatment for myofascial pain disorder: a comparative study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:429–434
Ga H, Choi JH, Park CH, Yoon HJ (2007) Acupuncture needling versus lidocaine injection of trigger points in myofascial pain syndrome in elderly patients - a randomised trial. Acupunct Med 25:130–136
Shen YF, Goddard G: The short-term effects of acupuncture on myofascial pain patients after clenching. 2007, 7:256-264.
Venâncio RDA, Ph D, Pereira FG, Ph D, Zamperini C (2008) Different substances and dry needling injections in patients with myofascial pain and headaches. Cranio 26:96–103
Ernberg M, Hedenberg-Magnusson B, List T, Svensson P (2011) Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: a randomized, controlled, double-blind multicenter study. Pain 152(9):1988–1996
Nilsson H, Vallon D, Ekberg EC (2011) Long-term efficacy of resilient appliance therapy in TMD pain patients: a randomised, controlled trial. J Oral Rehabil 38(10):713–721
Conti PC, de Alencar EN, da Mota Correa AS, Lauris JR, Porporatti AL, Costa YM (2012) Behavioural changes and occlusal splints are effective in the management of masticatory myofascial pain: a short-term evaluation. J Oral Rehabil 39(10):754–760
Dıraçoğlu D, Vural M, Karan A, Aksoy C (2012) Effectiveness of dry needling for the treatment of temporomandibular myofascial pain: a double-blind, randomized, placebo controlled study. J Back Musculoskelet Rehabil 25:285–290
Kalamir A, Graham PL, Vitiello AL, Bonello R, Pollard H (2013) Intra-oral myofascial therapy versus education and self-care in the treatment of chronic, myogenous temporomandibular disorder : a randomised, clinical trial. Chiropr Man Ther 21:1
La Touche R, Fernández-De-Las-Peñas C, Fernández-Carnero J, Escalante K, Angulo-Díaz-Parreño S, Paris-Alemany A, Cleland JA (2009) The effects of manual therapy and exercise directed at the cervical spine on pain and pressure pain sensitivity in patients with myofascial temporomandibular disorders. J Oral Rehabil 36:644–652
Alencar F, Viana P, Zamperini C, Becker A (2014) Patient education and self-care for the management of jaw pain upon awakening: a randomized controlled clinical trial comparing the effectiveness of adding pharmacologic treatment with cyclobenzaprine or tizanidine. J Oral Facial Pain Headache 28:119–127
Haddad C, Godoy LD, Motta LJ, Porta K, Fernandes S, Mesquita-ferrari RA, Deana AM, Bussadori SK (2015) Effect of low-level laser therapy on adolescents with temporomandibular disorder : a blind randomized controlled pilot study. J Oral Maxillofac Surg 73:622–629
Gonzalez-Perez LM, Infante-Cossio P, Granados-Nunez M, Urresti-Lopez FJ, Lopez-Martos R, Ruiz-Canela-Mendez P: Deep dry needling of trigger points located in the lateral pterygoid muscle: efficacy and safety of treatment for management of myofascial pain and temporomandibular dysfunction. Medicina Oral Patología Oral y Cirugia Bucal 2015:e326-e333.
Celakil T, Muric A, Roehlig BG, Evlioglu G: Management of pain in TMD patients : bio-oxidative ozone therapy versus occlusal splints. CRANIO® 2017, 9634:1-9.
Magri LV, Carvalho VA: Effectiveness of low-level laser therapy on pain intensity , pressure pain threshold , and SF-MPQ indexes of women with myofascial pain. Lasers in Medical Science 2017.
Seifi M, Ebadifar A, Kabiri S, Badiee MR, Abdolazimi Z, Amdjadi P (2017) Comparative effectiveness of low level laser therapy and transcutaneous electric nerve stimulation on temporomandibular joint disorders. J Lasers Med Sci 8:S27–S31
Ondo WG, Simmons JH, Shahid MH, Hashem V, Hunter C, Jankovic J: Onabotulinum toxin-A injections for sleep bruxism. Neurology 2018:https://doi.org/10.1212/WNL.0000000000004951.
Raeissadat SA, Rayegani SM, Sadeghi F, Rahimi Dehgolan S (2018) Comparison of ozone and lidocaine injection efficacy vs dry needling in myofascial pain syndrome patients. J Pain Res 11:1273–1279
Wright E, Anderson G, Schulte J (1995) A randomized clinical trial of intraoral soft splints and palliative treatment for masticatory muscle pain. J Orofac Pain 9(2):192–199
McMillan AS, Nolan A, Kelly PJ (1997) The efficacy of dry needling and procaine in the treatment of myofascial pain in the jaw muscles. J Orofac Pain 11(4):307–314
Carlson CR, Bertrand PM, Ehrlich AD, Maxwell AW, Burton RG (2001) Physical self-regulation training for the management of temporomandibular disorders. J Orofac Pain 15(1):47–55
Goddard G, Karibe H, McNeill C, Villafuerte E (2002) Acupuncture and sham acupuncture reduce muscle pain in myofascial pain patients. J Orofac Pain 16(1):71–76
Kamanli A, Kaya A, Ardicoglu O, Ozgocmen S, Zengin FO, Bayik Y (2005) Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int 25(8):604–611
Guarda-Nardini L, Manfredini D, Salamone M, Salmaso L, Tonello S, Ferronato G (2008) Efficacy of botulinum toxin in treating myofascial pain in bruxers: a controlled placebo pilot study. Cranio 26(2):126–135
Alencar F Jr, Becker A (2009) Evaluation of different occlusal splints and counselling in the management of myofascial pain dysfunction. J Oral Rehabil 36(2):79–85
Oz S, Gokcen-Rohlig B, Saruhanoglu A, Tuncer EB (2010) Management of myofascial pain: low-level laser therapy versus occlusal splints. J Craniofac Surg 21(6):1722–1728
Shen YF, Younger J, Goddard G, Mackey S (2009) Randomized clinical trial of acupuncture for myofascial pain of the jaw muscles. J Orofac Pain 23(4):353–359
Pramod GV, Shambulingappa P, Shashikanth MC, Lele S (2011) Analgesic efficacy of diazepam and placebo in patients with temporomandibular disorders: a double blind randomized clinical trial. Indian J Dent Res 22(3):404–409
Silva ROFd, Conti PCR, Araújo CdRP, Silva RdS: Evaluation of dry needling and 0.5% lidocaine injection therapies in myofascial pain trigger points in masticatory muscles. Dental Press Journal of Orthodontics 2012, 17:113-118.
Zhang F-Y, Wang X-G, Dong J, Zhang J-F, Lü Y-L (2013) Effect of occlusal splints for the management of patients with myofascial pain: a randomized, controlled, double-blind study. Chin Med J 126:2270–2275
Ahrari F, Madani AS, Ghafouri ZS, Tuner J (2014) The efficacy of low-level laser therapy for the treatment of myogenous temporomandibular joint disorder. Lasers Med Sci 29(2):551–557
Costa YM, Porporatti AL, Stuginski-Barbosa J, Bonjardim LR, Conti PC (2015) Additional effect of occlusal splints on the improvement of psychological aspects in temporomandibular disorder subjects: a randomized controlled trial. Arch Oral Biol 60(5):738–744
Sabatke S, Scola RH, Paiva ES, Kowacs PA (2015) Injecction of trigger points in the temporal muscles of patients with miofascial syndrome. Arq Neuro-Psiquiatr 73:861–866
Jadhao VA, Lokhande N, Habbu SG, Sewane S, Dongare S, Goyal N (2017) Efficacy of botulinum toxin in treating myofascial pain and occlusal force characteristics of masticatory muscles in bruxism. Indian J Dent Res 28(5):493–497
Nitecka-Buchta A, Walczynska-Dragon K, Batko-Kapustecka J, Więckiewicz M (2018) Comparison between collagen and lidocaine intramuscular injections in terms of their efficiency in decreasing myofascial pain within masseter muscles: a randomized, single-blind controlled trial. Pain Res Manag 2018:1–10
Wahlund K, List T, Larsson B (2003) Treatment of temporomandibular disorders among adolescents: a comparison between occlusal appliance, relaxation training, and brief information. Acta Odontol Scand 61(4):203–211
Guarda-Nardini L, Stecco A, Stecco C, Masiero S, Manfredini D (2012) Myofascial pain of the jaw muscles: comparison of short-term effectiveness of botulinum toxin injections and fascial manipulation technique. Cranio 30(2):95–102
Oliveira-Campelo NM, Rubens-Rebelatto J, MartÍn-Vallejo FJ, Alburquerque-SendÍn F, Fernández-de-las-Peñas C (2010) The immediate effects of atlanto-occipital joint manipulation and suboccipital muscle inhibition technique on active mouth opening and pressure pain sensitivity over latent myofascial trigger points in the masticatory muscles. J Orthop Sports Phys Ther 40(5):310–317
Demirkol N, Sari F, Bulbul M, Demirkol M, Simsek I, Usumez A (2015) Effectiveness of occlusal splints and low-level laser therapy on myofascial pain. Lasers Med Sci 30(3):1007–1012
Emshoff R, Bosch R, Pumpel E, Schoning H, Strobl H (2008) Low-level laser therapy for treatment of temporomandibular joint pain: a double-blind and placebo-controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105(4):452–456
da Silva Faria CA: Dry needling in the management of myofascial trigger points in the orofacial area. Porto, Portugal: Universidade do Porto; 2014.
van Grootel RJ, Buchner R, Wismeijer D, van der Glas HW (2017) Towards an optimal therapy strategy for myogenous TMD, physiotherapy compared with occlusal splint therapy in an RCT with therapy-and-patient-specific treatment durations. BMC Musculoskelet Disord 18(1):76
List T, Helkimo M, Karlsson R (1993) Pressure pain thresholds in patients with craniomandibular disorders before and after treatment with acupuncture and occlusal splint therapy: a controlled clinical study. J Orofac Pain 7(3):275–282
Michelotti A, Steenks MH, Farella M, Parisini F, Cimino R, Martina R (2004) The additional value of a home physical therapy regimen versus patient education only for the treatment of myofascial pain of the jaw muscles: short-term results of a randomized clinical trial. J Orofac Pain 18(2):114–125
Naikmasur V, Bhargava P, Guttal K, Burde K (2008) Soft occlusal splint therapy in the management of myofascial pain dysfunction syndrome: a follow-up study. Indian J Dent Res 19(3):196–203
Nitecka-Buchta A, Buchta P, Tabenska-Bosakowska E, Walczynska-Dragon K, Baron S: Myorelaxant effect of bee venom topical skin application in patients with RDC/TMD Ia and RDC/TMD Ib: a randomized, double blinded study. Biomed Res Int 2014, 2014:296053.
Al Quran FA, Kamal MS (2006) Anterior midline point stop device (AMPS) in the treatment of myogenous TMDs: comparison with the stabilization splint and control group. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(6):741–747
Sancakli E, Gokcen-Rohlig B, Balik A, Ongul D, Kipirdi S, Keskin H (2015) Early results of low-level laser application for masticatory muscle pain: a double-blind randomized clinical study. BMC Oral Health 15(1):131
Day JA, Stecco C, Stecco A (2009) Application of fascial manipulation technique in chronic shoulder pain–anatomical basis and clinical implications. J Bodyw Mov Ther 13(2):128–135
Pedrelli A, Stecco C, Day JA (2009) Treating patellar tendinopathy with fascial manipulation. J Bodyw Mov Ther 13(1):73–80
Michelotti A, Iodice G, Vollaro S, Steenks MH, Farella M: Evaluation of the short-term effectiveness of education versus an occlusal splint for the treatment of myofascial pain of the jaw muscles. Journal of the American Dental Association (1939) 2012, 143(1):47-53.
Dworkin SF, Huggins KH, Wilson L, Mancl L, Turner J, Massoth D, LeResche L, Truelove E (2002) A randomized clinical trial using research diagnostic criteria for temporomandibular disorders-axis II to target clinic cases for a tailored self-care TMD treatment program. J Orofac Pain 16(1):48–63
Cane L, Schieroni MP, Ribero G, Ferrero M, Carossa S (1997) Effectiveness of the Michigan splint in reducing functional cervical disturbances: a preliminary study. Cranio 15(2):132–135
Daif ET (2012) Correlation of splint therapy outcome with the electromyography of masticatory muscles in temporomandibular disorder with myofascial pain. Acta Odontol Scand 70(1):72–77
List T, Helkimo M, Andersson S, Carlsson GE (1992) Acupuncture and occlusal splint therapy in the treatment of craniomandibular disorders. Part I. A comparative study. Swed Dent J 16(4):125–141
Al-Moraissi EA, Farea R, Qasem KA, Al-Wadeai MS, Al-Sabahi ME, Al-Iryani GM (2020) Effectiveness of occlusal splint therapy in the management of temporomandibular disorders: network meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg 49(8):1042–1056
Kuzmanovic Pficer J, Dodic S, Lazic V, Trajkovic G, Milic N, Milicic B (2017) Occlusal stabilization splint for patients with temporomandibular disorders: meta-analysis of short and long term effects. PLoS One 12(2):e0171296
Alkhutari AS, Alyahya A, Rodrigues Conti PC, Christidis N, Al-Moraissi EA (2021) Is the therapeutic effect of occlusal stabilization appliances more than just placebo effect in the management of painful temporomandibular disorders? A network meta-analysis of randomized clinical trials. J Prosthet Dent 126(1):24–32
Diederich NJ, Goetz CG (2008) The placebo treatments in neurosciences: new insights from clinical and neuroimaging studies. Neurology 71(9):677–684
Vase L, Riley JL 3rd, Price DD (2002) A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain 99(3):443–452
Dao TT, Lavigne GJ, Charbonneau A, Feine JS, Lund JP (1994) The efficacy of oral splints in the treatment of myofascial pain of the jaw muscles: a controlled clinical trial. Pain 56(1):85–94
Feine JS (2000) Treating chronic pain: how do we measure success? N Y State Dent J 66(2):34
Ohrbach R, Dworkin SF (1998) Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain 74(2):315–326
Villemure C, Bushnell CM (2002) Cognitive modulation of pain: how do attention and emotion influence pain processing? PAIN 95(3):195–199
Rudy TE, Turk DC, Kubinski JA, Zaki HS (1995) Differential treatment responses of TMD patients as a function of psychological characteristics. Pain 61(1):103–112
Suvinen TI, Reade PC, Hanes KR, Kononen M, Kemppainen P (2005) Temporomandibular disorder subtypes according to self-reported physical and psychosocial variables in female patients: a re-evaluation. J Oral Rehabil 32(3):166–173
Vlaeyen JW, Morley S (2005) Cognitive-behavioral treatments for chronic pain: what works for whom? Clin J Pain 21(1):1–8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not required.
Informed consent
Not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Originally, the article has been published online with an error in affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix A:
PRISMA NMA Checklist of Items to Include When Reporting A Systematic Review Involving a Network Meta-analysis
Supplementary file1 (DOCX 161 KB)
Appendix B:
PICOTS database search strategy
Supplementary file2 (DOC 37 KB)
Appendix C:
Characteristics of RCTs
Supplementary file3 (DOCX 38 KB)
Appendix D:
Summary of risk of bias
Supplementary file4 (DOCX 25 KB)
Appendix E1-E5:
Five tables with individual data regarding post-treatment pain intensity, maximum mouth opening capacity and mechanical sensitization.
Supplementary file5 (DOCX 103 KB)
Appendix F:
Five figures illustrating the ifplot inconsistency models regarding post-treatment pain intensity, maximum mouth opening capacity and mechanical sensitization.
Supplementary file6 (PPTX 5.53 MB)
Appendix G:
Five figures illustrating the surface under the cumulative ranking (SUCRA) regarding post-treatment pain intensity, maximum mouth opening capacity and mechanical sensitization.
Supplementary file7 (PPTX 7.73 MB)
Rights and permissions
About this article
Cite this article
Al-Moraissi, E.A., Conti, P.C.R., Alyahya, A. et al. The hierarchy of different treatments for myogenous temporomandibular disorders: a systematic review and network meta-analysis of randomized clinical trials. Oral Maxillofac Surg 26, 519–533 (2022). https://doi.org/10.1007/s10006-021-01009-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-021-01009-y