Abstract
Very recently, two new cage-like radicals (C59B and C59N) formed by a boron or nitrogen atom substituting one carbon atom of C60 were synthesized and characterized. In order to explore the structure–property relationships of combination the cage-like radical and alkali metal, the endohedral Li@C59B and Li@C59N are designed by lithium (Li) atom encapsulated into the cage-like radicals C59B and C59N. Further, the structures, natural bond orbital (NBO) charges, and nonlinear optical (NLO) responses of C59B, C59N, Li@C59B, and Li@C59N were investigated by quantum chemical method. Three density functional methods (BHandHLYP, CAM-B3LYP, and M05-2X) were employed to estimate their first hyperpolarizabilities (β tot) and obtained the same trend in the β tot value. The β tot values by BHandHLYP functional of the pure cage-like radicals C59B (1.30 × 103 au) and C59N (1.70 × 103 au) are close to each other. Interestingly, when one Li atom encapsulated into the electron-rich radical C59N, the β tot value of the Li@C59N increases to 2.46 × 103 au. However, when one Li atom encapsulated into the electron-deficient radical C59B, the β tot value of the Li@C59B sharply decreases to 1.54 × 102 au. The natural bond orbital analysis indicates that the encapsulated Li atom leads to an obvious charge transfer and valence electrons distribution plays a significant role in the β tot value. Further, frontier molecular orbital explains that the interesting charge transfer between the encapsulated Li atom and cage-like radicals (C59B and C59N) leads to differences in the β tot value. It is our expectation that this work will provide useful information for the design of high-performance NLO materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonlinear optical (NLO) materials have developed quickly in the past several decades, because of their wide applications in optoelectronics and photonics field [1–7]. Much effort has been devoted to find important factors to enhance the NLO response for designing some new high-performance NLO materials. These strategies include extending π-electron systems [2], twisting π-electron systems [8, 9], changing the relative position of donor and acceptor [10–12], increasing push–pull effects [5], doping alkali metal into organic compounds [13–18] etc. So far, alkali metal endohedral fullerenes show remarkable NLO response owing to extensive π-electron conjugation along with charge delocalization. Recently, workers have reported the NLO response of Li@C60Cl8 [19] and Na@C60C60@F [20], which may be beneficial for further designing and synthesizing NLO molecular materials.
Research has made clear that all-carbon fullerene (C60) strictly obey the isolated pentagon rule (IPR) [21], which avoids pentagon–pentagon contacts by surrounding each pentagon with five hexagons. So it is relatively stable and synthesizable. Recently, a large number of endohedral fullerenes have been reported, for example, C60 endohedral complexes with ions F−, Na+, Mg2+, Al3+ [22], atom Li [23–25], and Gd [26], or molecules LiF [27, 28], and NH4Cl [29] etc. In addition, the cage-like radicals (C59B and C59N) have been achieved and reported, which have attracted wide attention in fullerene science [30–33]. An interesting question arises: how does the endohedral effect of cage-like radical fullerenes influence their nonlinear optical properties?
In order to answer this question, we have paid attention to the cage-like radicals (C59B and C59N) and a new type of endohedral fullerene derivatives (Li@C59B and Li@C59N) formed by encapsulating one Li atom into the cage-like radicals (C59B and C59N). In the present work, the structures, natural bond orbital (NBO) charges, first hyperpolarizabilities, and frontier molecular orbitals of the four molecules are explored by using quantum chemical calculation. We hope the present work can provide new ideas for the design of new optical and photoelectric devices with high performances.
Computational details
The density functional theory (DFT) [34, 35] has been widely used to optimize the geometries of radicals and fullerene systems. In the present work, the optimized geometry structures of C59B, C59N, Li@C59B, and Li@C59N with all real frequencies have been carried out using the B3LYP functional combined with the 6-31G* basis set. On the other hand, considering relatively good accuracy and moderate computational costs, we have optimized the geometric structures of C59B and Li@C59B using the M05-class functional (M05-2X). It was found that the structure parameters calculated by the B3LYP functional are close to that calculated by the M05-2X functional (for more details see Table 1). Besides, NBO charges were also calculated at the B3LYP/6–31+G* level of theory.
In this work, to correct the basis-set superposition error in the bond energy calculation, the counterpoise (CP) correction was used. The interaction energy (E int) [36, 37] was calculated at the B3LYP/6-31G* level according to Eq. (1),
where E int is the difference between the energies of the Li atom (A) and C59X (B) and the sum of the energies of the Li@C59X(X = B, N) (AB).
Further, it is very necessary to choose a suitable method for evaluating their nonlinear optical properties. For a medium-size system, Champagne and Nakano pointed out that the BHandHLYP functional can reproduce the hyperpolarizability values provided by the more sophisticated single, double, and perturbative triple excitation coupled-cluster [CCSD(T)] method [38, 39]. In this work, the first hyperpolarizabilities were calculated at the BHandHLYP/6-31+G* level. In order to confirm the reliability and accuracy of this method, we also use the CAM-B3LYP [40] and M05-2X [41] methods to calculate the first hyperpolarizabilities. The results show that the β tot values obtained by the CAM-B3LYP and M05-2X functionals are close to that obtained by the BHandHLYP functional. Therefore, the BHandHLYP functional is satisfactory for calculating the first hyperpolarizabilities of the four molecules.
The polarizability (α 0) is determined by:
The first hyperpolarizability (β tot) is determined by:
In which
All calculations were performed by using the Gaussian 09 program package [42].
Results and discussion
Optimized geometries and interaction energies (E int)
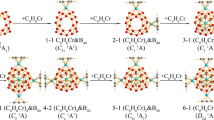
The optimized geometric structures of the four molecules at the B3LYP level are presented in Fig. 1. When a boron or nitrogen atom substitutes one carbon atom of the pristine buckminsterfullerene, C60(Ih) cage, the models of cage-like radicals (C59B and C59N) are formed. Further, the structures of Li@C59B and Li@C59N are obtained by encapsulating a Li atom into the cage-like radicals (C59B and C59N). The corresponding geometric parameters are given in Fig. 2. The C-B-C bond angle of the cage-like radical C59B are in the range of 106.3–118.6°, which is close to the C-N-C bond angle of the cage-like radical C59N (107.2–118.8°). Significantly, after encapsulating one Li atom, the C-N-C bond angles of Li@C 59N (106.8–118.5°) are slightly larger than that of the Li@C59B (104.4–117.0°). The bond lengths of the four molecules are clearly shown in Fig. 2. We can find that the C-B bond lengths of the C59B and Li@C59B are slightly larger than the C-N bond lengths of the C59N and Li@C59N. In addition, the Li-N distance of Li@C59N is about 3.980 Å, which is larger than the Li-B distance of the Li@C59B (2.221 Å).
To further evaluate the stabilities of the two molecules (Li@C59B and Li@C59N), we calculated the interaction energy (E int) at the B3LYP/6-31G* level of theory with a counterpoise correction. The results are listed in Table 2, the E int values of Li@C59B and Li@C59N are −35.21 kcal mol−1 and −63.66 kcal mol−1. This shows that the encapsulating one Li atom and the cages have strong attraction in the two molecules. Furthermore, the E int value of Li@C59N is larger than that of the Li@C59B, which demonstrates that Li@C59N is more stable. Numerous studies illustrate that the DFT method often underestimates the diffusion interaction [43, 44], which means that the actual E int values of the two molecules should be larger than the calculated values. Thus, the corresponding stability is also better than the calculated results.
Natural bond orbital (NBO) analysis
The NBO charges were calculated at the B3LYP/6-31+G* level and the results are shown in Table 2. As we know, the C60(Ih) has no charge transfer due to its centrosymmetric structure. However, for the cage-like radicals C59B, the charge of B atom is 0.702 and the negative charge mainly distributes three carbon atoms (C1, C2, C8) directly connected to the boron atom. Similarly, the charge of N atom is −0.362 and the positive charge mainly distributes three carbon atoms (C1, C2, C8) directly connected to the nitrogen atom in the cage-like radicals C59N. Further, encapsulating one Li atom into the cage-like radicals (C59B and C59N), the charges of Li atom of Li@C59B/Li@C59N are 0.555/0.535, which indicates that the degree of ionization of lithium atom is almost equal in the two cages. Compared to the molecules without one Li doping, the charge of B atom becomes 0.527 in the Li@C59B, while for Li@C59N, the charge of N atom (−0.369) is almost no change. The results show that the transferred electron of the Li atom partially distributes to the boron atom in the Li@C59B. For the Li@C59N, the transferred electron of the Li atom almost distributes to the carbon fragments. According to the above analysis, the encapsulated Li atom is an important factor causing the variation in charge transfer.
Furthermore, how does electron distribution influence the nonlinear optical properties? For the pure cage-like radicals C59B and C59N, the singlet electrons mainly distribute to three carbon atoms (C1, C2, C8) directly connected to the boron or nitrogen atom. The β tot values of the pure cage-like radicals C59B (1.30 × 103 au) and C59N (1.70 × 103 au) are close to each other. When one Li atom encapsulated into the cage-like radicals (C59B and C59N), the valence electrons of the Li atom almost distribute to the cage-like radical. The β tot value of Li@ C59N increases to 2.46 × 103 au. However, the β tot value of Li@C59B sharply decreases to 1.54 × 102 au.
Linear and nonlinear optical property
The polarizabilities (α 0) and the first hyperpolarizabilities (β tot) of the four molecules (C59B, C59N, Li@C59B, and Li@C59N) were calculated by BHandHLYP, CAM-B3LYP, and M05-2X functionals and the results are listed in Table 3. It is clear that each method obtained the close α 0 values. In addition, the α 0 values of C59B (5.40 × 102–5.44 × 102 au), C59N (5.45 × 102–5.41 × 102 au), Li@C59B (5.35 × 102–5.41 × 102 au), and Li@C59N (5.62 × 102–5.68 × 102au) are very close, which shows that the encapsulated Li atom has little effect on the polarizabilities.
According to Table 3 and Fig. 3, different methods obtained the same trend in the β tot value, so we chose the results of the BHandHLYP for further discussion. The first hyperpolarizabilities of the cage-like radicals C59B and C59N are 1.30 × 103 and 1.70 × 103 au, respectively. At the same time, the β tot values of the pure cage-like radicals C59B (1.30 × 103 au) and C59N (1.70 × 103 au) are close to each other. Further, when one Li atom encapsulated into electron-rich radical C59N, the β tot value of Li@C59N increases to 2.46 × 103 au. However, when one Li atom encapsulated into electron-deficient radical C59B, the β tot value of Li@C59B sharply decreases to 1.54 × 102 au. The results show that the encapsulated Li atom influences the static first hyperpolarizabilities sensitively.
The following two-level expression is employed to further understand the origin of the β tot values [45, 46].
The β tot value is proportional to the difference between the dipole moments of the ground state and the crucial excited state (Δμ), the oscillator strength (f 0), and the inverse of the third power of the transition energy (ΔE). According to the two-level model expression, the transition energy is the main factor in the first hyperpolarizability. The f 0, ΔE, and Δμ for the four molecules are obtained by B3LYP/6-31G* level of theory. Firstly, as shown in Table 3 and Fig. 4, the β tot value of the cage-like radical C59B (1.30 × 103 au) is close to the β tot value of the cage-like radical C59N (1.70 × 103 au), the ΔE values of the two molecules are nearly 1.73 eV. Secondly, the β tot value of Li@C59N (2.46 × 103 au) is obviously larger than that of the Li@C59B (1.54 × 102 au), which is inverse to the ΔE values (1.5815 eV for Li@C59N < 2.9530 eV for Li@C59B). Furthermore, the (f 0 · Δμ)/ΔE 3 values of four molecules are shown in Table 3. As one can see, the order of the (f 0 · Δμ)/ΔE 3 values is Li@C59N (1.03 × 102) > C59N (25.59) ≈ C59B (19.07) > Li@C59B (3.50 au). The calculated results show that the interesting encapsulated Li atom effect on the first hyperpolarizability is well explained by the three main factors ΔE, f 0 and Δμ.
Frontier molecular orbital analysis
Furthermore, the molecular orbitals of the considered transitions are shown in Fig. 5. Firstly, compared with the pure cage-like radicals C59B and Li@C59B, the considered transition of radical C59B is the SOMO → LUMO and the considered transition of Li@C59B is HOMO → LUMO+3. For the pure cage-like radicals C59B, the electron density of SOMO almost distributes to the C59B cage atoms excluding the boron atom. At the same time, the electron density mainly covers the boron atom and the neighboring carbon atoms in LUMO. On the contrary, for the Li@C59B, the electron density of HOMO mainly covers the boron atom and the neighboring carbon atom. At the same time, the electron density almost distributes to the C59B cage atoms excluding the boron atom in LUMO+3. Secondly, compared with the pure cage-like radical C59N and Li@C59N, the considered transition of the cage-like radicals C59N is the SOMO → LUMO+3 transition and the considered transition of Li@C59N is HOMO → LUMO+2 transition. For the pure cage-like radicals C59N, the electron density of SOMO mainly covers the nitrogen atom and the neighboring carbon atom, at the same time, the electron density almost distributes to the C59N cage atoms excluding the nitrogen atom in LOMO+3. However, for the Li@C59N, the electron density of HOMO almost distributes to the C59N cage atoms and the electron density mainly covers the carbon atoms which are distant from the nitrogen atom in LUMO+2. As mentioned above, the interesting charge transfer between the encapsulated Li atom and cage-like radicals (C59B and C59N) explain that the first hyperpolarizabilities show differences.
Conclusions
In the present paper, we focus on exploring the structures, stabilities, NBO charges, frontier molecular orbitals, linear, and nonlinear optical properties of the four molecules. The above investigations show that the E int values of Li@C59B and Li@C59N are negative, which shows that the encapsulating one Li atom and cages have strong attraction in the two molecules. The natural bond orbital analysis indicates that the encapsulated Li atom leads to an obvious charge transfer and the valence electrons distribution plays a significant role in the β tot value. Furthermore, frontier molecular orbital confirms that differences of the β tot values can mainly be attributed to charge transfer between the encapsulated Li atom and cage-like radicals (C59B and C59N). We used three DFT methods (BHandHLYP, CAM-B3LYP, and M05-2X) to calculate their first hyperpolarizabilities, and the three methods obtained the same order of the β tot value. Firstly, the α 0 values of the four molecules are close. Secondly, the β tot values of the pure cage-like radicals C59B and C59N are close to each other. Interestingly, when one Li atom encapsulated into the cage-like radicals C59B and C59N, the β tot value of Li@C59N increases. However, the β tot value of Li@C59B sharply decreases. It is clear that the encapsulated Li atom has a large contribution to the β tot value. We hope the present work can provide a novel strategy for enhancing the first hyperpolarizability by altering the molecular structure, which may be beneficial to experimentalists for designing high-performance nonlinear optical materials.
References
Eaton DF (1991) Science 253:281–287
Marder SR, Gorman CB, Meyers F, Perry JW, Bourhill G, Brédas J-L, Pierce BM (1994) Science 265:632–635
Zhong R-L, Zhang J, Muhammad S, Hu Y-Y, Xu H-L, Su Z-M (2011) Chem Eur J 17:11773–11779
Kanis DR, Ratner MA, Marks TJ (1994) Chem Rev 94:195–242
Coe BJ, Jones LA, Harris JA, Brunschwig BS, Asselberghs I, Clays K, Persoons A (2003) J Am Chem Soc 125:862–863
Pavanello M, Jalbout AF, Trzaskowski B, Adamowicz L (2007) Chem Phys Lett 442:339–343
Plaquet A, Champagne B, Castet F, Ducasse L, Bogdan E, Rodriguez V, Pozzo J-L (2009) New J Chem 33:1349–1356
Xu H-L, Li Z-R, Wu D, Ma F, Li Z-J, Gu FL (2009) J Phys Chem C 113:4984–4986
Xu H-L, Li Z-R, Wang F-F, Wu D, Harigaya K, Gu FL (2008) Chem Phys Lett 454:323–326
Hrobárik P, Hrobáriková V, Sigmundová I, Zahradník P, Fakis M, Polyzos I, Persephonis P (2011) J Org Chem 76:8726–8736
Hrobárik P, Sigmundová I, Zahradník P, Kasák P, Arion V, Franz E, Clays K (2010) J Phys Chem C 114:22289–22302
Hrobarik P, Zahradnik P, Fabian WMF (2004) Phys Chem Chem Phys 6:495–502
Chen W, Li Z-R, Wu D, Li Y, Sun C-C, Gu FL (2005) J Am Chem Soc 127:10977–10981
Xu H-L, Li Z-R, Wu D, Wang B-Q, Li Y, Gu FL, Aoki Y (2007) J Am Chem Soc 129:2967–2970
Liu Z-B, Zhou Z-J, Li Y, Li Z-R, Wang R, Li Q-Z, Li Y, Jia F-Y, Wang Y-F, Li Z-J, Cheng J-B, Sun C-C (2010) Phys Chem Chem Phys 12:10562–10568
Li Z-J, Wang F-F, Li Z-R, Xu H-L, Huang X-R, Wu D, Chen W, Yu G-T, Gu FL, Aoki Y (2009) Phys Chem Chem Phys 11:402–408
Wu H-Q, Sun S-L, Zhong R-L, Xu H-L, Su Z-M (2012) J Mol Model 18:4901–4907
Gao Y, Wu H-Q, Sun S-L, Xu H-L, Su Z-M (2015) J Mol Model 21:1–7
Wang L-J, Sun S-L, Zhong R-L, Liu Y, Wang D-L, Wu H-Q, Xu H-L, Pan X-M, Su Z-M (2013) RSC Adv 3:13348–13352
Ma F, Li Z-R, Zhou Z-J, Wu D, Li Y, Wang Y-F, Li Z-S (2010) J Phys Chem C 114:11242–11247
Kroto HW (1987) Nature 329:529–531
Cioslowski J, Fleischmann ED (1991) J Chem Phys 94:3730–3734
Aoyagi S, Nishibori E, Sawa H, Sugimoto K, Takata M, Miyata Y, Kitaura R, Shinohara H, Okada H, Sakai T, Ono Y, Kawachi K, Yokoo K, Ono S, Omote K, Kasama Y, Ishikawa S, Komuro T, Tobita H (2010) Nat Chem 2:678–683
Aoyagi S, Sado Y, Nishibori E, Sawa H, Okada H, Tobita H, Kasama Y, Kitaura R, Shinohara H (2012) Angew Chem Int Ed 51:3377–3381
Fukuzumi S, Ohkubo K, Kawashima Y, Kim DS, Park JS, Jana A, Lynch VM, Kim D, Sessler JL (2011) J Am Chem Soc 133:15938–15941
Bolskar RD, Benedetto AF, Husebo LO, Price RE, Jackson EF, Wallace S, Wilson LJ, Alford JM (2003) J Am Chem Soc 125:5471–5478
Hu YH, Ruckenstein E (2005) J Am Chem Soc 127:11277–11282
Cioslowski J (1991) J Am Chem Soc 113:4139–4141
Ma F, Li Z-R, Xu H-L, Li Z-J, Wu D, Li Z-S, Gu FL (2009) ChemPhysChem 10:1112–1116
Guo T, Jin C, Smalley RE (1991) J Phys Chem 95:4948–4950
Pradeep T, Vijayakrishnan V, Santra AK, Rao CNR (1991) J Phys Chem 95:10564–10565
Chen F, Singh D, Jansen SA (1993) J Phys Chem 97:10958–10963
Muhr HJ, Nesper R, Schnyder B, Kötz R (1996) Chem Phys Lett 249:399–405
Hermosilla L, Calle P, García de la Vega JM, Sieiro C (2005) J Phys Chem A 109:1114–1124
Hermosilla L, Calle P, García de la Vega JM, Sieiro C (2005) J Phys Chem A 109:7626–7635
Alkorta I, Elguero J (1999) J Phys Chem A 103:272–279
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Nakano M, Kishi R, Nitta T, Kubo T, Nakasuji K, Kamada K, Ohta K, Champagne B, Botek E, Yamaguchi K (2005) J Phys Chem A 109:885–891
Champagne B, Botek E, Nakano M, Nitta T, Yamaguchi K (2005) J Chem Phys 122:114315
Tawada Y, Tsuneda T, Yanagisawa S, Yanai T, Hirao K (2004) J Chem Phys 120:8425–8433
Zhao Y, Schultz NE, Truhlar DG (2006) J Chem Theory Comput 2:364–382
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant KN, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Ailaham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2009) Gaussian 09, revision A.02. Gaussian Inc, Wallingford
Shields AE, van Mourik T (2007) J Phys Chem A 111:13272–13277
van Mourik T, Karamertzanis PG, Price SL (2006) J Phys Chem A 110:8–12
Oudar JL (1977) J Chem Phys 67:446–457
Oudar JL, Chemla DS (1977) J Chem Phys 66:2664–2668
Acknowledgments
The authors gratefully acknowledge financial support from National Science Foundation of China (NSFC)(21473026), the Science and Technology Development Planning of Jilin Province (201201062 and 20140101046JC), the Computing Center of Jilin Province provided essential support and H.-L.X. acknowledges support from the Hong Kong Scholars Program (XJ2013007). And Project funded by China Postdoctoral Science Foundation 2014M560227).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, FW., Zhong, RL., Sun, SL. et al. Charge transfer and first hyperpolarizability: cage-like radicals C59X and lithium encapsulated Li@C59X (X=B, N). J Mol Model 21, 258 (2015). https://doi.org/10.1007/s00894-015-2808-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2808-9