Abstract
Alkaliphilic fungi are fundamentally different from alkalitolerant ones in terms of mechanisms of adaptation. They accumulate trehalose in cytosol and phosphatidic acids (PA) in the membrane lipids, whereas alkalitolerants contain these compounds in low amounts. But it is unclear how the composition of osmolytes and lipids changes during cytodifferentiation. In this article the composition of lipids and soluble cytosol carbohydrates in the mycelium and fruit bodies of the alkaliphilic fungus Sodiomyces alkalinus was studied. In the mycelium, mannitol and trehalose dominated, while in fruit bodies only trehalose was predominant. Phosphatidylcholines (PC), PA and sterols were major membrane lipids of the mycelium, while PC and sterols were predominant in fruit bodies. The degree of fatty acids unsaturation of the main mycelium phospholipids (PC and PA) increased with age, while that of PC did not change regardless of the developmental stage. In young mycelium, storage lipids were represented mainly by free fatty acids, and in mature mycelium and fruit bodies—by triacylglycerols. Fruit bodies contained three times less membrane lipids and twice as many storage lipids as mycelium. Trehalose was the main cytosol carbohydrate in the mycelium and fruit bodies, which confirms its key value for alkaliphily.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkalitolerance is widespread among various groups of fungi, while alkaliphily is a rare phenomenon. Obligate alkaliphilic fungi grow optimally at pH above 8, and are incapable of growth at pH below 5 (Grum-Grzhimaylo et al. 2016). The natural habitats of alkaliphilic fungi with hyper-alkaline conditions (pH ≥ 10) are relict (Zavarzin et al. 1999) and include soda solonchaks, soda lakes and their littoral soils, rich in saltwort leaf debris. Such habitats are located all over the world, although, as a rule, they are small in area (Bilanenko et al. 2005; Grum-Grzhimaylo et al. 2013, 2016). In addition to pH stress, organisms, inhabiting soda lakes, have to survive under fluctuating osmotic and temperature conditions, that vary drastically with a change of drought and rain, heat and freezing.
The investigated species S. alkalinus was isolated from littoral soils of small soda lakes and soda solonchaks located in Kenya, the Altai and Mongolia. This fungus has a dormancy period in its life cycle, when it forms enclosed fruit bodies (cleistothecia) and dormant ascospores with a thick cell wall to survive without water, or when it becomes inaccessible. Life cycle of S. alkalinus and some possible mechanisms of its adaptation to high pH combined with salinization have been investigated recently at cytomorphological level (Kozlova 2006), while the biochemical mechanisms have not been yet studied sufficiently (Bondarenko et al. 2017, 2018).
Osmolytes, low-molecular compounds, represented in fungi by sugar alcohols, trehalose and, sometimes, amino acids—are known to be involved in adaptation to various stress factors (Yancey 2005). The importance of protective disaccharide trehalose for alkaliphily was demonstrated for the first time in alkaliphilic fungus S. tronii (Bondarenko et al. 2017). Trehalose level reached 5–10% of dry weight at all growth stages, which is comparable with its level in thermophiles (Ianutsevich et al. 2016b) and in mesophiles in response to heat shock (Tereshina et al. 2011). A comparative study of osmolyte composition under different pH in alkaliphiles (S. magadii and S. alkalinus) and alkalitolerants (Acrostalagmus luteoalbus and Chordomyces antarcticus) confirms the hypothesis of the key role of trehalose in alkaliphily, while in alkalitolerants trehalose was a minor component and did not participate in adaptation to ambient pH (Bondarenko et al. 2018).
The maintenance of functional state of membranes is of crucial importance for adaptation to various stress factors. The study of the membranes composition helps to understand the adaptation mechanisms of extremophiles developed over the course of evolution. Membrane protection mechanisms include not only changes of lipid composition and structure (formation of rafts, caveolae, Lɛ—membranes, rich in non-bilayer phospholipids), but although their stabilization by protective carbohydrates, in particular trehalose (Vigh et al. 2005; Alvarez et al. 2007; Balogh et al. 2013; Csoboz et al. 2013; Glatz et al. 2016). In S. tronii, in addition to large amount of trehalose, phosphatidic acids (PA) were found to be one of the major membrane phospholipids in growth dynamics (Bondarenko et al. 2017). It was previously shown that PA level in membrane lipids of mycelial fungi increases significantly in response to various stress factors (Tereshina et al. 2011; Ianutsevich et al. 2016a), while thermophilic fungi have a high PA proportion at all growth stages (Ianutsevich et al. 2016b).
The previous research was focused mainly on the lipid and carbohydrate composition of alkaliphiles in growth dynamics and under adverse pH conditions, but not at different developmental stages. The objective of this work was to study the composition of membrane lipids and soluble cytosol carbohydrates in the mycelium and fruit bodies of alkaliphilic fungus S. alkalinus.
Materials and methods
Strains, media, and cultivation
Strain: original isolate (F11, type) Sodiomyces alkalinus comb. nov. (Bilanenko and M. Ivanova, Grum-Grzhim et al. 2013, CBS 110278) (Plectosphaerellaceae, Ascomycota), originated from the North-Eastern Mongolia (Choibalsans region, Shar-Burdyin Lake). It was isolated from cortical argillaceous loam (sample pH—10.65; soluble salt content—4.9 g/100 g; carbonate alkalinity—10.46 mmol/100 g; bicarbonate alkalinity—20.94 mmol/100 g; total alkalinity—48.16 mmol/100 g. The strain F11-2 under study was isolated from single uninucleate conidium from the original F11 culture (Bilanenko et al. 2005).
For the cultivation of fungus we used alkaline agar, pH 10.0–10.5 (g/l): Na2CO3—24, NaHCO3—6, NaCl—5, KNO3—1, K2HPO4—1, MgSO4x7H2O—0.5, yeast extract—1, starch—15, Difco agar—20, malt extract (Maltax 10 standard, Finland)—17. The final medium was prepared by mixing 1:1 of the liquid medium and 4% agar at 50 °C to prevent caramelization of the agar. Soda buffer allowed to maintain stable alkaline pH within the range 10.0–10.5 (Bilanenko et al. 2005). The cultures were grown on 90 mm Petri dishes, on the cellophane films placed on the agar surface. The Petri dishes were incubated for 3.5 or 16 days at 25 °C in darkness. After the incubation, mycelium was removed from cellophane films with a scalpel. The biomass obtained was rinsed with distilled water to remove sparse conidia, then frozen immediately at − 20 °C. In 16-day cultures the marginal mycelium (0.5–1.0 cm from the edge of the colony) and mature fruit bodies were separated gently by scalpel from the rest of the colony. The purity of every fraction was controlled microscopically.
Lipid and carbohydrate analysis
Lipid and carbohydrate analyses were performed as described earlier (Ianutsevich et al. 2016b). Briefly, lipids were extracted by the Nichols method with phospholipase-deactivating isopropanol, separated by two-dimensional (polar lipids) and one-dimensional (neutral lipids) thin-layer chromatography (TLC) and quantified using standard compounds, densitometry method (DENS software). To determine the composition of fatty acids, their methyl esters were obtained and analyzed by GLC. Soluble cytosol carbohydrates were extracted with boiling water, proteins and charged compounds were removed, trimethylsilyl derivatives of sugars were obtained and analyzed by GLC.
For each variant, experiments were performed three times (n = 3). The differences among the means were compared using one-way ANOVA (P ≤ 0.05). Post hoc Tukey HSD test was used for pairwise comparison between mycelium and fruit bodies’ variants. Statistical analyses were carried out using MS Office Excel. In the figures and tables an asterisk (*) represents significant difference (P ≤ 0.05), each data point is a mean value ± SE (n = 3).
Results
The life cycle of alkaliphilic fungus S. alkalinus includes the vegetative (mycelium), asexual (conidiation) and sexual (fructification) stages (Fig. 1) (Kozlova 2006). In the present study fungal colonies at the age of 3.5 days were represented solely by young (3.5 days) vegetative mycelium, without neither conidia nor ascomata. At the age of 16 days, the colonies consisted of sterile vegetative mycelium at the edge, and developed abundant fructification with a small amount of aerial mycelium in its center, most of which transformed into ascomata at this developmental stage. Conidia were sparse. At the age of 16 days, the colonies consisted of mature mycelium: sterile and vegetative at the edge, and developing abundant fructification with a small amount of aerial mycelium in the center, most of which transformed into ascomata at this developmental stage.
Life cycle of S. alkalinus (Kozlova 2006). a–c Anamorph: a mycelium, b conidiophores, c conidium; d–i teleomorph: d ascogonium, e, f ascogonium, covered with investing hyphae, g young ascocarp developing its ascogenous system consisting of uninucleate and binucleate ascogenous cells (AC) and young asci (A), P peridium, h ascocarp with asci and single ascospores maturing in the common (combined) epiplasm, i mature bicellular ascospore
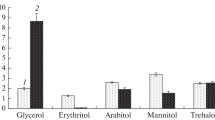
Soluble carbohydrates at the different stages of S. alkalinus life cycle
Soluble cytosol carbohydrates are known to participate in adaptation to heat, osmotic and cold impacts and dehydration by stabilizing macromolecules and cell membranes (Jennings 1985; Yancey 2005), but there are few studies on their participation in adaptation to ambient pH (Bondarenko et al. 2017, 2018).
Study of soluble cytosol carbohydrates composition in S. alkalinus showed that at all investigated developmental stages mannitol, trehalose and arabitol were major carbohydrates, while glucose, inositol, erythritol, and glycerol were the minor ones (Fig. 2). In both young and mature mycelium, the amount of carbohydrates reached 9–11% of dry weight, their composition being similar: mannitol and trehalose were predominant (35–40% of total carbohydrates). Fruit bodies with ascospores contained the same carbohydrates, but the amount of mannitol decreased sharply (threefold) as compared to the mycelium, while the level of trehalose raised twofold, making this disaccharide the sole dominant carbohydrate (70–75% of the total carbohydrates). Meanwhile, the amount of carbohydrates in fruit bodies was practically the same as that in the mycelium.
The composition of main soluble cytosol carbohydrates of mycelium and fruit bodies with ascospores S. alkalinus. The fungus was grown for 3.5 and 16 days on alkaline agar under optimal conditions. In the 16-day culture, the mycelium and fruiting bodies were separated. Soluble cytosol carbohydrates were extracted four times with boiling water, proteins and charged compounds were removed, trimethylsilyl derivatives of sugars were obtained and analyzed by GLC. In the mycelium of the fungus, regardless of age, mannitol and trehalose dominated, whereas in the fruit bodies only trehalose was predominant. The amount of arabitol varied slightly
Membrane and storage lipids at the different stages of differentiation in S. alkalinus
Under optimal growth conditions, the mycelium membrane lipids were generally represented by phospholipids and sterols (St), while sphingolipids were minor compounds (Fig. 3). The major phospholipids were phosphatidylcholines (PC), phosphatidic acids (PA) and phosphatidylethanolamines (PE), the minor ones included phosphatidylserines (PS), phosphatidylinositols (PI), lysophosphatidylethanolamines (LPE) and lysophosphatidylcholines (LPC). The amount of membrane lipids in the mature 16 days mycelium increased slightly (by 20%), compared with the young one (Table 1). The major mycelium phospholipids, along with PC, were PA (their relative content reaching 33%). The membrane lipid profiles in the young and mature mycelia were similar, the only difference was the increase in the proportions of PC and sterols in the mature mycelium, while PA decreased. The proportion of PE did not change and did not exceed 10%. The membrane lipid profile of ascomata differed significantly from that of the mycelium, the amount of lipids having been three times lower in the latter one. PC and sterols were predominant (37% of the total), while the proportion of PA decreased sharply from 33% in the mycelium to 6% in ascomata. Thus, PC and sterols dominated in membrane lipids of fruit bodies, which indicated a shift of predominant membrane lipids.
Composition of membrane lipids of mycelium and fruit bodies with ascospores S. alkalinus. The fungus was grown for 3.5 and 16 days on alkaline agar under optimal conditions. In the 16-day culture, the mycelium and fruiting bodies were separated. Polar lipids were extracted by the Nichols method with phospholipase deactivating isopropanol and mixture of isopropanol and chloroform, separated by two-dimensional TLC and quantified using phosphatidylcholine, glycoceramide and ergosterol standards, followed by densitometry method (DENS software). In the mycelium of the fungus of different ages, PC, PA and St prevailed, while in ascospores—PC and St. The proportion of PE did not significantly change
Lipid bilayer viscosity depends on the degree of unsaturation (DoU) of the main membrane phospholipids. DoU of phospholipids usually decreased over the course of fungal growth, while in dormant structures it depended on the depth of dormancy (Tereshina et al. 2002). The fatty acid composition was individually analyzed for each of the main phospholipids. The main fatty acids of all phospholipids were linoleic (C18:2), oleic (C18:1), and palmitic (C16:0) acids (Table 2). In mature mycelium DoU of all phospholipids increased, compared with the young one, due to the increase in linoleic acid proportion. In contrast to the mycelium, DoU of the main phospholipid (PC) in fruit bodies containing ascospores, varied insignificantly.
Storage lipids of the fungus were represented by triacylglycerols (TAG), diacylglycerols (DAG), free fatty acids (FFA), and sterol esters (Fig. 4). In young mycelium, the dominant lipids were FFA (55% of the total), while in mature mycelium—TAG (52%). In mature mycelium, in comparison to the young one, the amount of storage lipids increased 1.5-fold (Table 1); the proportion of TAG increased threefold and the relative content of FFA decreased, while the proportion of DAG did not change. Ascomata lipid profile was similar to that of the mature mycelium and the main patterns stayed the same, i.e., the proportion of TAG increased to 65% in ascomata, while the amount of storage lipids doubled and reached 15% of dry weight, in comparison with mature mycelium.
Composition of storage lipids of mycelium and fruit bodies with ascospores S. alkalinus. The fungus was grown for 3.5 and 16 days on alkaline agar under optimal conditions. In the 16-day culture, the mycelium and fruit bodies were separated. Neutral lipids were extracted by the Nichols method, separated by one-dimensional TLC and quantified using ergosterol and triacylglycerols standards, followed by densitometry method (DENS software). In the 3.5-day mycelium, FFA dominated in the composition of the storage lipids, and in the mature mycelium and ascospores—TAG, while the proportion of DAG did not change
Discussion
It was demonstrated earlier, that a unique feature of S. alkalinus is early lysis of cell walls of asci and release of immature ascospores inside the centrum of cleistothecium (enclosed fruit body), where they complete their maturation within the united cytoplasm of centrum pseudoparenchyma cells and epiplasms of asci (Kozlova et al. 2018). Conidia are cells with thin hyaline membranes, intended for distribution, but not for survival. The intensity of conidiogenesis was found to vary significantly in different strains of S. alkalinus. The investigated strain S. alkalinus F11-2 is characterized by very low production of conidia and abundant fructification, which makes it possible to isolate a sufficiently pure fraction of mature cleistothecia with ascospores for biochemical analysis.
Soluble cytosol carbohydrates of both mycelium and cleistothecia with ascospores were mainly represented by mannitol, trehalose, and arabitol. Predominant carbohydrates of the mycelium were mannitol and trehalose (up to 45% of the total), while in cleistothecia it was trehalose, its relative content increasing to 75% at the expense of a sharp decrease in mannitol, while the relative content of arabitol did not change. It is worth noting that the amount of trehalose increased during development from 4% of dry weight in the mycelium to 8–10% in ascomata. The fact that trehalose is one of the dominant carbohydrates at all developmental stages has confirmed the idea of the key role of trehalose in alkaliphily, which we suggested earlier (Bondarenko et al. 2017). It should also be noted that arabitol level in the mycelium is close to that in fruit bodies, which suggests its importance for S. alklinus in hyper-alkaline ambient conditions. Meanwhile, mannitol, being the dominant carbohydrate in the mycelium, became a minor component in ascomata. These data are consistent with the results obtained for two other obligate alkaliphilic species of the Sodiomyces genus—S. tronii and S. magadii, which showed increase in mannitol and arabitol levels in fungal mycelium as the result of the raise of pH up to 10.2 (Bondarenko et al. 2017, 2018). It is known that most of fungal spores contain high amounts of trehalose (Tereshina et al. 2002; Rubio-Texeira et al. 2016), whereas in the mycelium of non-extremophilic fungi this carbohydrate is present in trace amounts. Recently, in ascospores of the Neosartorya fisheri (Aspergillus fisheri), which have the highest thermal stability among eukaryotic cells, in addition to trehalose and mannitol, trehalose-based tri-, tetra- and pentasaccharides have been found, associated with a process of vitrification in spores (Wyatt et al. 2015). The main function of trehalose is considered to be protection under various stress conditions (Elbein et al. 2003; Crowe 2007; Iturriaga et al. 2009; Patel and Williamson 2016; Rubio-Texeira et al. 2016; Glatz et al. 2016). The main mechanism of trehalose action is suggested to be water replacement and (or) vitrification under dehydration, which can occur under desiccation and other stresses. This disaccharide is unique, because it protects not only macromolecules of the cell, but also the membranes.
Cytologic study of dormant spores showed that there is a great variety in composition and number of intracellular membrane structures. For example, in the arthrospores of Geotrichum candidum, mitochondria and endoplasmic reticulum (ER) were found, in addition to the nucleus, whereas in arthrospores of Mucor, Golgi apparatus and ER were absent (Barrera 1986). As shown earlier, there are following membrane structures in ascospores of the studied fungus: mitochondria, microbodies, small vacuoles, individual ER cisternae, although small in number (Kozlova 2006). During ascospores maturaion, the number of their membrane structures decreases, while the number of lipid droplets increases.
Ascospores of the investigated fungus germinate easily, without additional external stimuli, which indicates the exogenous type of dormancy (Kozlova 2006). According to our data, TAG and DAG dominate in ascospores, accounting for about 15% of dry weight. Lipids are valuable storage compounds since they are sources of both energy and metabolic water. It is interesting to note that membrane lipids in ascospores contain almost all the components typical for vegetative cells—the entire spectrum of phospholipids, sphingolipids, and sterols, but in smaller amounts. It is worth noting that in S. alkalinus the dominance of phosphatidic acids is observed only in vegetative mycelium, whereas in ascomata they are present in trace amount. These data support our hypothesis about a special role of this phospholipid in the cells of alkaliphilic fungi during active growth stage (Bondarenko et al. 2017). Taking into account the previously obtained data on the dominance of PA in the cells of thermophilic fungi (Ianutsevich et al. 2016b) and the increase in the PA level in response to various stress effects in mesophilic fungi (Tereshina et al. 2011; Ianutsevich et al. 2016a) a protective effect of PA in fungi cells may be hypothesized. High proportion of PA in extremophile cells can reflect different processes: PA-mediated signaling, structural rearrangement of membranes, formation of membrane regions rich in non-bilayer lipids, intensive vesicle formation, active endo- and exocytosis (Kooijman et al. 2003; Vigh et al. 2005; McMahon and Gallop 2005; Alvarez et al. 2007; Balogh et al. 2013; Csoboz et al. 2013; Glatz et al. 2016).
In the fruit bodies of S. alkalinus there were three times less membrane lipids than in the mycelium, and their ratio was different. Sterols, along with PC, were dominant in the membrane lipids of the fruit bodies, and together with PC and PA—in the mycelium, i.e., were one of the main components of membranes at all studied stages of development. It is believed that in the course of evolution there is a trend from cholesterol (in earliest diverging fungi) to ergosterol (in later diverging fungal species), which leads to the dominance of ergosterol in asco- and basidiomycetes (Weete et al. 2010). In fungal cells sterols perform not only structural, but also regulatory functions. They affect membrane viscosity, along with sphingolipids participate in the formation of membrane microdomains and rafts (Alvarez et al. 2007). The importance of sterols in such processes as growth and reproduction, formation of biofilms, virulence (Rella et al. 2016; Rodrigues 2018) has been shown. Using the mutant GL7, it was shown that the yeast cell cycle terminates at the G1 stage in the absence of ergosterol (Dahl and Dahl 1988). It has been shown for the first time that plasma membrane sterols are immunoactive compounds (Rodrigues 2018) and are involved in the formation of biofilm matrix (Rella et al. 2016). Considering the multifunctionality of sterols and their dominance among membrane lipids of ascospores, it can be assumed that these compounds are necessary for the early stages of ascospores germination.
The variety of lipid composition in fungal spores can be illustrated by the example of Neurospora crassa: in ascospores, the predominant lipids are triacylglycerols, while in macroconidia phospholipids dominate, and in microconidia—sterols (Goodrich-Tanrikulu et al. 1998). Aspergillus niger conidia are notable for containing significant amounts of choline sulfate, which is a source of choline for the synthesis of phospholipids, and sulfur—for the synthesis of sulfur-containing proteins (Sussman and Halvorson 1966; Turian 1976). In fungi qualitative fatty acids composition of mycelium often differs to that of spores. In some fungi, spores contain fatty acids not typical for mycelium: 10-epoxydecanoic acid is found in uredospores, behenic acid—in conidia of Sphaerotheca humuli, branched and cyclic fatty acids—in Erysiphe graminis. On the contrary, in the fungi of genus Mucor qualitative fatty acids’ composition of spores does not differ from that of vegetative mycelium. In the S. alkalinus strain under study no differences in qualitative fatty acid composition of ascospores and the mycelium were detected. Moreover, fatty acid composition of PC, predominant membrane phospholipids of ascospores, was similar to that of mycelium; the DoU did not differ significantly as well.
Thus, at the developmental stages studied, in soluble carbohydrates high trehalose content was detected, which supports the assumption about a specific role of this carbohydrate in alkaliphily. The dominance of PA in membrane lipids of mycelium indicates the great importance of these non-bilayer phospholipids for subsistence under hyper-alkaline conditions. In fruit bodies with ascospores, large amounts of trehalose, TAG (in storage lipids), and high proportion of PC and sterols (in membrane lipids) were detected.
Abbreviations
- CL:
-
Cardiolipins
- DAG:
-
Diacylglycerols
- DoU:
-
Degree of unsaturation
- FFA:
-
Free fatty acids
- HS:
-
Heat shock
- LPE:
-
Lysophosphatidylethanolamines
- PA:
-
Phosphatidic acids
- PC:
-
Phosphatidylcholines
- PE:
-
Phosphatidylethanolamines
- PI:
-
Phosphatidylinositols
- PS:
-
Phosphatidylserines
- SL:
-
Sphingolipids
- St:
-
Sterols
- TAG:
-
Triacylglycerols
References
Alvarez FJ, Douglas LM, Konopka JB (2007) Sterol-rich plasma membrane domains in fungi. Eukaryot Cell 6:755–763. https://doi.org/10.1128/EC.00008-07
Balogh G, Péter M, Glatz A, Gombos I, Török Z, Horváth I, Harwood JL, Vigh L (2013) Key role of lipids in heat stress management. FEBS Lett 587:1970–1980. https://doi.org/10.1016/j.febslet.2013.05.016
Barrera CR (1986) Formation and germination of fungal arthroconidia. Crit Rev Microbiol 12:271–292
Bilanenko E, Sorokin D, Georgieva M, Kozlova M (2005) Heleococcum alkalinum, a new alkalitolerant ascomycete from saline soda soils. Mycotaxon 91:497–507
Bondarenko SA, Ianutsevich EA, Danilova OA, Grum-Grzhimaylo AA, Kotlova ER, Kamzolkina OV, Bilanenko EN, Tereshina VM (2017) Membrane lipids and soluble sugars dynamics of the alkaliphilic fungus Sodiomyces tronii in response to ambient pH. Extremophiles 21:743–754. https://doi.org/10.1007/s00792-017-0940-4
Bondarenko SA, Ianutsevich EA, Sinitsyna NA, Georgieva ML, Bilanenko EN, Tereshina VM (2018) Dynamics of the cytosol soluble carbohydrates and membrane lipids in response to ambient pH in alkaliphilic and alkalitolerant fungi. Microbiology 87:21–32. https://doi.org/10.1134/S0026261718010034
Crowe JH (2007) Trehalose as a “chemical chaperone”: fact and fantasy. Adv Exp Med Biol 594:143–158. https://doi.org/10.1007/978-0-387-39975-1_13
Csoboz B, Balogh GE, Kusz E, Gombos I, Peter M, Crul T, Gungor B, Haracska L, Bogdanovics G, Torok Z, Horvath I, Vigh L (2013) Membrane fluidity matters: hyperthermia from the aspects of lipids and membranes. Int J Hyperth 29:491–499. https://doi.org/10.3109/02656736.2013.808765
Dahl C, Dahl J (1988) Cholesterol and cell function. CRC Press Inc., Boca Raton
Elbein AD, Pan YT, Pastuszak I, Carroll D (2003) New insights on trehalose: a multifunctional molecule. Glycobiology 13:17R–27R. https://doi.org/10.1093/glycob/cwg047
Glatz A, Pilbat A, Németh GL, Vince-Kontár K, Jósvay K, Hunya Á, Udvardy A, Gombos I, Péter M, Balogh G, Horváth I, Vígh L, Török Z (2016) Involvement of small heat shock proteins, trehalose, and lipids in the thermal stress management in Schizosaccharomyces pombe. Cell Stress Chaperones 21:327–338. https://doi.org/10.1007/s12192-015-0662-4
Goodrich-Tanrikulu M, Howe K, Stafford A, Nelson MA (1998) Changes in fatty acid composition of Neurospora crassa accompany sexual development and ascospore germination. Microbiology 144:1713–1720. https://doi.org/10.1099/00221287-144-7-1713
Grum-Grzhimaylo AA, Debets AJM, van Diepeningen AD, Georgieva ML, Bilanenko EN (2013) Sodiomyces alkalinus, a new holomorphic alkaliphilic ascomycete within the Plectosphaerellaceae. Persoonia Mol Phylogeny Evol Fungi 31:147–158. https://doi.org/10.3767/003158513X673080
Grum-Grzhimaylo AA, Georgieva ML, Bondarenko SA, Debets AJM, Bilanenko EN (2016) On the diversity of fungi from soda soils. Fungal Divers 76:27–74. https://doi.org/10.1007/s13225-015-0320-2
Ianutsevich EA, Danilova OA, Groza NV, Tereshina VM (2016a) Membrane lipids and cytosol carbohydrates in Aspergillus niger under osmotic, oxidative, and cold impact. Microbiol (Russian Federation) 85:302–310. https://doi.org/10.1134/S0026261716030152
Ianutsevich EA, Kotlova ER, Danilova OA, Tereshina VM, Groza NV (2016b) Heat shock response of thermophilic fungi: membrane lipids and soluble carbohydrates under elevated temperatures. Microbiology 162:989–999. https://doi.org/10.1099/mic.0.000279
Iturriaga G, Suárez R, Nova-Franco B (2009) Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci 10:3793–3810. https://doi.org/10.3390/ijms10093793
Jennings DH (1985) Polyol Metabolism in Fungi. Adv Microb Physiol 25:149–193. https://doi.org/10.1016/S0065-2911(08)60292-1
Kooijman EE, Chupin V, de Kruijff B, Burger KNJ (2003) Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic 4:162–174. https://doi.org/10.1034/j.1600-0854.2003.00086.x
Kozlova MV (2006) Life cycle in a haloalkalitolerant ascomycete Heleococcum alkalinum Bilanenko et Ivanova/Zhiznenniy tsikl galoalkalotolerantnogo askomiceta Heleococcum alkalinum Bilanenko et Ivanova (Russian)/Dissertation, 03.02.12 (Mycology), p 210. Lomonosov Moscow State University, Biological Faculty, Moscow, Russia
Kozlova MV, Bilanenko EN, Grum-Grzhimaylo AA, Kamzolkina OV (2018) An unusual sexual stage in the alkalophilic ascomycete Sodiomyces alkalinus. Fungal Biol. https://doi.org/10.1016/j.funbio.2018.11.010
McMahon HT, Gallop JL (2005) Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438:590–596. https://doi.org/10.1038/nature04396
Patel TK, Williamson JD (2016) Mannitol in plants, fungi, and plant–fungal interactions. Trends Plant Sci 21:486–497. https://doi.org/10.1016/j.tplants.2016.01.006
Rella A, Farnoud AM, Del Poeta M (2016) Plasma membrane lipids and their role in fungal virulence. Prog Lipid Res 61:63–72. https://doi.org/10.1016/j.plipres.2015.11.003
Rodrigues ML (2018) The multifunctional fungal ergosterol. MBio 9:1–5. https://doi.org/10.1128/mBio.01755-18
Rubio-Texeira M, Van Zeebroeck G, Thevelein JM (2016) 10 trehalose metabolism: enzymatic pathways and physiological functions. In: Hoffmeister D (ed) Biochemistry and molecular biology. Springer International Publishing, Cham, pp 191–277
Sussman AS, Halvorson HO (1966) Spores their dormancy and germination. Harper & Row, New York, p 354
Tereshina VM, Memorskaia AS, Kochkina GA, Feofilova EP (2002) Dormant cells in the developmental cycle of Blakeslea trispora: distinct patterns of the lipid and carbohydrate composition. Mikrobiologiia 71:684–689. https://doi.org/10.1023/A:1021432007070
Tereshina VM, Memorskaya AS, Kotlova ER (2011) The effect of different heat influences on composition of membrane lipids and cytosol carbohydrates in mycelial fungi. Microbiology 80:455–460. https://doi.org/10.1134/S0026261711040199
Turian G (1976) Spores in Ascomycetes, their controlled differentiation. In: Weber DJ, Hess WM (eds) The fungal spore. Form and function. Wiley, New York, pp 716–786
Vigh L, Escribá PV, Sonnleitner A, Sonnleitner M, Piotto S, Maresca B, Horváth I, Harwood JL (2005) The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res 44:303–344. https://doi.org/10.1016/j.plipres.2005.08.001
Weete JD, Abril M, Blackwell M (2010) Phylogenetic distribution of fungal sterols. PLoS One 5:e10899. https://doi.org/10.1371/journal.pone.0010899
Wyatt TT, van Leeuwen MR, Golovina EA, Hoekstra FA, Kuenstner EJ, Palumbo EA, Snyder NL, Visagie C, Verkennis A, Hallsworth JE, Wösten HAB, Dijksterhuis J (2015) Functionality and prevalence of trehalose-based oligosaccharides as novel compatible solutes in ascospores of Neosartorya fischeri (Aspergillus fischeri) and other fungi. Environ Microbiol 17:395–411. https://doi.org/10.1111/1462-2920.12558
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830. https://doi.org/10.1242/jeb.01730
Zavarzin GA, Zhilina TN, Kevbrin VV (1999) The alkaliphilic microbial community and its functional diversity. Microbiology 68:503–521
Acknowledgements
The study was partially supported by the Russian Foundation for Basic Research (project 18-04-00488).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Albers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kozlova, M.V., Ianutsevich, E.A., Danilova, O.A. et al. Lipids and soluble carbohydrates in the mycelium and ascomata of alkaliphilic fungus Sodiomyces alkalinus. Extremophiles 23, 487–494 (2019). https://doi.org/10.1007/s00792-019-01100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-019-01100-z