Abstract
Quinoline alkaloids are abundant in the Rutaceae, and many have exhibited cytotoxic activity. Because structurally related antitumor alkaloids such as camptothecin and fagaronine are known to function as intercalative topoisomerase poisons, it is hypothesized that cytotoxic Stauranthus alkaloids may also serve as intercalative topoisomerase inhibitors. To test this hypothesis theoretically, ten Stauranthus quinoline alkaloids were examined for potential intercalation into DNA using a molecular docking approach. Four of the alkaloids (stauranthine, skimmianine, 3′,6′-dihydroxy-3′,6′-dihydrostauranthine, and trans-3′,4′-dihydroxy-3′,4′-dihydrostauranthine) were able to intercalatively dock consistently into DNA. In order to probe the intermolecular interactions that may be responsible for intercalation of these quinoline alkaloids, density functional calculations have been carried out using both the B3LYP and M06 functionals. M06 calculations indicated favorable π–π interactions between either skimmianine or stauranthine and the guanine–cytosine base pair. Furthermore, the lowest-energy face-to-face orientation of stauranthine with guanine is consistent with favorable dipole–dipole orientations, favorable electrostatic interactions, and favorable frontier molecular orbital interactions. Likewise, the lowest-energy face-to-face orientation of stauranthine with the guanine–cytosine base pair reveals favorable electrostatic interactions as well as frontier molecular orbital interactions. Thus, not only can quinoline alkaloids dock intercalatively into DNA, but the docked orientations are also electronically favorable.

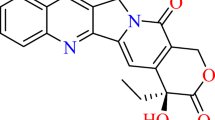
Lowest-energy face-to-face π–π interaction between stauranthine and guanine
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are numerous antineoplastic agents that function by way intercalating into double-stranded B-DNA [1]. These intercalating antitumor drugs are typically planar aromatic heterocycles and function by forming a ternary cleavable complex with DNA and various DNA polymerases such as topoisomerases I and II [2–5]. Such compounds include the topoisomerase I poisons camptothecin and its derivatives [6], nitidine and fagaronine [7], as well as the topoisomerase II inhibitors mitoxantrone [8], ellipticine [9], and cryptolepine [10].
Quinoline alkaloids are abundant in the Rutaceae, and have been suggested to serve as signature compounds for this family [11–13]. A number of these compounds are planar aromatic heterocycles that have shown cytotoxic activity (for example, see [14–19]). The cytotoxic quinoline alkaloid lunacridine has been found to inhibit topoisomerase II [20], and a series of cytotoxic quinolines that inhibit topoisomerases have been synthesized [21–23]. Similarly, the makaluvamines [24] and pyridoacridines [25] are marine alkaloids that exhibit both topoisomerase I and topoisomerase II inhibition [26, 27]. In our group, using activity-directed preparative chromatographic methods, we have isolated a number of cytotoxic quinoline alkaloids from Stauranthus perforatus, and identified them using NMR techniques [28, 29]. Because of the structural similarities to known intercalating antitumor compounds, we hypothesize that the cytotoxic activities of quinoline alkaloids are due to DNA intercalation and/or inhibition of topoisomerase activity leading to apoptosis.
In order to test this hypothesis, we have carried out a molecular docking analysis of Stauranthus quinoline alkaloids with DNA to examine the steric requirements and constraints of intercalation of the quinoline alkaloids between DNA base pairs (BP). X-ray crystal structures have been solved for various intercalators with DNA, including daunomycin [30], idarubicin [31], iododoxorubicin [32], morpholino-doxorubicins [33], 9-amino-[N-(2-dimethylamino)ethyl]acridine-4-carboxamide [34], actinomycin D [35], nogalamycin [36], bis-daunomycin [37], cryptolepine [38], and ellipticine [39]. Similarly, NMR structures of intercalated complexes of DNA have been solved, including nogalamycin [40] and bis-daunorubicin [41]. In addition, the crystal structures of the ternary complex of human topoisomerase I, a 22 bp DNA duplex, with the intercalaters topotecan [42] and camptothecin [43], have been reported. Analogous molecular docking investigations have been reported previously for camptothecin and camptothecin derivatives with the DNA-topoisomerase I complex [44], as well as substituted 9-arylacridines with the DNA-topoisomerase I complex [45].
We further hypothesize that, in addition to planar π intercalation, the π-stacked complexes are stabilized further by favorable, complementary frontier molecular orbital interactions [46, 47]. That is, the highest occupied molecular orbital (HOMO) of the C–G base pair has the correct nodal properties to overlap favorably with the lowest unoccupied molecular orbital (LUMO) of the intercalating quinoline alkaloids. Ab initio calculations at the MP2/6-31G*(0.25) level have been used to model the π stacking interactions between the known intercalators ethidium, daunomycin, ellipticine, and 4,6′-diaminide-2-phenylindole with adenine–thymine (AT) and cytosine–guanine (CG) [48]. These calculations revealed stable π–π interactions between the intercalators and the base pairs. DFT/B3LYP methods, on the other hand, were found to fail completely, and showed no energy minima. Ellipticine and derivatives have been similarly investigated [49]. Both hydrogen-bonding and π stacking interactions between psoralens and DNA base pairs have been examined using DFT/B3LYP as well as MP2 methods [50]. Ab initio MP2/6-31G** calculations predicted stable π–π interactions between either psoralen or 8-methoxypsoralen and the AT base pair; DFT/B3LYP, however, did not show an energy minimum. Kumar and co-workers [51] have examined the interaction of the intercalating carcinogen benzo(a)pyrene and its metabolites with the C–G base pair using the self-consistent-charge, density functional tight-binding (SCC-DFTB-D) method, augmented by an empirical London dispersion energy term. The optimized molecular geometries of the π–π complexes were in good agreement with experimental structures, and interaction energies were comparable with MP2 calculated energies. Similarly, π stacking interactions of mitoxantrone with adenine–thymine (AT) and cytosine–guanine (CG) have been modeled using the DFTB approximate DFT method, and mitoxantrone showed greater binding with CG [52]. Jena and Mishra [53] have calculated the molecular properties of camptothecin and analogs using DFT/B3LYP, and have predicted topoisomerase I activity based on the calculated properties. These workers have not, however, looked at π–π interactions between camptothecin and DNA base pairs.
In this present work, we have carried out ab initio investigations of the electronic π–π interactions between quinoline alkaloids and DNA base pairs using density functional theory (DFT) at the M06/6-31G* level. Aromatic π–π interactions have been the subject of numerous theoretical investigations into a number of interactions [54, 55], including benzene dimer (see [56–62] for recent high-level computational investigations), uracil dimer [63], naphthalene dimer [58], and nucleotide base pairs [64–66].
In order to account for electron correlations in these models, ab initio calculations have included very large basis sets with coupled cluster calculations with single and double substitutions with noniterative triple excitations [CCSD(T)] [56, 57, 60]. MP2 calculations have been shown to overestimate the effects of electron correlation in π–π interactions [67–70]. These high level ab initio methods are very computationally demanding, however, and not suitable for molecular interactions of larger, biologically important molecules. In an effort to circumvent the computational costs, DFT methods have been employed. However, the widely used B3LYP and BLYP methods fail completely for π–π interactions [71–77]. Recently, DFT methods have been augmented with dispersion terms [58, 59, 61, 62, 76–79] to correct for these failures. Newly developed functionals such as MPWB1K [80], M05-2X [81], and M06-L [82] seem to sufficiently describe non-covalent interactions including π–π stacking [83–87].
Computational methods
Molecular docking
Molecular structures for the alkaloids were built using Spartan ’08 for Windows [88], and geometries optimized using the MMFF 94 force field [89]. DNA–alkaloid docking studies were carried out based on the crystal structure of DNA crystallized with ellipticine (PDB: 1z3f) [39], the NMR structure of DNA complexed with bis-daunorubicin (PDB: 1al9) [41], and crystal structures of DNA-topoisomerase I with topotecan (PDB: 1k4t) [42], camptothecin (PDB: 1t8i), and the synthetic indenoisoquinoline MJ-II-38 (PDB: 1sc7) [43]. The crystal structures were downloaded from the Protein Data Bank (PDB) using ArgusLab 4.0.1 [90]. These PDB structures provide a selection of DNA structures with known planar aromatic intercalators as well as crystal structures of DNA-topoisomerase I complexes. All solvent molecules and the co-crystallized inhibitor were removed from the structures to provide sterically unimpeded cavities for ligand docking. Molecular docking calculations for the quinoline alkaloids at the intercalation sites of DNA were undertaken using the ArgusDock docking algorithm of ArgusLab 4.0.1 [90]. A box of 20 × 20 × 20 Å (large enough to completely encompass the cavity/intercalation site) was centered around the intercalation site in order to allow each alkaloid ligand to explore potential binding poses. Larger box sizes were examined, but these only increased the number of non-intercalative binding poses. Different orientations of the alkaloids were examined and divided based on their energy values.
Ab initio molecular structures and energies
The calculations were carried out using Spartan ’08 for Windows [88]. Both the popular B3LYP [91, 92] and the recently developed M06 [82] functionals, were used together with the 6-31G* basis set [93] for the optimization of all stationary points in the gas phase. Frequency calculations were used to characterize stationary points as minima. All enthalpies are zero-point (ZPE) and thermally corrected. π–π interactions were carried out using Spartan ’08 for Windows at the M06/6-31G* level. Several different orientations of the alkaloids (skimmianine and stauranthine and the C–G base pair (or G alone in the case of stauranthine), as indicated by the molecular docking preferred orientations, were carried out with complete geometry optimization.
Results and discussion
Ten quinoline alkaloids were examined for potential intercalation into DNA (Fig. 1). Molecular docking analysis was carried out using structures of intercalated DNA (PDB access numbers 1z3f and 1al9) as well as topoisomerase I/DNA complexes (PDB access numbers 1k4t, 1t8i, and 1sc7). The intercalating interactions of the quinoline alkaloids are summarized in Table 1. Four quinoline alkaloids, stauranthine (Fig. 2), skimmianine (Fig. 3), 3′,6′-dihydroxy-3′,6′-dihydrostauranthine, and trans-3′,4′-dihydroxy-3′,4′-dihydrostauranthine were able to dock intercalatively into all five DNA structures, while two, veprisine and 6′-hydroxy-3′-ketostauranthine, docked successfully into four and three structures, respectively. The other quinoline alkaloids in this study presumably did not intercalate due to steric effects of non-coplanar appended substituents (see Fig. 4 for example). The intercalating docking energies of the quinoline alkaloids from this study are comparable to those for the known intercalating ligands camptothecin and ellipticine (Table 1). While skimmianine and stauranthine show average binding energies of around 5.1 kcal mol−1, 3′,6′-dihydroxydihydrostauranthine and 3′,4′-dihydroxydihydrostauranthine have average binding energies nearly equal to that of camptothecin (5.6, 5.4, and 5.5 kcal mol−1, respectively).
The electronic structures and properties of the quinoline alkaloids, as well as the AT and CG base pairs, have been calculated using DFT, B3LYP/6-31G* and M06/6-31G*. Molecular electronic descriptors are summarized in Table 2. The importance of these descriptors in the development of QSAR models has been reviewed [94–96], and utilized to examine anticancer activities of potential intercalators [97, 98]. In this present work, the molecular descriptors of the quinoline alkaloids that correlate best with molecular docking intercalation are the energies of the HOMO, the van der Waals surface areas, the ovalities, and the polarizabilities. That is, the best intercalating quinoline alkaloids tend to have higher E HOMO, smaller surface areas, lower ovalities, and lower polarizabilities. Interestingly, hydrophobicity, as measured by log P or E hydration do not seem to correlate with intercalation.
Dipole–dipole interactions [99–101], electrostatic interactions [102, 103], and van der Waals interactions [104–106], as well as frontier molecular orbital interactions [107–110], may be responsible for energetically favorable intercalation of planar aromatic compounds into DNA. Using the lower-energy intercalated docked poses of skimmianine and stauranthine as a guide, different face-to-face orientations of skimmianine with guanine–cytosine and stauranthine with guanine were computationally examined at the M06/6-31G* level. Based on frontier molecular orbital theory [111], the important interactions of intercalated ligands with the base pairs are expected to be the LUMO of the quinoline alkaloid with the HOMO of the purine base [107, 108]. The HOMO of the C–G base pair is higher in energy than that of A–T, and should exhibit better interaction with the intercalated ligands. DNA intercalators generally show a preference for CG-rich regions [112–115]. Atom numbering schemes for stauranthine, skimmianine, and guanine–cytosine are presented in Fig. 5. Atomic charges, based on Mulliken population analysis, are listed in Table 3.
The lowest-energy intercalation docking orientation (pose) from the molecular docking analysis for stauranthine corresponds to the M06/6-31G* lowest-energy orientation of stauranthine with guanine (Fig. 6). This particular orientation allows for favorable electrostatic interactions between stauranthine and guanine (see Fig. 7) as well as favorable dipole–dipole interactions (Fig. 7). In addition, there appears to be favorable complementary frontier molecular orbital overlap between the LUMO of stauranthine and the HOMO of guanine–cytosine in this orientation. Thus, for example, the LUMO lobe centered on C5 of stauranthine overlaps with the HOMO lobe centered on C6–N2 of guanine, the lobe on C3–C4 of stauranthine overlaps with the lobe centered on C3 of guanine, the lobe centered on C10 of stauranthine overlaps with the lobe located on N3 of guanine, and the lobe on C11 of stauranthine overlaps with the lobe on N4 of guanine (see Fig. 8).
The lowest-energy intercalation pose (ArgusDock) of skimmianine is consistent with two low-energy orientations of face-to-face M06/6-31G* structures (Fig. 9). The orientation shown in Fig. 9B is the lowest-energy (M06/6-31G*) face-to-face orientation of skimmianine with the cytosine–guanine base pair (4.4 kcal mol−1 lower in energy than the orientation in Fig. 9C. In this orientation (Fig. 9B), there are somewhat favorable dipole–dipole and electrostatic interactions between the skimmianine and the C–G base pair (see Fig. 10). For example, there are favorable electrostatic interactions between C1 of skimmianine and O1 of C–G, C3 of skimmianine and N1 of C–G, C6 of skimmianine and C3 of C–G, O2 of skimmianine and C2 of C–G, and O4 of skimmianine and C10 of C–G, in this orientation. Additionally, there seem to be some favorable frontier molecular orbital interactions available in this orientation. Thus, the lobe of the LUMO of skimmianine located on C11 would overlap with the HOMO lobe of guanine centered on C3, the skimmianine LUMO lobe on C2-C6 overlaps with the guanine HOMO lobe on C2–N3, and the lobe on C3 of skimmianine overlaps with the lobe on N4 of guanine (see Fig. 11).
Summary and conclusions
Analysis of molecular docking of Stauranthus alkaloids into DNA suggests that some of these compounds may act as intercalating ligands with DNA. Skimmianine, stauranthine, 3′,4′-dihydroxydihydrostauranthine, and 3′,6′-dihydroxydihydrostauranthine, each docked intercalatively into all five DNA crystal structures examined. Veprisine and 6′-hydroxy-3′-ketostauranthine docked into four and three DNA structures, respectively, while 3′,4′-dihydroxydihydroveprisine docked successfully into two DNA structures. Three of the quinoline alkaloids, 5-hydroxy-1-methyl-2-phenyl-4-quinolone, 6′-hydroxy-3′-ketoveprisine, and 3′,6′-dihydroxydihydroveprisine, did not intercalate successfully into any of the DNA crystal structures. Two of the quinoline alkaloids, stauranthine and skimmianine, were examined further in terms of π–π interactions with guanine–cytosine using DFT(M06/6-31G*). The lowest energy docked poses (from the ArgusDock molecular docking analysis) of these two alkaloids are structurally consistent with the lowest-energy face-to-face π-stacking orientations as revealed by the DFT (M06) calculations. The orientations of the quinoline alkaloids with guanine–cytosine are such that there are favorable dipole–dipole, electrostatic, and frontier molecular orbital interactions (alkaloid LUMO with guanine HOMO) interactions, and these may serve to stabilize the π–π interactions between the intercalating ligand and the base pairs. We conclude, therefore, that intercalation of quinoline alkaloids is determined by steric interactions (as revealed in the ArgusDock molecular docking analysis), and that a combination of π–π electronic interactions serve to orient the intercalating ligands with respect to the base pairs of DNA.
References
Wink M (2007) Alkaloids 64:1–47
Liu LF (1989) Annu Rev Biochem 58:351–375
Wilstermann AM, Osheroff N (2003) Curr Top Med Chem 3:321–338
Pommier Y, Pourquier P, Fan Y, Strumberg D (1998) Biochim Biophys Acta 1400:83–106
Burden DA, Osheroff N (1998) Biochim Biophys Acta 1400:139–154
Li QY, Zu YG, Shi RZ, Yao LP (2006) Curr Med Chem 13:2021–2039
Wang LK, Johnson RK, Hecht SM (1993) Chem Res Toxicol 6:813–818
Fox ME, Smith PJ (1990) Cancer Res 50:5813–5818
Stiborova M, Rupertova M, Schmeiser HH, Frei E (2006) Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 150:13–23
Bonjean K, De Pauw-Gillet MC, Defresne MP, Colson P, Houssier C, Dassonneville L, Bailly C, Greimers R, Wright C, Quetin-Leclercq J, Tits M, Angenot L (1998) Biochemistry 37:5136–5146
Seigler DS (1977) Plant systematic and alkaloids. In: Manske RHF (ed) The alkaloids, vol XVI. Academic, New York, pp 1–82
Hu J, Zhang WD, Shen YH, Zhang C, Xu L, Liu RH, Wang B, Xu XK (2007) Biochem Syst Ecol 35:114–117
Boyd DR, Sharma ND, Loke PL, Malone JF, McRoberts WC, Hamilton JTG (2007) Org Biomol Chem 5:2983–2991
Svoboda GH, Poore GA, Simpson PJ, Boder GB (1966) J Pharm Sci 55:758–768
Wu TS, Wang ML, Jong TT, McPhail AT, McPhail DR, Lee KH (1989) J Nat Prod 52:1284–1289
Cui B, Chai H, Dong Y, Horgen FD, Hansen B, Madulid DA, Soejarto DD, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD (1999) Phytochemistry 52:95–98
Chaturvedula VSP, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DGI (2003) J Nat Prod 66:532–534
Chen JJ, Fang HY, Duh CY, Chen IS (2005) Planta Med 71:470–475
Jansen O, Akhmedjanova V, Angenot L, Balansard G, Chariot A, Ollivier E, Tits M, Frédérich M (2006) J Ethnopharmacol 105:241–245
Prescott TAK, Sadler IH, Kiapranis R, Maciver SK (2007) J Ethnopharmacol 109:289–294
Kaczmarek L, Peczyńska-Czoch W, Osiadacz J, Mordarski M, Sokalski WA, Boratński J, Marcinkowska E, Glazman-Kuśnierczyk H, Radzikowski C (1999) Bioorg Med Chem 7:2457–2464
Osiadacz J, Majka J, Czarnecki K, Peczyńska-Czoch W, Zakrzewska-Czerwińska J, Kaczmarek Ł, Sokalski WA (2000) Bioorg Med Chem 8:937-943
Chen YL, Hung HM, Lu CM, Li KC, Tzeng CC (2004) Bioorg Med Chem 12:6539–6542
Carney JR, Scheuer PJ, Kelly-Borges M (1993) Tetrahedron 49(38):8483–8486
Molinski TF (1993) Chem Rev 93:1825–1838
McDonald LA, Eldredge GS, Barrows LR, Ireland CM (1994) J Med Chem 37:3819–3827
Dias N, Vezin H, Lansiaux A, Bailly C (2005) Top Curr Chem 253:89–108
Setzer WN, Setzer MC, Schmidt JM, Moriarity DM, Vogler B, Reeb S, Holmes AM, Haber WA (2000) Planta Med 66:493–494
Setzer WN, Vogler B, Bates RB, Schmidt JM, Dicus CW, Nakkiew P, Haber WA (2003) Phytochem Anal 14:54–59
Nunn CM, Van Meervelt L, Zhang SD, Moore MH, Kennard O (1991) J Mol Biol 222:167–177
Dautant A, Langlois d’Estaintot B, Gallois B, Brown T, Hunter WN (1995) Nucleic Acids Res 23:1710–1716
Berger I, Su L, Spitzner JR, Kang C, Burke TG, Rich A (1995) Nucleic Acids Res 23:4488–4494
Gao YG, Wang AH (1995) J Biomol Struct Dyn 13:103–117
Adams A, Guss JM, Collyer CA, Denny WA, Wakelin LPG (1999) Biochemistry 38:9221–9233
Robinson H, Gao YG, Yang XL, Sanishvili R, Joachimiak A, Wang AHJ (2001) Biochemistry 40:5587–5592
Smith CK, Davies GJ, Dodson EJ, Moore MH (1995) Biochemistry 34:415–425
Hu GG, Shui X, Leng F, Priebe W, Chaires JB, Williams LD (1997) Biochemistry 36:5940–5946
Lisgarten JN, Coll M, Portugal J, Wright CW, Aymami J (2002) Nature Struct Biol 9:57–60
Canals A, Purciolas M, Aymami J, Coll M (2005) Acta Crystallogr Sect D 61:1009–1012
Williams HEL, Colgrave ML, Searle MS (2002) Eur J Biochem 269:1726–1733
Robinson H, Priebe W, Chaires JB, Wang AH (1997) Biochemistry 36:8663–8670
Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Stewart LJ (2002) Proc Natl Acad Sci USA 99:15387–15392
Staker BL, Feese MD, Cushman M, Pommier Y, Zembower D, Stewart L, Burgin AB (2005) J Med Chem 48:2336–2345
Lauria A, Ippolito M, Almerico AM (2007) J Mol Model 13:393–400
Bhowmik S, Bagchi A, Ghosh R (2008) Int J Integr Biol 2:8–14
Byler KG (2001) Frontier molecular orbital interactions between intercalating quinoline alkaloids and DNA base pairs: an ab initio investigation. MS Thesis, University of Alabama in Huntsville
Nakatani K, Matsuno T, Adachi K, Hagihara S, Saito I (2001) J Am Chem Soc 123:5695–5702
Řeha D, Kabeláč M, Ryjáček F, Šponer J, Šponer JE, Elstner M, Suhai S, Hobza P (2002) J Am Chem Soc 124:3366–3376
Dračinský M, Castaño O (2004) Phys Chem Chem Phys 6:1799–1805
El-Gogary TM, Koehler G (2007) THEOCHEM 808:97–109
Kumar A, Elstner M, Suhai S (2003) Int J Quant Chem 95:44–59
Riahi S, Ganjali MR, Dinarvand R, Karamdoust S, Bagherzadeh K, Norouzi P (2008) Chem Biol Drug Des 71:474–482
Jena NR, Mishra PC (2007) J Mol Model 13:267–274
Hobza P, Šponer J (1999) Chem Rev 99:3247–3276
Hunter CA, Lawson KR, Perkins J, Urch CJ (2001) J Chem Soc Perkin Trans 2:651–669
Tsuzuki S, Honda K, Uchimaru T, Mikami M, Tanabe K (2002) J Am Chem Soc 124:104–112
Sinnokrot MO, Valeev EF, Sherrill CD (2002) J Am Chem Soc 124:10887–10893
Sato T, Tsuneda T, Hirao K (2005) J Chem Phys 123:104307
Podeszwa R, Bukowski R, Szalewicz K (2006) J Phys Chem A 110:10345–10354
DiStasio RA, von Helden G, Steele RP, Head-Gordon M (2007) Chem Phys Lett 437:277–283
Jha PC, Rinkevicius Z, Ågren H, Seal P, Chakrabarti S (2008) Phys Chem Chem Phys 10:2715–2712
Bludský O, Rubeš M, Soldán P, Nachtigall P (2008) J Chem Phys 128:114102
Pitoňák M, Riley KE, Neogrády P, Hobza P (2008) Chem Phys Chem 9:1636–1644
Dabkowska I, Gonzalez HV, Jurečka P, Hobza P (2005) J Phys Chem A 109:1131–1136
Cooper VR, Thonhauser T, Langreth DC (2008) J Chem Phys 128:204102
Šponer J, Riley KE, Hobza P (2008) Phys Chem Chem Phys 10:2595–2610
Jaffe RL, Smith GD (1996) J Chem Phys 105:2780–2788
Hobza P, Selzle HL, Schlag EW (1996) J Phys Chem 100:18790–18794
Tsuzuki S, Uchimaru T, Matsumura K, Mikami M, Tanabe K (2000) Chem Phys Lett 319:547–554
Tsuzuki S, Lüthi HP (2001) J Chem Phys 114:3949–3957
Milet A, Korona T, Moszynski R, Kochanski E (1999) J Chem Phys 111:7727–7735
Elstner M, Hobza P, Frauenheim T, Suhai S, Kaxiras E (2001) J Chem Phys 114:5149–5155
Cybulski SM, Bledson TM, Toczyłowski RR (2002) J Chem Phys 116:11039–11040
Mourik TV, Gdanitz RJ (2002) J Chem Phys 116:9620–9623
Cybulski SM, Seversen CE (2005) J Chem Phys 122:014117
Grimme S (2006) J Comput Chem 27:1787–1799
Jurečka P, Černý J, Hobza P, Salahub DR (2007) J Comput Chem 28:555–569
Wu Q, Yang W (2002) J Chem Phys 116:515–524
Antony J, Grimme S (2006) Phys Chem Chem Phys 8:5287–5293
Zhao Y, Truhlar DG (2004) J Phys Chem A 108:6908–6918
Zhao Y, Schultz NE, Truhlar DG (2006) J Chem Theory Comput 2:364–382
Zhao Y, Truhlar DG (2006) J Chem Phys 125:194101
Zhao Y, Truhlar DG (2005) Phys Chem Chem Phys 7:2701–2705
Dkhissi A, Blossey R (2007) Chem Phys Lett 439:35–39
Stepanian SG, Karachevtsev MV, Glamazda AYu, Karachevtsev VA, Adamowicz L (2008) Chem Phys Lett 459:153–158
Gu J, Wang J, Leszczynski J, Xie Y, Schaefer HF (2008) Chem Phys Lett 459:164–166
Wong BM (2009) J Comput Chem 30:51–56
Spartan ’08 for Windows (2006) Wavefunction, Irvine, CA
Halgren TA (1996) J Comp Chem 17:490–519
Thompson MA (2004) ArgusLab 4.0.1. Planaria Software LLC, Seattle, WA
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hehre WJ, Radom L, PvR S (1986) Ab initio molecular orbital theory. Wiley, New York
Chermette H (1999) J Comput Chem 20:129–154
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793–1874
Sarkar U, Roy DR, Chattaraj PK, Parthasarathi R, Padmanabhan J, Subramanian V (2005) J Chem Sci 117:599–612
Chen JC, Qian L, Wu WJ, Chen LM, Zheng KC (2005) THEOCHEM 756:167–172
Chen JC, Shen Y, Liao S, Chen LM, Zheng KC (2007) Int J Quant Chem 107:1468–1478
Berman HM, Young PR (1981) Annu Rev Biophys Bioeng 10:87–114
Xiao S, Lin W, Wang C, Yang M (2001) Bioorg Med Chem Lett 11:437–441
El-Gogary TM, Koehler G (2009) THEOCHEM 895:57–64
Müller W, Crothers DM (1975) Eur J Biochem 54:267–277
Hunter CA, Lawson KR, Perkins J, Urch CJ (2001) J Chem Soc Perkin Trans 2:651–669
Boger DL, Invergo DJ, Coleman RS, Zarrinmayeh H, Kitos PA, Collins-Thompson S, Leong T, McLaughlin LW (1990) Chem Biol Interact 73:29–52
Haq I (2002) Arch Biochem Biophys 403:1–15
Baginski M, Fogolari F, Briggs JM (1997) J Mol Biol 274:253–267
Rehn C, Pindur U (1996) Monats Chem 127:645–658
Nakatani K, Matsuno T, Adachi K, Hagihara S, Saito I (2001) J Am Chem Soc 123:5695–5702
Mei WJ, Liu J, Zheng KC, Lin LJ, Chao H, Li AX, Yun FC, Ji LN (2003) Dalton Trans 2003:1352–1359
Nowak K, Wysocki S (2004) THEOCHEM 682:191–199
Fukui K, Yonezawa T, Shingu H (1952) J Chem Phys 20:722–725
Pullman B (1991) Anticancer Drug Design 6:95–105
Trotta E, D’Ambrosio E, Ravagnan G, Paci M (1995) Nucleic Acids Res 23:1333–1340
Rehn C, Pundur U (1996) Monats Chem 127:631–644
Lisgarten JN, Coll M, Portugal J, Wright CW, Aymami J (2002) Nature Struct Biol 9:57–60
Ghose AK, Pritchett A, Crippen GM (1988) J Comput Chem 9:80–90
Cramer CJ, Truhlar DG (1992) J Comput Chem 13:1089–1097
Chambers CC, Hawkins GD, Cramer CJ, Truhlar DG (1996) J Phys Chem 100:16385–16398
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Byler, K.G., Wang, C. & Setzer, W.N. Quinoline alkaloids as intercalative topoisomerase inhibitors. J Mol Model 15, 1417–1426 (2009). https://doi.org/10.1007/s00894-009-0501-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0501-6