Abstract
The enzyme topoisomerase I (topo I), which is essential for cell replication, transiently causes a DNA single strand break and makes a complex with it. The anti-cancer agent camptothecin (CPT) binds to the topo I–DNA complex and stabilizes it, preventing resealing of the broken DNA strand and cell growth. Considering the structural factors of CPT that are believed to be involved in stabilizing the topo I–DNA complex via hydrogen bonding and stacking interactions, designs of two new analogues of CPT (topo I inhibitors) have been suggested. The molecular geometries of CPT, two of its analogues and certain other related molecules included in the study were fully optimized in both gas phase and aqueous media at the B3LYP/6-311++G(d,p) level of density functional theory. Solvation effects of aqueous media were treated using the polarizable continuum model (PCM). Net CHelpG charges and surface molecular electrostatic potentials (MEP) near the atomic sites of the molecules were studied. Structural analogy and surface MEP values suggests that the two new CPT analogues studied here would be potent topoisomerase I inhibitors.

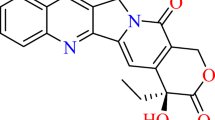
Optimized structures of CPT and two of its new analogues, 10 and 11.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Topoisomerase I (topo I) is an enzyme that causes transient DNA single strand breaks, that are rejoined later, and makes a complex with the broken DNA strand [1, 2]. For this reason, topo I is vital for DNA replication, transcription and certain other nuclear processes [1, 2]. The anticancer drug camptothecin (CPT) binds to the topo I–DNA complex, thereby inhibiting rejoining of the broken strand. It has been found that CPT and its derivatives intercalate between DNA base pairs at the site of a single strand break and stabilize the topo I–DNA complex, thus preventing the rejoining of the broken DNA strand and stopping cancer cell growth [3].
The CPT molecule consists of five rings out of which four (A, B, D and E) are six-membered and one (C) is five-membered (Fig. 1a). It has two different forms that make stable complexes with the topo I–DNA complex. In one form, the E ring is closed (lactone form) (Fig. 1a) while in the other, this ring is open (carboxylate form) [2]. The lactone form of CPT has a greater potency towards non-proliferation of tumor cells than the carboxylate form which is generally inactive [2, 4]. It is known that CPT is neither stable at physiological pH nor soluble in water. As a result, it does not work properly inside living systems. To deal with these problems, several attempts have been made to obtain different CPT [5–8] and non-CPT [9] topo I inhibitors. Different substitutions were made at the A and B rings of CPT to enhance its stability in human plasma or blood [2, 4, 5,10]. It was found that due to the existence of a intramolecular hydrogen bond between the hydrogen atom of the 9-OH group and the O8 atom present in the lactone form of E ring (Fig. 1a), CPT can undergo hydrolysis easily at physiological pH [7, 8, 11]. As a result, the active lactone E ring is converted to the inactive carboxylate form [7, 8]. Bailly et al. [7] have shown that replacement of the six-membered E ring of CPT by a seven-membered E ring (homoCPT) where there is no significant hydrogen bonding between the H atom of the 9-OH group and the O8 atom leads to enhancement of the stability of the system. It was also shown that the homoCPT has superior antitumor activities than CPT in vivo [12]. Other CPT analogues with modified E rings have also been suggested [4, 6, 8].
When the C3H group of the D ring of CPT (Fig. 1a) is replaced by an N3 atom to give 3-azaCPT, the resulting analogue of CPT stabilizes the topo I–DNA complex to the same extent as CPT and is also soluble in water [4, 13]. It was also found that 3-azaCPT effectively suppresses super-coiling relaxation of DNA [4]. In order to design new CPT analogues, it is important to know the binding modes of CPT that stabilize the topo I–DNA complex. Several models have been proposed in this context in which it was suggested that CPT stabilizes the topo I–DNA complex mainly by (1) hydrogen-bonding interactions between the atoms attached to the D and E rings of CPT and topoisomerase I and (2) stacking interactions, mainly between the C ring and the adjacent DNA base pairs [1, 3, 4, 14]. The X-ray crystal structure of an A-ring-substituted lactone form of CPT derivative (topotecan) complexed with topo I–DNA showed that there is only one hydrogen bond between the hydrogen atom of the 9-OH group and an oxygen atom of Asp533 of topo I [15].
The formation of 8-oxoguanine (8OG) is a common oxidative form of DNA damage found in biological systems [16, 17]. 8OG makes Hoogsteen-type base pairing with adenine that can lead to mutation and cancer [18]. It has been shown that 8OG suppresses DNA-cleavage activity of the topo I enzyme by stabilizing the inactive conformation of the topo I–DNA complex [19]. In previous work from our laboratory, it was suggested that replacement of the N7H7 group of 8OG by an oxygen atom to give 7-O-8OG can destabilize the mispairing between 8OG and adenine [20]. Further, due to the structural similarity between guanine and 7-O-8OG in the Watson–Crick base pairing region, the latter molecule can be incorporated into DNA in place of guanine. Thus 7-O-8OG may have anti-mutagenic properties [20].
Considering the above facts, we have tried to design some new analogues of CPT. The starting point of the present study was the 7-O-8OG molecule because of the structural similarity between the imidazole ring of 7-O-8OG and the E ring of CPT. We have examined the structures and properties of several derivatives of 7-O-8OG obtained by systematic substitution of the different functional groups present in CPT to 7-O-8OG, followed by fusion of the C, B and A rings of CPT to the substituted 7-O-8OG molecule. In this way we have obtained two close analogues of CPT that differ from CPT only in the D and E rings.
Computational methodology
The geometries of CPT, 8OG, 7-O-8OG and their derivatives (11 molecules in total, Figs. 1 and 2) were fully optimized in the gas phase at the B3LYP/6-311++G(d,p) level of density functional theory (DFT). [21, 22] Geometry optimization was also performed for ten of the 11 molecules in aqueous media treating solvation using the polarizable continuum model (PCM). [23] Geometry optimization could not be completed for 8 in aqueous media because of convergence failure. In this case, a single point solvation calculation was performed using the PCM model and the gas-phase optimized geometry. Vibrational frequency analysis in the gas phase was carried out for all the molecules except 1, 10 and 11 because they were too large. No vibrational frequency analysis was performed in aqueous media. For visualization of the optimized structures, the GaussView program (versions 2.1 and 3.09) was used [24]. All the calculations were performed using the Windows versions of the Gaussian 98 (G98W) [25] and Gaussian 03 (G03W) [26] programs. Molecular electrostatic potentials (MEP) at the van der Waals surfaces of the molecules near various atomic sites were obtained using the MEP-fitted CHelpG charges using a software developed locally.
Results and discussion
Structures of CPT and other molecules
Optimized bond lengths and bond angles of CPT (1) in both the gas phase and aqueous media are shown in Table 1. The optimized geometrical parameters of the molecules in the gas phase and aqueous media are very similar. This shows that the aqueous medium has a small effect on the molecular geometries (Table 1). The optimized bond lengths and bond angles of CPT were compared with the X-ray crystallographic results obtained for camptothecin iodoacetate [27]. The root mean square (RMS) difference between the calculated bond lengths of CPT in the gas phase and aqueous media and the crystallographic bond lengths of camptothecin iodoacetate is ∼0.032 Å, while the corresponding difference for bond angles is ∼2.4 °. Thus, the agreement between the calculated and observed bond lengths and bond angles is satisfactory. The differences between the calculated and crystallographic values of bond lengths and bond angles can be ascribed largely to packing forces present in crystals. The optimized structures of CPT (1) and 8OG derivatives (2–11) are shown in Figs. 1 and 2.
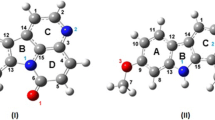
In Fig. 1a, structure of the lactone form of CPT (1) is shown. For the D and E rings of CPT, we have adopted the same atomic numbering scheme as that used for guanine or 8OG (2). Atoms of other parts of CPT and its derivatives are numbered sequentially. All the rings of CPT are coplanar except the six-membered E ring, which is nonplanar, the C5C4C9C8, C4C9C8O8 and C4C5C23O7 dihedral angles being about 39°, 140° and 32°, respectively. There is a weak intramolecular hydrogen bond between the H atom of the 9-OH group and the O8 atom, the H(9-OH)-O8 distance being 2.046 and 2.187 Å in gas phase and aqueous medium, respectively (Table 1). This hydrogen bond is involved in the hydrolysis of CPT at pH ≥ 7.0 that leads to conversion of the active lactone form to the inactive carboxylate form. [7, 8] There are some similarities between CPT (1) and 8OG (2) (Fig. 1a,b) as follows. (1) Both molecules have a nitrogen atom at the 1 position (N1) and (2) A CO group is attached to each of the six- and five-membered rings of 8OG (C6O6 and C8O8 groups, respectively) similar to those present in CPT (Fig. 1a,b) [1, 27]. The rings of 8OG are coplanar (Fig. 1b). The structure of 7-O-8OG (3) is shown in Fig. 1c. The main structural difference between 8OG and 7-O-8OG is that the N7H7 group of the former is replaced by an oxygen atom (O7) in the latter.
The structure of a derivative of 7-O-8OG (4) in which the N9H group present in 3 is replaced by a carbon atom to which a hydrogen atom and an OH group are attached is shown in Fig. 1d. This substitution was made in 3 to obtain 4 as there is a carbon atom at the 9th position of CPT to which an OH group is attached and the H atom of this OH group makes a hydrogen bond with one of the amino acids of topo I, which is involved in stabilizing the topo I–DNA complex [1, 3, 14, 17, 28]. In an experimental study, a model of CPT analogue-topo I–DNA complex was suggested, according to which the oxygen atom of the 9-OH group is involved in hydrogen bonding with two different amino acids while its hydrogen atom is involved in hydrogen bonding with another amino acid of the topo I enzyme [29]. When the 9-OH group of CPT was replaced by a 9-NH2, 9-Cl or 9-Br group, it was found that all the resulting molecules stabilized the topo I–DNA complex but less efficiently than CPT [4, 29]. These facts indicate the importance of the 9-OH group present in the CPT. Previous studies have also shown that the presence of both the lactone E ring of CPT and the 9-OH group are very important in stabilizing the topo I–DNA complex [30–33]. We have 9-H, 9-CH3, 9-C2H5 and 9-C3H7 groups as well as the 9-OH group, in 3, 5, 6 and 7, respectively (Figs. 1c–e and 2a,b). These groups were attached to C9 in order to analyze the role of the 9-C2H5 group in CPT.
The C, B and A rings of CPT were fused to molecule 6 sequentially in order to evaluate the perturbation they cause to different molecular properties like dipole moment, charge distribution and surface MEP. When a five-membered C ring, which is present in CPT, is fused to the D ring of 6 at the N1C2 bond, we obtain 8, the structure of which is shown in Fig. 2c. The structure of 9, in which a pyridine ring (B ring) is fused to the C ring of 8 at the C12C13 bond is shown in Fig. 2d. When a benzene ring (A ring) is fused to the B ring of 9 at the C16C21 bond, we obtain 10, the structure of which is shown in Fig. 2e.
There are only two structural differences between CPT (1) and 10. (i) The E ring in the former is six-membered and nonplanar while in the latter, the corresponding ring is five-membered and planar. (ii) The D ring of 10 contains an N3 atom instead of the C3H group present in CPT. Thus, 10 differs from 3-azaCPT [4] only with respect to the E ring. It is interesting to note that both CPT and 3-azaCPT [4] have intramolecular hydrogen bonding between the H atom of the 9-OH group and the O8 atom, while in 10, the corresponding intramolecular hydrogen bonding is absent (distance H(9-OH)-O8 = 2.708 and 2.730 Å in gas phase and aqueous medium, respectively). This indicates that 10 would be stable at physiological pH.
When the N3 atom of 10 is replaced by a C3H group, 11 results, the structure of which is shown in Fig. 2f. Eleven differs from CPT and homoCPT [7] only with respect to the E ring. Thus, 11 is a close analogue of CPT (1) and homoCPT [7]. As intramolecular hydrogen bonding between the H atom of the 9-OH group and the O8 atom is absent in 11 (distance H(9-OH)-O8 = 2.657 and 2.734 Å in gas phase and aqueous medium, respectively) (Table 1), it would also be stable at physiological pH. Further, as the A, B, C and D rings of 10 and 11 are similar to those of CPT, these two molecules are also expected to be involved in stacking interactions with the topo I–DNA complex in a similar manner as CPT.
Solvation energies and dipole moments
Geometry optimizations performed in aqueous media for all molecules (except 8) enable us to compare their stabilities, solubilities and other important properties in aqueous media. Solvation energies (defined as the sum of electrostatic and non-electrostatic interaction energies between the solvent and solutes) and dipole moments of all the molecules studied here (1–11) in aqueous media are shown in Table 2. The solvation energies of the molecules in aqueous media are found to be fairly large, between ∼−19 and ∼−34 kcal mol−1 (Table 2). We also find that the solvation energies of 10 and 11 are larger than that of CPT (1) by about 3 and 1 kcal mol−1, respectively (Table 2). The calculated dipole moments of these three molecules in both the gas phase and aqueous media also follow the same order i.e. 10 ≥ 11 > 1 (Table 2). Thus, it seems that 10 would be somewhat more soluble than 11 and CPT (1) in aqueous media.
Surface MEP and drug activities
To study the relative hydrogen-bonding strengths of a series of molecules approximately, one can use molecular electrostatic potentials (MEP) or molecular electric fields (MEF) near the corresponding atomic sites at the van der Waals surfaces [31, 32]. Surface MEP or MEF can be obtained using CHelpG point charges. Nearly linear relationships have been found between the MEP or MEF and experimentally measured hydrogen-bond donating or accepting abilities of molecules [34–37]. The use of CHelpG charges are quite appropriate in this context as these are derived by fitting of surface MEP values. As the hydrogen bonding strength between topo I and CPT or its analogues would be an important factor in controlling the activity of the molecules as drugs, MEP values obtained using CHelpG charges can be used as a reliable descriptor of drug activity.
Surface MEP values near the selected atomic sites of the molecules computed using CHelpG charges obtained using optimized geometries in the gas phase and aqueous media are shown in Table 3. We make the following observations from the MEP values (Table 3):
-
(1)
The MEP values near the different atomic sites in aqueous media are usually appreciably larger than those in gas phase. This is due to an appreciable modification of charge distribution in going from the gas phase to aqueous media, as shown by the calculated CHelpG charges. It may also be considered as arising from a cooperative effect between the solvent and the solutes, due to which the negative and positive regions of the solutes are enhanced. However, the qualitative trends shown by the MEP values in the gas phase and aqueous media are similar. The MEP values suggest that all the hydrogen bonds between the drug molecules and the topo I–DNA complex would usually be stronger in aqueous media than those in gas phase.
-
(2)
The MEP magnitudes in aqueous media near O6, O7, O9 and the H atom of the 9-OH group are larger in 7 than those near the corresponding sites of 5 and 6. However, the MEP magnitude near O8 in 7 in aqueous medium is least among the three molecules. These results show that appreciable effects on hydrogen bonding involving the above mentioned atomic sites in aqueous media would be caused by substitutions of the CH3, C2H5 and C3H7 groups at the C9 position.
-
(3)
According to a model proposed by Redinbo et al. [1], O6, O7, O8 and the H atom of the 9-OH group of CPT would be involved in hydrogen bonding with the topo I–DNA complex. It is also proposed that O9 and N15 atoms of CPT would be involved in hydrogen bonding with topo I–DNA complex [3, 29]. Considering the fact that 10 and 11 would stabilize the topo I–DNA complex in a similar manner as CPT, it is essential to compare the expected hydrogen-bond strengths involving the above atoms on the basis of MEP values [31, 32]. On this basis (Table 3), it seems that the hydrogen-bond strengths involving the four oxygen atoms and one nitrogen atom where these atoms would serve as hydrogen bond acceptors would follow the order O6 > O8 > O7 > O9 > N15. The H atom of the 9-OH group would be a hydrogen bond donor. This property of the H atom of the 9-OH group corresponds to the positive surface MEP values near this site in the different molecules (Table 3).
-
(4)
Sums of MEP magnitudes near the appropriate sites in the different molecules can be treated as overall descriptors of hydrogen-bonding strength or drug activity. The sums of MEP magnitudes near the O6, O7, O8, O9 and N15 atoms in 1, 10 and 11 in the gas phase follow the order 10 (231.8) ≅ 11 (231.8) > 1 (224.3), while in aqueous media, the order changes to 10 (330.2) > 11 (292.9) > 1 (232.9), where the MEP values in kcal mol−1 are given in parentheses (Table 3). The MEP values near the H atom of the 9-OH group in both the gas phase and aqueous media follow the order 11 > 10 > 1. The surface MEP value near the H atom of the 9-OH group of CPT [1] is appreciably smaller than the corresponding MEP values in 10 and 11 (Table 3). This is because in the case of CPT, there is an intramolecular hydrogen bond between the H atom of 9-OH group and the O8 atom (Table 1). Due to the influence of the O8 atom of CPT, the positive surface MEP value near the H atom of the 9-OH group is reduced.
-
(5)
As solvation energies are appreciable (Table 2), the effects of aqueous media should be considered in dealing with the MEP values and activities of the molecules as drugs. On the basis of the MEP values in aqueous media, it appears that molecules 10 and 11 would make strong hydrogen bonds with the topo I–DNA complex.
Conclusions
We arrive at the following conclusions from the present study. Out of the eleven molecules of which the geometries and surface MEP have been studied here, two i.e. 10 and 11 are close analogues of CPT. There is a five-membered E ring in each of 10 and 11 while there is a six-membered E ring in CPT. The six-membered E rings of CPT is nonplanar while the corresponding five-membered E rings of 10 and 11 are planar. Due to the nonplanarity of the E ring in CPT, an intramolecular hydrogen bond is formed between the hydrogen atom of the 9-OH group and the O8 atom, due to which the molecule undergoes hydrolysis at physiological pH easily. The potency of CPT may also be lowered for this reason. This effect is not present in 10 and 11 and, therefore, these molecules would be stable at physiological pH. On the basis of the calculated surface MEP values in aqueous media near the atoms of 10 and 11 that are believed to be involved in stabilizing the topo I–DNA complex via hydrogen bonding, it appears that 10 and 11 would make strong hydrogen bonds with the topo I–DNA complex. Further, as the A, B, C and D rings of 10 and 11 are similar to those of CPT, these molecules are expected to be involved in stacking interactions with the topo I–DNA complex in a similar manner as CPT. Thus, due to close structural analogy with CPT, the absence of the intramolecular hydrogen bonds mentioned above and surface MEP values, it appears that 10 and 11 would be potent topo I inhibitors. However, this remains to be verified experimentally.
References
Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WGJ (1988) Science 279:1504–1513
Sanna N, Chillemi G, Grandi A, Castelli S, Desideri A, Barone V (2005) J Am Chem Soc 127:15429–15436
Kerrigan JE, Pilch DS (2001) Biochem 40:9792–9798
Hecht SM (2005) Curr Med Chem-Anti-Cancer Agents 5:353–362
Veadu B, Woynarowski JM, Manikumar G, Wani MC, Wall ME, Hoff DDV, Wadkins R (2000) Mol Pharmacol 57:243–251
Rahier NJ, Eisenhauer BM, Gao R, Thomas SJ, Hecht SM (2005) Bioorg Med Chem 13:1381–1386
Bailly C, Lansiaux A, Dassonneville L, Demarquay D, Coulomb H, Huchet M, Lavergne O, Bigg DCH (1999) Biochem 38:15556–15563
Rahier NJ, Eisenhauer BM, Gao R, Jones SH, Hecht SM (2004) Org Lett 6:321–324
Wang L-K, Rogers BD, Hecht SM (1996) Chem Res Toxicol 9:75–83
Lavergene O, Lesueur-Ginot L, Pla Rodas F, Bigg DCH (1997) Biorg Med Chem Lett 7:2235–2238
Bailly C (2003) Crit Rev Oncol Hematol 45:91–108
Lesueur-Ginot L, Demarquay D, Kiss R, Kasprzyk PG, Dassonneville L, Bailly C, Camara J, Lavergne O, Bigg DCH (1999) Can Res 59:2939–2943
Cheng K, Rahier NJ, Eisenhauer BM, Gao R, Thomas SJ, Hecht SM (2004) J Am Chem Soc 127:838–839
Fan Y, Weinstein JN, Kohn KW, Shi LM, Pommier YJ (1998) Med Chem 41:2216–2226
Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Stewart L (2002) Proc Natl Acad Sci (USA) 99:15387–15392
Jena NR, Mishra PC (2005) J Phys Chem B 109:14205–14218
Jena NR, Mishra PC, J Comput Chem (accepted)
Cheng X, Kelso C, Hornak V, Santos CDI, Grollman AP, Simmerling C (2005) J Am Chem Soc 127:13906–13918
Lesher D-TT, Pommier Y, Stewart L, Redinbo MR (2002) Proc Natl Acad Sc (USA) 99:12102–12107
Singh AK, Mishra PC (2005) Int J Quant Chem 102:343–351
Becke AD (1996) J Chem Phys 104:1040–1046
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Cossi M, Barone V, Cammi R, Tomaso J (1996) Chem Phys Lett 255:327–335
Frisch AE, Dennington RD, Keith TA, Nielsen AB, Holder AJ (2003) GaussView, Rev 3.9. Gaussian, Pittsburg PA
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratman RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu C, Liashenko A, Piskorz P, Komaromi, I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Gonzales C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98. Gaussian Inc, Pittsburgh PA
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03. Gaussian, Wallingford CT
McPhail AT, Sim GA (1968) J Chem Soc B 923–928
Wang X, Zhou X, Hecht SM (1999) Biochem 38:4374–4381
Laco GS, Collins JR, Luke BT, Kroth H, Sayer JM, Jerina DM, Pommier Y (2002) Biochem 41:1428–1435
Cagir A, Jones, SH, Eisenhauer BM, Hecht SM (2003) J Am Chem Soc 125:13628–13629
Cagir A, Jones SH, Eisenhauer BM, Gao R, Hecht SM (2004) Bioorg Med Chem Lett 14:2051–2054
Cagir A, Jones SH, Eisenhauer BM, Gao R, Thomas SJ, Hecht SM (2004) Bioorg Med Chem 12:6287–6299
Rahier NJ, Eisenhauer BM, Gao R, Thomas SJ, Hecht SM (2005) Bioorg Med Chem 13:1381–1386
Mohan CG, Kumar A, Mishra PC (1995) J Mol Struct (Theochem) 332:171–176
Mishra PC, Kumar A (1995) Topics Curr Chem 174:27–43
Murray JS, Politzer PJ (1992) Chem Res (S) 110–111
Hagelin H, Brinck T, Berthelot M, Murray JS, Politzer P (1995) Can J Chem 73:483–488
Acknowledgements
The authors thank the Council of Scientific and Industrial Research (New Delhi) and the University Grants Commission (New Delhi) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jena, N.R., Mishra, P.C. A theoretical study of some new analogues of the anti-cancer drug camptothecin. J Mol Model 13, 267–274 (2007). https://doi.org/10.1007/s00894-006-0157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-006-0157-4