Abstract
The geometries, relative stabilities of some 4(7) and 5(6) substituted 2-hydroxybenzimidazole derivatives were calculated with full geometry optimization using AM1 and PM3 in aqueous phase. With the exception of molecules 4, 6 and 7 for all the 4(7) and 5(6) substituted 2-hydroxybenzimidazole derivatives the 3H and keto forms were found to be favored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taking into account the utilization of the benzimidazole derivatives in the synthesis of heat-resistant fibers, which are also used in the manufacture of parachutes, conveyer belts, heat-insulating material and asbestos replacements, they strongly influence progress in aerospace and aeronautics technology. Use of some benzimidazole molecules, such as 5-nitrobenzimidazole, as antifogging substances, photoemulsion stabilizers and as a fungicides significantly increase the importance of benzimidazole derivatives and led our research group to investigate their structure–reactivity relationships at both experimental and theoretical levels. [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]
We now report on the substituent effects on tautomerism of 4(7) and 5(6) substituted 2-hydroxybenzimidazole derivatives to fill the gap in the literature and hoping to give some clues to those researchers who will attempt to obtain alternatives in synthesis and use in the above mentioned applications. As one can immediately notice, there exist two kinds of prototautomerism in 4(7) or 5(6) substituted 2-hydroxybenzimidazole derivatives and these are annular and ring–chain tautomerisms (Scheme 1).
It was reported in the literature that the substitution in position 2 of the benzimidazole molecule has no influence on annular tautomerism (i.e. K T) and the symmetry was C s. [13] Substituents in position 4(7) and 5(6) exercise a relatively remote perturbation. Therefore K T does not differ much from 1 unless an unusual (attractive or repulsive) interaction occurs between a subsitituent in the 7-position and the proton bound to the nitrogen atom (N1). Experimental studies on annular tautomerism of unsubstituted and 4(7) and 5(6) substituted benzimidazoles have been reported in the literature [13, 14] but we did not come across any detailed theoretical study on this subject. On the other hand, a few experimental [13] and theoretical [10, 11, 12, 13] results on ring–chain tautomerism for unsubstituted and for 4(7) and 5(6) substituted 2-hydroxybenzimidazoles have also been reported. However no systematic theoretical work exists yet.
Method
Theoretical calculations were carried out at the restricted Hartree–Fock level (RHF) using AM1 and PM3 semi-empirical SCF-MO methods in the MOPAC 7.0 program [14] implemented on an Intel Pentium Pro 200-MHz computer. All the structures were optimized to a gradient norm of <0.1 kcal mol−1 Å−1 in the aqueous phase. The initial estimates of the geometries of all the structures were obtained by a molecular mechanics program of PCMODEL 3.1 from Serena Software.
Results and discussion
Relative stabilities and tautomerism
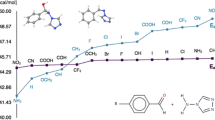
The aqueous phase semi-empirical AM1 and PM3 computed thermodynamic and stability data for 4(7) and 5(6) substituted 2-hydroxybenzimidazole derivatives are given Tables 1 and 2, respectively.
Annular tautomerism
When the annular tautomerism of 2-hydroxybenzimidazole derivatives is considered, it seems that the presence of potentially tautomeric hydroxyl group at 2C of the benzimidazole molecule has no influence on relative stability values, RS, and the value of tautomeric equilibria constants, K T, is about unity as indicated in the literature [13] (Tables 2 and 3). The calculated RS and K T1 values for \( a1 \rightleftharpoons a3 \) equilibria, which represent the annular 1H 3H for 2-hydroxbenzimidazole derivatives, were found to be close to zero and unity, respectively, in most cases (Scheme 1). This result fits the literature reports very nicely. [13] In same cases, however, considerable deviations were obtained from the zero value of RS and unity of K T1. These deviations were presumably due to the unusual interactions between the substituent and the proton bound to the nitrogen atom as in the 7-nitro-2-hydroxybenzimidazole molecule and indicates the effect of chain tautomerism on annular tautomerism (Scheme 2).
The RS values for 7-nitro-2-hydroxybenzimidazole were found to be 0.69 and −1.15 kcal mol−1 for \(a1 \rightleftharpoons a3 \) equilibrium with the AM1 and PM3 methods, respectively. These are very small but contradictory values; the first value indicates that the 3H form is favored over 1H weakly for this molecule. From these values, which are close to the zero, we can say that 1H≡3H forms as for the other 4(7) substituted 2-hydroxybenzimidazole molecules. RS results for the \(a1 \rightleftharpoons a3 \) equilibrium, which are 0.2 and 0.41 kcal mol−1 with the AM1 and PM3 methods, respectively, might well indicative that the 3H form is favored due to full the conjugative effect of NO2, which is an electron-withdrawing group, by withdrawing electrons from the imidazole ring and rendering the formation of 3H keto form more favorable (Scheme 3).
This conclusion can be justified by looking at the K T2 and K T3 values of 5(6)-nitro-2-hydroxybenzimidazole, which are smaller than the K T2 and K T3 values of 4(7)-nitro-2-hydroxybenzimidazoles molecules (Table 3).
Ring–chain tautomerism
The AM1 and PM3 aqueous phase calculated ring–chain tautomeric equilibrium constants, K T values, are collected in Table 3. As one can easily see the K T2 and K T3 values suggest the oxo forms for all studied compounds are overwhelmingly favored, as stated in the literature. A small drop in K T2 values is observed when a strong electron-withdrawing (like NO2) or electron-donating (like OCH3) substituent moves from 4(7) C to 5(6) C, which obviously leads stronger interactions as mentioned earlier between the hydrogen atom of the imidazole ring and the substituent by causing a longer through conjugation over the whole ring. All these observations fit well with the literature. [13]
Conclusion
It seems that the AM1 and PM3 aqueous phase calculations may let us predict the possible tautomeric form in neutral solutions, which may provide invaluable knowledge about the structure and activity of the substituted benzimidazoles for planning syntheses to use for a specific purpose. An attempt to compare the success of the two methods by searching a correlation between the AM1 and PM3 data revealed that in most cases there exists a perfect correlation with a regression of around unity (i.e. R 2≅1) (see Figs. 1, 2, 3), which in turn indicates that only one of the methods of AM1 or PM3 can safely be used in such research, and the molecules that deviate (such as 4, 6 and 8) behave abnormally due to the substituents.
References
Öğretir C, Demirayak Ş (1986) Doğa Kim 10:112–117
Öğretir C, Demirayak Ş (1986) Doğa Kim 10:118–124
Öğretir C, Demirayak Ş (1986) Doğa Kim 10:193–196
Öğretir C, Demirayak Ş (1986) Chim Acta Turc 14:199–211
Öğretir C, Demirayak Ş (1986) Chim Acta Turc 14:285–298
Öğretir C, Demirayak Ş (1990) Chim Acta Turc 18:285–293
Öğretir C, Demirayak Ş (1990) Chim Acta Turc 18:119–124
Öğretir C, Yarligan S (1996) J Mol Struct (THEOCHEM) 366:227–231
Öğretir C, Pütün E, Özbay N (1996) Chim Acta Turc 24:185–188
Öğretir C, Açıkkalp E, Yıldız K, Yarlıgan S (2001) J Mol Struct (THEOCHEM) 536:155–160
Öğretir C, Kanışkan N (2002) J Mol Struct (THEOCHEM) 583:137–144
Yarlıgan S, Öğretir C, Kaynak B, Esenoğlu E (2002) J Mol Struct (THEOCHEM) 586:9–16
Elguero J, Marzin C, Katritzky AR, Linda P (1976) Adv Heterocycl Chem. In: Katrizky AR, Boulton AJ (eds) The tautomerizm of heterocycles, Supplement 1. Academic Press, New York, pp 277–446
Stewart JJP(1993) MOPAC 7.0, QCPE. University of Indiana Bloomington, USA
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Öğretir, C., Yarlıgan, S., Berber, H. et al. A theoretical study of substituent effects on tautomerism of 2-hydroxybenzimidazoles. J Mol Model 9, 390–394 (2003). https://doi.org/10.1007/s00894-003-0150-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-003-0150-0