Abstract
Alkaliphily, the ability of an organism to thrive optimally at high ambient pH, has been well-documented in several lineages: archaea, bacteria and fungi. The molecular mechanics of such adaptation has been extensively addressed in alkaliphilic bacteria and alkalitolerant fungi. In this study, we consider an additional property that may have enabled fungi to prosper at alkaline pH: altered contents of membrane lipids and cytoprotectant molecules. In the alkaliphilic Sodiomyces tronii, we showed that at its optimal growth pH 9.2, the fungus accumulates abundant cytosolic trehalose (4–10% dry weight) and phosphatidic acids in the membrane lipids, properties not normally observed in neutrophilic species. At a very high pH 10.2, the major carbohydrate, glucose, was rapidly substituted by mannitol and arabitol. Conversely, lowering the pH to 5.4–7.0 had major implications both on the content of carbohydrates and membrane lipids. It was shown that trehalose dominated at pH 5.4. Fractions of sphingolipids and sterols of plasma membranes rapidly elevated possibly indicating the formation of membrane structures called rafts. Overall, our results reveals complex dynamics of the contents of membrane lipids and cytoplasmic sugars in alkaliphilic S. tronii, suggesting their adaptive functionality against pH stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In natural habitats, pH can vary in a wide range from very acidic (pH 0–1) to very alkaline (pH 11–12). While acidic habitats are not often encountered in nature, alkaline habitats are more widespread and typified by soda lakes and its surroundings called soda soils with stable pH values of 10–12 (Muruga and Anyango 2013). Such environments harbour a diversity of microorganisms that have adapted to persist and thrive in these harsh conditions. A vibrant spectrum of bacteria from all major trophic groups have been shown to populate soda lakes and soils, accounting for the presumed complete nutrient recycle (Jones et al. 1998; Zavarzin et al. 1999; Sorokin et al. 2014). In addition to dense cyanobacterial presence, eukaryotic species, like diatoms and green algae supply the bulk of carbon, ranking soda lakes among the most productive ecosystems in the world (Duckworth et al. 1996).
Fungal presence in soda soils have been seldomly addressed earlier by (Steiman et al. 2004) and (Elíades et al. 2006), and more recently with systematic approaches using morphology characters, growth tests and DNA information (Grum-Grzhimaylo et al. 2013; Grum-Grzhimaylo and Georgieva 2013; Grum-Grzhimaylo et al. 2016). These studies reveal that fungi recovered from very alkaline soda soils differ in their preference toward ambient pH. The large fraction of these fungi comprise alkalitolerant species, while the smaller proportion are truly alkaliphilic—a rare property of fungi that have growth optimum at pH >8 while lacking the ability to grow at pH below 5. The ascomycetous family Plectosphaerellaceae appears to harbour many alkalitolerant and alkaliphilic species (Grum-Grzhimaylo et al. 2016), with members of Sodiomyces genus recovered from soda soils of Mongolia, Russia, Kenya and Tanzania, showing the most extreme alkaliphilic adaptation. Currently, the genus contains three species: S. alkalinus, S. magadii, S. tronii.
Alkaliphilic fungi provide a unique potential for studying adaptations towards extremely high ambient pH. Previous studies addressed the fungal response to elevated ambient pH using neutrophilic species: Aspergillus niger (Hesse et al. 2002), A. nidulans (Denison 2000), Fusarium oxysporum (Caracuel et al. 2003), Neurospora crassa (Sanders and Slayman 1982), and Ustilago maydis (Benito et al. 2009). It has been demonstrated that fungi are capable of maintaining proper intracellular pH upon changes of ambient pH. pH homeostasis seems to be regulated by the P-type Na+- and H+-ATPases, electroneutral Na+/H+ antiporters, as well as the vacuolar V-type Na+-ATPases and mitochondrial F1Fo-ATPases. The intricate mechanism of pH-sensing and signal transduction has been extensively studied in neutrophilic A. nidulans, a fungus with wide pH tolerance (Peñalva et al. 2008). The key role is attributed to the transcription factor PacC that mediates the expression of numerous target genes upon pH change (Espeso and Arst 2000; Arst and Peñalva 2003; Peñalva et al. 2008). Subsequent studies demonstrated the conservative nature of PacC among different physiological groups of fungi, indicating early evolution of this pathway (Davis 2003; Rollins 2003; Zou et al. 2010; Landraud et al. 2013; Zhang et al. 2013; Grum-Grzhimaylo 2015). Additionally, alkaline stress was shown to invoke antioxidant defence enzymes in Chaetomium globosum (Ravindran and Naveenan 2011).
In the current study, we address the alkaliphily phenomenon in fungi from a different perspective. We hypothesise that extremely high (or low) ambient pH invokes a general stress response that alters the contents of the plasma membranes and compatible solutes in cytosol. It has been shown before that stress conditions like desiccation, osmotic and thermal shock, trigger global changes in the chemical composition of the cell. Major defence strategies against these stresses involves synthesis of heat shock proteins and antioxidants, but also functional maintenance of membranes and cytoplasmic macromolecules (Piper 1993; Richter et al. 2010; Péter et al. 2017). The recent discovery of domain configuration of membranes, such as rafts, caveolae and detergent-insoluble membrane fractions, shed a new light on the adaptations to stress. Previous work on these membrane structures have demonstrated their involvement in the regulation of activity of heat-shock proteins, G-proteins, enzymes, and other important cellular attributes related to stress response (Vigh et al. 2005; Alvarez et al. 2007; Balogh et al. 2013; Csoboz et al. 2013; Glatz et al. 2016). Furthermore, an increase of non-bilayer lipids in the cellular membrane, such as phosphatidylethanolamines (PE), phosphatidic acids (PA) and monoglycosyldiglycerides can lead to the formation of the so-called Lɛ membranes. These structures appears to stabilize peripheral proteins that are nested within the membranes, through the acyl groups of phospholipids. Substantial increase of the PA levels in the cellular membranes content upon cold, heat, osmotic and oxidative shocks have been demonstrated in a number of fungi, such as A. niger, Cunninghamella japonica, Pleurotus ostreatus and others (Tereshina et al. 2011; Yanutsevich et al. 2014; Ianutsevich et al. 2016a; Ianutsevich et al. 2016b).
Alternatively, membrane stability during stress can be achieved by small cytoplasmic protectant molecules. For example, trehalose, a non-reducing disaccharide, appears to be multifunctional, but primarily is believed to act as a membrane and macromolecules protectant upon several stress types (Elbein et al. 2003; Crowe 2007; Iturriaga et al. 2009). An array of other compounds, like polyols, various sugars (including trehalose) and carboxamines, are compatible solutes that can accumulate in large quantities, and function as osmoprotectants and thermostabilizers during desiccation, osmotic and heat shocks (Jennings 1984; Hohmann 2002; DasSarma and DasSarma 2012). For example, halophilic and halotolerant fungal species, those that prefer or can tolerate high salt concentrations, accumulate arabitol and glycerol upon exposure to high salts (Kogej et al. 2007; Smolyanyuk et al. 2013).
The profile of the commonly known protectant molecules, as well as cellular membrane contents upon exposure to various pH, has not been addressed before. Here, we use the alkaliphilic fungus Sodiomyces tronii and show its response in the accumulation of commonly known protectant compounds and membrane lipids contents at its optimal and sub-optimal growth pH. The presented insights may help explain the pH tolerance in neutrophilic species, as well as the adaptation to extremely high pH in alkaliphilic species.
Methods
Strain, media and growth
The alkaliphilic filamentous fungus Sodiomyces tronii (Plectosphaerellaceae, Ascomycota) strain CBS 137620, recovered from soda soil of Magadi Lake, was used throughout this study (Grum-Grzhimaylo et al. 2016). This strain, as opposed to the type strain, started to sporulate asexually (no development of fruit bodies was observed) only after 20 days if grown on alkaline agar (AA, pH 10.2) medium (Grum-Grzhimaylo et al. 2013). This property allowed us to collect pure mycelium without the admixture of spores, which is important for assessing the chemical state of living mycelium. Routine sub-culturing of the fungus was performed using AA medium.
For elucidating the growth rate at various pH, we performed growth experiments using the so-called race tubes (Perkins and Pollard 1986). Several growth media based on citrate, phosphate and carbonate buffers were generated that ranged in pH: 4.0, 5.4, 7.0, 9.2, 10.2 (±0.2). The contents of the media and buffers composition were followed from Grum-Grzhimaylo et al. 2016, except that the nutrient component of the medium contained 200 ml/l wort, instead of 17 g/l malt extract powder. We inoculated the race tubes with a mycelium plug (1 mm) from one side, and followed the growth front throughout the tube over a month at 32 °C (dark).
Temperature preference of S. tronii was assessed by growing the fungus on the modified AA medium: we lowered the pH to 9.2 using appropriate carbonate buffer. The growth tests were performed in triplicate in Petri dishes incubated at 15.0, 23.5, 28.0, 32.5, 36.0, 40.0, 45.0 and 50.0 °C in the dark. Inoculation of the plates was done with 5 mm agar plugs that contained mycelium. Diameters of the colonies were measured every 2–4 days until they reached the edge of the plate.
The effect of NaCl on growth was performed at 32 °C using wort agar (200 ml/l wort, 20 g/l agar) based on various pH (5.4, 7.0, 9.2, 10.2) and NaCl content (0, 0.05, 0.1, 0.2, 0.3 M and additional 0, 0.5, 1.0, 1.5, 2.0 M concentrations at optimal pH 9.2). Inoculation was done with a mycelium plug (1 mm) placed in the centre of the plate, and growth expansion was followed as described above.
The effect of buffer molarity on growth was determined at optimal conditions (pH 9.2, 32 °C, 0 M NaCl). Inoculation was done with a mycelium plug (1 mm) placed in the centre of a plate, and growth expansion was followed as described above. Growth rate was calculated as mm/day during linear growth of the fungus.
For the biochemical analyses, we pre-grew the fungus as follows. A mycelial suspension from a parental AA fungus plate was prepared, which was then surface inoculated onto a fresh plate with modified AA medium (pH 9.2, no NaCl), and put at 32 °C for 4–6 days. After pre-growth, we cut out 1 mm mycelium agar plugs and centre-inoculated them onto Petri dishes with a cellophane membrane placed on top of the medium. We incubated these plates for 4, 7, 10, 17 days (wrapped with Parafilm), and then used the mycelium for the subsequent membrane and cytoplasmic sugars analyses. The mycelium for the investigation of the pH effect on these traits was prepared in a similar way on the mycelium grown at pH values of 5.4, 7.0, 9.2, 10.2 for 10 days.
Lipid analysis
The fungal mycelium was separated from the solid medium using a scalpel and detected gravimetrically. For lipid analysis, the weighted portion of the biomass was immediately homogenized in isopropanol by pestle and mortar, and incubated at 70 °C for 30 min. Thereupon, the lipids were extracted using the method described in (Nichols 1963), which involved extraction with isopropanol and the isopropanol–chloroform mixture (1:1 and 1:2) at 70 °C, evaporation in a rotary evaporator, and extraction of the residue with chloroform–methanol (1:1) supplemented with 5% sodium chloride solution and water to remove water-soluble substances. After separating the mixture by allowing it to stand overnight or by centrifugation, the chloroform layer was dried by passing it through water-free sodium sulphate, evaporated, and desiccated with a vacuum pump. The resulting pellet was dissolved in a small amount of chloroform–methanol (1:1) and stored at –21 °C. The composition of neutral lipids was assessed using ascending thin layer chromatography on glass plates with silica gel 60 (Merck, Germany). To separate neutral lipids, the hexane:diethyl ether: acetic acid (85:15:1) system (Kates 1972) was used. Phospholipids and glycolipids were separated with the systems (Benning et al. 1995) for two-dimensional TLC. The lipids (100–200 μg) were applied to a plate. To develop the stains, the chromatograms were sprayed with 5% sulfuric acid in ethanol with subsequent heating to 180 °C. Phospholipids were identified using individual markers and qualitative tests for amino groups (with ninhydrin), choline-containing phospholipids (with the Dragendorff reagent), and glycolipids (with α-naphthol). Neutral lipids were identified with individual markers for mono-, di-, and triglycerides, sterols (ergosterol), free fatty acids, and hydrocarbons (Sigma, United States). Sphingolipids were detected in the glycolipid fraction by the saponification method (Kates 1972). Quantitative analysis of the lipids was performed using the Dens software (Lenchrom, Russia). Lipid quantities were determined using the following standards: phosphatidylcholine (Sigma, United States) for phospholipids, a glycoceramide mixture (Larodan, Sweden) for sphingolipids, and ergosterol (Sigma, United States) for sterols. To determine the fatty acid composition of the phospholipids, the main phospholipids were isolated by TLC. After methanolysis (2.5% H2SO4 in methanol, 2 h at 80 °C), the fatty acid methyl ethers were determined using a Kristall 5000.1 gas chromatograph (Chromatek, Russia) on an Optima-240 0.25 μm, 60 m, 0.25 mm capillary column (Macherey–Nagel GmbH & Co., Germany). During the chromatography, the temperature was gradually increased from 130 to 240 °C. Fatty acids were identified using the Supelco 37 Component FAME Mix (mixture of fatty acid methyl esters) (Supelco, United States).
Carbohydrate analysis
To determine the soluble carbohydrate composition of the mycelium, sugars were extracted with boiling water for 20 min in four steps. Proteins were removed from the resulting extract (Somogyi 1945). The carbohydrate extract was further purified from charged compounds using a combined column with the Dowex-1 (acetate form) and Dowex 50 W (H+) ion exchange resins. Carbohydrate composition was determined by GLC using trimethylsilyl sugar derivatives obtained from the lyophilized extract (Brobst 1972). The internal standard was α-methyl-D-mannoside (Merck). Chromatography was carried out on a Kristall 5000.1 gas chromatograph (Chromatek, Russia) with a ZB-5 30 m, 0.32 mm, 0.25 μm capillary column (Phenomenex, United States). The temperature was increased from 130 to 270 °C at a rate of 5–6 °C/min. Glucose, mannitol, arabitol, inositol, and trehalose (Sigma, United States) were used as standards.
Statistical analyses
The experiments were performed in biological triplicates. The influence of incubation period and pH on soluble sugars and lipids was determined by one-way ANOVA (n = 3) using R (R Core Team 2016).
Results
Optimal growth conditions for S. tronii
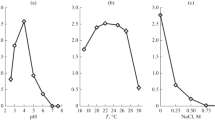
Prior to assessing the pH effect on the lipid and sugar contents, we first sought to establish pH and NaCl concentration optimal for the growth of S. tronii CBS 137620. Our results demonstrate its maximal linear growth rate at pH 9.2 (about 7 mm/day), and no growth at pH below 5, confirming the alkaliphilic nature of S. tronii (Fig. 1). During propagation of S. tronii, no pH shifts in the tested media were detected. At these conditions, the colony has the following morphological traits: white, fluffy, 5 mm height, smooth colony rim, clear colony reverse, no exudate. Although drastically affecting growth rate, pH changes do not invoke significant morphology alterations, only making the fungal colonies somewhat less fluffy.
At the optimal pH of 9.2, the fungus grows well in a wide range of temperatures from 25 to 45 °C, showing maximum growth at 32 °C (Fig. 2). Sodiomyces tronii does not grow at temperature above 50 °C, however, residual propagation was seen at temperature below 15 °C.
The effect of NaCl on S. tronii growth was assessed on citrate, phosphate and carbonate buffers at four pH values –5.4, 7.0, 9.2, 10.2. In all cases the addition of NaCl (0.05–0.3 M) did not stimulate the growth of the fungus (Fig. 3a). Elevated NaCl content in neutral medium (pH 7.0) resulted in greater growth inhibition, than in other media tested. At optimal pH of 9.2, S. tronii was capable of growth with up to 2 M of NaCl concentration in the medium (Fig. 3b). Furthermore, increasing the concentration of (bi-)carbonate buffer with pH 9.2 from 0.1 to 0.7 M in the medium negatively affected the growth of S. tronii (Fig. 4). Thus, we established a few parameters optimal for the growth of the fungus: pH 9.2 at 0.1 M buffer, 32 °C, no NaCl.
a The effect of NaCl on the growth of S. tronii at various pH. The fungus was incubated in Petri dishes with the medium based on 0.1 M carbonate buffer (pH 9.2, 10.2), phosphate for pH 7.0, and citric for pH 4.0, 5.4. Temperature was set to 32 °C and the growth was registered over 10 days. Mean values are displayed (n = 3, ±SD). b The effect of NaCl on the growth of S. tronii at optimal pH of 9.2 and temperature of 32 °C over 10 days period. Mean values are displayed (n = 3, ±SD)
At these optimal conditions the colony of S. tronii CBS 137620 developed asexual sporulation after 17 days of incubation. During our experimentations sexual sporulation was not detected. Lack of asexual sporulation in the first couple of weeks and ability to grow at sub-optimal pH, renders S. tronii a convenient model species for studying lipid and sugar contents in response to various ambient pH values. Absence of sporulation is important as spore admixing would affect our subsequent biochemical assays, hardening the interpretation of the results.
Cytoplasmic soluble carbohydrates at various ambient pHs
As mentioned earlier, soluble cytoplasmic sugars play a role in adaptations upon heat, osmotic, cold shocks and desiccation, protecting macromolecules and cellular membranes (Jennings 1984; Yancey 2005; Iturriaga et al. 2009; Ianutsevich et al. 2016a). However, whether soluble sugars account for adaptations to the unfavourable ambient pH, remains to be shown. Here, we tracked the composition of membrane lipids and cytoplasmic sugars both over the time course of 17 days and at various pH. At optimal pH and temperature (pH 9.2 at 32 °C), on the 4th, 6th, 10th, and 17th day of growth the cytoplasmic sugars content varied from 10 to 14% (w/w) (Fig. 5). Glucose and trehalose proved to be the major sugars, together with mannitol to a lesser extent (Online resource 1). Glycerol, inositol and erythritol were present in trace amounts (data not shown). By the first week of incubation, glucose comprised half of the soluble sugars in the mixture, however, as the growth progressed further, the glucose amount decreased twofold at the end of incubation (p < 0.05). Of note, high trehalose content (>5% w/w) was observed from the early growth stages, further increasing to 70% of the entire sugar mix by the end of the incubation (p < 0.05).
The main cytosol sugar composition during the growth of S. tronii at optimal conditions (pH 9.2, 32 °C, no NaCl). Samples were analysed on the 4th, 7th, 10th, and 17th day of growth. With time, trehalose content was increasing (p < 0.05), while glucose was decreasing (p < 0.05). Mean values are displayed (n = 3, ± SD)
pH dramatically affected the cytoplasmic sugar repertoire in S. tronii. Elevating pH by 1 unit from optimum resulted in increased mannitol fraction (p < 0.001), making it the dominating sugar (50% of the total soluble sugars in cytosol (Fig. 6). While the amount of mannitol increased, the glucose fraction went down dramatically at pH 10.2 (p < 0.05). Trehalose fractions did not change much, but arabitol concentration increased tenfold (p < 0.05). Dropping ambient pH to neutral did not induce major changes in soluble sugars content, when compared to pH 9.2. However, decreasing pH further down to 5.4 resulted in the overall reduction of soluble sugars and a dramatic drop (sixfold) in glucose content (p < 0.05). Low pH did not significantly change the trehalose content when compared to pH 9.2, thus rendering it as a dominating sugar with the fraction of 80% of total sugars.
The main cytosol sugar composition of S. tronii at optimal conditions (pH 9.2, 32 °C, no NaCl) after 10 days of growth. The pH elevation to 10.2 resulted in fivefold increase of the mannitol fraction (p < 0.001), and decrease in glucose (p < 0.05). Lowering pH led to an overall reduction of soluble sugars and dramatic drop in glucose content (p < 0.05). Mean values are displayed (n = 3, ±SD)
Overall our results demonstrate replacement of a dominant cytoplasmic sugar across different pH tested. At very alkaline pH of 10, mannitol and trehalose were dominant, while at pH 9.2 and 7.0—glucose and trehalose were dominant. Acidic pH of 5.4 only invoked trehalose. We hypothesize these dynamics are adaptive to various ambient pH values in S. tronii.
Membrane and storage lipids at various ambient pH
Another way to protect cellular membranes is altering its contents in response to unfavourable conditions (Piper 1993). We sought to assess the dynamics of the cellular membrane lipids in response to various environmental pH.
At optimal pH, phospholipids and sterols were dominating in the membranes with a minority of sphingolipids (SL) (Fig. 7). Dominating phospholipids were phosphatidylethanolamines (PE), phosphatidylcholines (PC), and phosphatidic acids (PA), while minority comprised phosphatidylserines (PS), phosphatidylinositols (PI), lysophosphatidylethanolamines (LPE), and lysophosphatidylcholines (LPC). PE and PC fractions did not change significantly over the 17 days of incubation. Of note, PA were dominating in the beginning of the cultivation, but then were replaced by an increasing amount of sterols (St) (p < 0.05).
Membrane lipids composition during the growth of S. tronii in Petri dishes at optimal conditions (pH 9.2, 32 °C, no NaCl). Samples were analysed on the 4th, 7th, 10th, and 17th day of growth. Early development was associated with the dominance of PA, PC, PE, and St. As the growth progressed, PA fraction decreased with the concurrent increase in St (p < 0.05). Mean values are displayed (n = 3, ±SD). PE phosphatidylethanolamines, PC phosphatidylcholines, CL cardiolipins, PA phosphatidic acids, PS phosphatidylserines, PI + LPE phosphatidylinositols + lysophosphatidylethanolamines, LPC lysophosphatidylcholines, SL sphingolipids, St sterols
Membrane lipid contents at pH 10.2 was not much different from that of at pH 9.2. But a pH drop to 7.0 or 5.4 resulted in the dramatic increase in St (p < 0.05) and SL (p < 0.01), with concurrent decrease PC fraction (p < 0.05) (Fig. 8). The total amount of membrane lipids remained more or less constant at different pHs (data not shown).
Membrane lipids composition in S. tronii at various pH after 10 days of growth (no NaCl, 32 °C). The fungus was incubated in Petri dishes with the medium based on 0.1 M carbonate buffer (pH 9.2, 10.2), phosphate for pH 7.0, and citric for pH 4.0, 5.4. The pH increase to 10.2 did not significantly change the membrane lipid content. Lowering pH to 7 and 5.4 caused an increase in SL and St (p < 0.05) with the concurrent reduction of PC (p < 0.05). Mean values are displayed (n = 3, ±SD). PE phosphatidylethanolamines, PC phosphatidylcholines, CL cardiolipins, PA phosphatidic acids, PS phosphatidylserines, PI + LPE phosphatidylinositols + lysophosphatidylethanolamines, LPC lysophosphatidylcholines, SL sphingolipids, St sterols
Viscosity of the membrane lipid bilayer is determined by the degree of unsaturation of acyl residues in phospholipids. To address the hypothesis that membrane viscosity protection (Sinensky 1974) may play a role in the adaptation to ambient pH, we chromatographically isolated three main phospholipids and analysed its fatty acids composition. Our analysis showed that throughout cultivation the degree of unsaturation of fatty acids was increasing in PE (p < 0.05) and PC (p < 0.01), but decreasing in PA (p < 0.001). The overall degree of fatty acids unsaturation remained constant (Table 1). Dominating fatty acids among all phospholipids were linoleic (C18:2), oleic (C18:1), and palmic (C16:0). The pH effect on the unsaturation degree of PC and PE were not pronounced (Table 2) as of total lipids (Online resource 2). In general, the degree of unsaturation of lipids reduced at the extreme values on the pH spectrum (p < 0.05).
Storage lipids in fungi comprise triacylglycerols (TAG), diacylglycerols (DAG), and free fatty acids (FFA). The pH increase to 10.2 did not affect the composition of storage lipids, when compared to pH 9.2 (Fig. 9). However, lowering pH resulted in the 1.5–2 times increase of acylyc lipids, mostly by TAG contribution (p < 0.05).
Storage lipids composition in S. tronii at different pH after 10 days of growth (no NaCl, 32 °C). The fungus was incubated in Petri dishes with the medium based on 0.1 M carbonate buffer (pH 9.2, 10.2), phosphate for pH 7.0, and citric for pH 4.0, 5.4. The dominance of TAG was observed at every pH tested, with significant increase upon acidification (p < 0.05). Mean values are displayed (n = 3, ±SD). FFA free fatty acid, TAG triacylglycerols, DAG diacylglycerols
In conclusion, we demonstrated dominance of phosphatidic acids in an actively growing S. tronii colony. The unsaturation analyses of fatty acids in PE and PC suggests their minor response to ambient pH, indicating that membrane viscosity remains constant upon the pH change. Rapid membrane and storage lipids dynamics at low pH (elevated ST, St, TAG) compared to high pH indicate that acid stress manifests more a pronounced response in S. tronii than alkaline stress.
Discussion
The alkaliphilic fungus Sodiomyces tronii was isolated from the edge of one of the most alkaline (pH 12) soda lakes—Magadi Lake of south Kenya. The climate conditions of that area alternate between the rainy period and the extreme drought season with temperatures exceeding 40 °C (Muruga and Anyango 2013). Microorganisms that populate such an environment presumably have evolved under several abiotic stresses: elevated pH and temperature, periodic drought and flooding, hence fluctuating osmotic pressures. As all these factors affect the performance of a given organism, our first goal was to establish optimal growth conditions for S. tronii.
We confirmed that S. tronii is an obligate alkaliphilic species, having a growth optimum at pH around 9.2 (Fig. 1). Elevating pH further up caused hindered growth, a feature that enabled us study the fungal response to extremely alkaline sub-optimal pH. Our experiments display S. tronii as thermotolerant species growing optimally at 32.5 °C, and tolerating temperatures exceeding 45 °C (Fig. 2). Although having rather high temperature optimum, S. tronii cannot be tagged as thermophilic species, as it is still capable of growth at temperature as low as 15 °C (Cooney and Emerson 1966). NaCl supplementation in the media did not stimulate the growth regardless of buffer composition (Fig. 3a). At optimal pH, growth was arrested at 2 M NaCl concentration in the media. Taken together, these results classify S. tronii as weak halotolerant (Fig. 3b). Also, increased molarity of a buffer resulted in reduced growth (Fig. 4). Wide salt and buffer molarity tolerance capabilities suggest S. tronii has adapted to fluctuating osmotic conditions due to environmental dynamics, like periodical drought or flooding of soda soils.
In the past years, there has been a growing realization that organic osmolites can partake in stress adaptations that involve water deficits, such as anhydrobiosis, freezing, osmotic, cold, and heat stresses (Yancey 2005; Glatz et al. 2016). Most of these compounds exhibit cytoprotectant properties, promoting antioxidant defence and macromolecule stabilization, which suggests a wider metabolic role of osmolites than previously considered. For example, trehalose is believed to take part in various protective mechanisms: carbon storage, osmolite, membrane protector at stress conditions, protein folding, antioxidant (Elbein et al. 2003; Crowe 2007; Iturriaga et al. 2009). Polyols are another type of compounds that exhibit protective properties. It is widely accepted that their function is maintaining proper osmotic pressure (Hohmann 2002), however, there is growing evidence that polyols participate in many other cellular processes. Glycerol, for example, was detected in high quantities in halophilic yeasts (Kogej et al. 2007; Gunde-Cimerman et al. 2009; Zajc et al. 2014) and shown to reduce the formation of reactive oxygen species (ROS), as well as maintaining proper redox potential in the cell (Ansell et al. 1997). One of our aims here was to test whether pH stress induces changes in osmolite contents in S. tronii. At optimal pH growth conditions (pH 9.2) and throughout all growth stages, S. tronii contained large proportion of soluble sugars (10–16% dry weight), predominantly glucose and trehalose, not mannitol, as in other studied ascomycetes. Trehalose content in the mycelium was very high (5–8% dry weight), rising up to 70% of total sugars as mycelium aged (Fig. 5). Such a pattern is comparable to that of thermophilic fungus Myceliophthora thermophila (Ianutsevich et al. 2016b), which had similar trehalose content. Furthermore, both species have glucose as major cytosol sugar. High levels of trehalose throughout the life cycle of S. tronii indicates its possible involvement in the adaptation to multiple stress factors. We then studied the cytoplasmic sugar dynamics of S. tronii at extremely high ambient pH, as well as lower sub-optimal pH. Interestingly, varying ambient pH not only affected the overall sugar content, but also dramatically changed its composition, which indirectly suggests involvement of different sugars in the adaptation to pH-fluctuating conditions. The dominance of trehalose signify its involvement in response to acidic stress. It is worth noting that at pH levels we tested, trehalose remained the dominant sugar and accumulation of other sugars were achieved by substituting glucose, and not trehalose (Fig. 6).
Previous studies showed that intracellular pH seems to be neutral upon exposures to lower-than-optimum pH in a related alkaliphilic fungus, S. alkalinus (Grum-Grzhimaylo 2015). This implies effective machinery for maintaining proper cellular pH. Apart from proton pumps, carbohydrate metabolism has been suggested to promote cell pH homeostasis. Polyols may possibly act as proton sinks that can also partake in maintaining cellular redox potential (Jennings 1984). This hypothesis is based largely on a fact that polyols are synthesized using NADPH and NADH. Even though it is unknown whether intracellular pH is stably maintained in S. tronii, we note a substantial increase of mannitol concentration upon elevated ambient pH exposure (Fig. 6). It should be pointed out that in our experiments elevated pH was achieved by a different carbonate/bicarbonate ratio, therefore, keeping osmotic pressure constant. We hence conclude that the increase in mannitol is a response to elevated pH, indirectly suggesting its involvement in pH homeostasis. Mannitol can act as a physiological buffering agent that helps maintaining proper cellular redox potential and enzymes activity.
One of the key attributes towards tolerating various stress factors is keeping the active physiological state of cellular membranes. Membrane protection can be achieved, among other ways, by altering its contents. In S. tronii, lipid membrane composition was similar if compared to other fungi (A. niger, Cunninghamella japonica, Pleurotus ostreatus) (Tereshina et al. 2011) However, an unusual feature was dominance of phosphatidic acids (PA) in the membranes at early growth stage (Fig. 7). With age, their relative content subsided and substituted by sterols. Conical shape of the PA molecules and the ability to aggregate promote membrane curvature, which is indicative to the formation of vesicles (Kooijman et al. 2003; McMahon and Gallop 2005). This in turn suggests active exocytosis, endocytosis, and hence membrane renewal. We previously showed that PA increase was observed only upon heat shocks in three mesophilic fungi, however, a thermophilic one maintained constant high PA contents throughout the entire life cycle (Yanutsevich et al. 2014; Ianutsevich et al. 2016b). Even though in S. tronii high PA levels were only observed during the trophophase, we suspect PA to be involved in general stress response in this fungus.
Neutral and acidic pH caused substantial changes in the membrane lipid composition in S. tronii: decrease of major PC with the concurrent increase of sphingolipids and sterols (Fig. 8). Such pH-response in the membrane lipid dynamics were dissimilar compared to responses in other fungi upon heat, cold, osmotic and oxidation shocks (Tereshina et al. 2011; Ianutsevich et al. 2016b). It has been hypothesized that glycolipids, such as sphingolipids, can form hydrogen bonds with the heads of phospholipid molecules enhancing their stability (Yu et al. 1986). Sphingolipids are in fact multifunctional molecules that partake in various cellular processes (Cheng et al. 2001; Levery et al. 2002; Siskind 2005), including their signalling role upon heat shock (Jenkins 2003). Sterols also accumulated upon acid pH in S. tronii. They appear to be essential and serve a structural function stabilizing membranes (Beck et al. 2007). Concurrent increase of sphingolipids and sterols upon pH stress leads us to suspect the formation of the so-called rafts, which are the membrane complexes composed of sphingolipids, sterols and integral proteins. The functional state of proteins within those membrane complexes are maintained by lipid–lipid, protein–lipid and protein–protein interactions (Alvarez et al. 2007; Simons and Sampaio 2011). The discovery of these mosaic membrane structures shed a new light on the regulation of membrane functionality and gene expression (Vigh et al. 2005; Alvarez et al. 2007). In S. tronii, we suspect rafts also play a role in response to acid pH stress.
To conclude, we found complex dynamics of the composition of the cytosolic and membrane lipids upon exposure to various ambient pH. At the optimal pH of 9.2, S. tronii accumulated abundant trehalose (5–8%) in cytosol and phosphatidic lipids in the membranes, a property not normally seen in neutrophilic fungi (Tereshina et al. 2011). We showed the dominance of trehalose upon the acidic pH shock. Conversely, mannitol and arabitol accumulation was coincidental with the pH increase. Acidic pH induces significant accumulation of sphingolipids and sterols in the membranes, which is indicative of the membrane rafts formation. The demonstrated dynamics of lipid and carbohydrates upon pH stress reflects intricate regulation and adaptation mechanisms to various ambient pHs in the alkaliphilic fungi.
Abbreviations
- PA:
-
Phosphatidic acids
- PC:
-
Phosphatidylcholines
- PE:
-
Phosphatidylethanolamines
- CL:
-
Cardiolipins
- PS:
-
Phosphatidylserines
- PI:
-
Phosphatidylinositols
- LPE:
-
Lysophosphatidylethanolamines
- LPC:
-
Lysophosphatidylcholines
- SL:
-
Sphingolipids
- St:
-
Sterols
References
Alvarez FJ, Douglas LM, Konopka JB (2007) Sterol-rich plasma membrane domains in fungi. Eukaryot Cell 6:755–763. doi:10.1128/EC.00008-07
Ansell R, Granath K, Hohmann S et al (1997) The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J 16:2179–2187. doi:10.1093/emboj/16.9.2179
Arst HN, Peñalva MA (2003) pH regulation in Aspergillus and parallels with higher eukaryotic regulatory systems. Trends Genet 19:224–231. doi:10.1016/S0168-9525(03)00052-0
Balogh G, Péter M, Glatz A et al (2013) Key role of lipids in heat stress management. FEBS Lett 587:1970–1980. doi:10.1016/j.febslet.2013.05.016
Beck JG, Mathieu D, Loudet C et al (2007) Plant sterols in “rafts”: a better way to regulate membrane thermal shocks. FASEB J 21:1714–1723. doi:10.1096/fj.06-7809com
Benito B, Garciadeblás B, Pérez-Martín J, Rodríguez-Navarro A (2009) Growth at high pH and sodium and potassium tolerance in media above the cytoplasmic pH depend on ENA ATPases in Ustilago maydis. Eukaryot Cell 8:821–829. doi:10.1128/EC.00252-08
Benning C, Huang ZH, Gage DA (1995) Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch Biochem Biophys 317:103–111. doi:10.1006/abbi.1995.1141
Brobst KM (1972) Gas–liquid chromatography of trimethylsilyl derivatives: analysis of corn syrup. In: Whistler RL, BeMiller JN (eds) General carbohydrate method. Academic, New York, pp 3–8
Caracuel Z, Casanova C, Roncero MIG et al (2003) pH response transcription factor PacC controls salt stress tolerance and expression of the P-type Na+-ATPase Ena1 in Fusarium oxysporum. Eukaryot Cell 2:1246–1252. doi:10.1128/EC.2.6.1246
Cheng J, Park T, Fischl AS et al (2001) Cell cycle progression and cell polarity require sphingolipid biosynthesis in Aspergillus nidulans. Mol Cell Biol 21:6198–6209. doi:10.1128/MCB.21.18.6198
Cooney DG, Emerson R (1966) Thermophilic fungi. An account of their biology, activities, and classification. Z Allg Mikrobiol 6:137. doi:10.1002/jobm.19660060212
Crowe JH (2007) Trehalose as a “chemical chaperone”. In: Csermely P, Vígh L (eds) Molecular aspects of the stress response: chaperones, membranes and networks. Springer, New York, pp 143–158
Csoboz B, Balogh GE, Kusz E et al (2013) Membrane fluidity matters: hyperthermia from the aspects of lipids and membranes. Int J Hyperth 29:491–499. doi:10.3109/02656736.2013.808765
DasSarma S, DasSarma P (2012) Halophiles. eLS. doi:10.1002/9780470015902.a0000394.pub3
Davis D (2003) Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr Genet 44:1–7. doi:10.1007/s00294-003-0415-2
Denison SH (2000) pH regulation of gene expression in fungi. Fungal Genet Biol 29:61–71. doi:10.1006/fgbi.2000.1188
Duckworth AW, Grant WD, Jones BE, Van Steenbergen R (1996) Phylogenetic diversity of soda lake alkaliphiles. FEMS Microbiol Ecol 19:181–191. doi:10.1016/0168-6496(96)00003-7
Elbein AD, Pan YT, Pastuszak I, Carroll D (2003) New insights on trehalose: a multifunctional molecule. Glycobiology 13:17–27. doi:10.1093/glycob/cwg047
Elíades LA, Cabello MN, Voget CE (2006) Contribution to the study of alkalophilic and alkali-tolerant Ascomycota from Argentina. Darwiniana 44:64–73
Espeso EA, Arst HN (2000) On the mechanism by which alkaline pH prevents expression of an acid-expressed gene. Mol Cell Biol 20:3355–3363
Glatz A, Pilbat A, Németh GL, Vince-kontár K (2016) Involvement of small heat shock proteins, trehalose, and lipids in the thermal stress management in Schizosaccharomyces pombe. Cell Stress Chaperones 21:327–338. doi:10.1007/s12192-015-0662-4
Grum-Grzhimaylo AA (2015) On the biology and evolution of fungi from soda soils. Dissertation, Wageningen University, Wageningen
Grum-Grzhimaylo AA, Georgieva ML (2013) Are alkalitolerant fungi of the Emericellopsis lineage (Bionectriaceae) of marine origin? IMA Fungus 4:213–228. doi:10.5598/imafungus.2013.04.02.07
Grum-Grzhimaylo AA, Debets AJM, van Diepeningen AD et al (2013) Sodiomyces alkalinus, a new holomorphic alkaliphilic ascomycete within the Plectosphaerellaceae. Persoonia Mol Phylogeny Evol Fungi 31:147–158. doi:10.3767/003158513X673080
Grum-Grzhimaylo AA, Georgieva ML, Bondarenko SA et al (2016) On the diversity of fungi from soda soils. Fungal Divers 76:27–74. doi:10.1007/s13225-015-0320-2
Gunde-Cimerman N, Ramos J, Plemenitaš A (2009) Halotolerant and halophilic fungi. Mycol Res 113:1231–1241. doi:10.1016/j.mycres.2009.09.002
HeckerYancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830. doi:10.1242/jeb.01730
Hesse SJA, Ruijter GJG, Dijkema C, Visser J (2002) Intracellular pH homeostasis in the filamentous fungus Aspergillus niger. Eur J Biochem 269:3485–3494. doi:10.1046/j.1432-1033.2002.03042.x
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372
Ianutsevich EA, Danilova OA, Groza NV, Tereshina VM (2016a) Membrane lipids and cytosol carbohydrates in Aspergillus niger under osmotic, oxidative, and cold impact. Microbiology 85:302–310. doi:10.1134/S0026261716030152
Ianutsevich EA, Danilova OA, Groza NV et al (2016b) Heat shock response of thermophilic fungi: membrane lipids and soluble carbohydrates under elevated temperatures. Microbiology. doi:10.1099/mic.0.000279
Iturriaga G, Suárez R, Nova-Franco B (2009) Trehalose metabolism: from osmoprotection to signaling. Int J Mol Sci 10:3793–3810. doi:10.3390/ijms10093793
Jenkins GM (2003) The emerging role for sphingolipids in the eukaryotic heat shock response. Cell Mol Life Sci 60:701–710. doi:10.1007/s00018-003-2239-0
Jennings DH (1984) Polyol metabolism in fungi. Adv Microb Physiol 25:149–193
Jones BE, Grant WD, Duckworth AW, Owenson GG (1998) Microbial diversity of soda lakes. Extremophiles 2:191–200. doi:10.1007/s007920050060
Kates M (1972) Techniques of lipidology: isolation, analysis and identification of lipids. In: Work TS, Work E (eds) Laboratory techniques in biochemistry and molecular biology. North-Holland, Amsterdam, pp 267–610
Kogej T, Stein M, Volkmann M et al (2007) Osmotic adaptation of the halophilic fungus Hortaea werneckii: role of osmolytes and melanization. Microbiology 153:4261–4273. doi:10.1099/mic.0.2007/010751-0
Kooijman EE, Chupin V, de Kruijff B, Burger KNJ (2003) Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic 4:162–174
Landraud P, Chuzeville S, Billon-Grande G et al (2013) Adaptation to pH and role of PacC in the rice blast fungus Magnaporthe oryzae. PLoS One. doi:10.1371/journal.pone.0069236
Levery SB, Momany M, Lindsey R et al (2002) Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett 525:59–64
McMahon HT, Gallop JL (2005) Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438:590–596. doi:10.1038/nature04396
Muruga B, Anyango B (2013) A survey of extremophilic bacteria in Lake Magadi, Kenya. Am J Mol Cell Biol 2:14–26. doi:10.7726/ajmcb.2013.1002
Nichols BW (1963) Separation of the lipids of photosynthetic tissues: improvements in analysis by thin-layer chromatography. Biochim Biophys Acta Spec Sect Lipids Relat Subj 70:417–422. doi:10.1016/0926-6542(63)90060-X
Peñalva MA, Tilburn J, Bignell E, Arst HN (2008) Ambient pH gene regulation in fungi: making connections. Trends Microbiol 16:291–300. doi:10.1016/j.tim.2008.03.006
Perkins DD, Pollard VC (1986) Linear growth rates of strains representing ten Neurospora species. Fungal Genet Newsl 33:41–43. doi:10.1017/CBO9781107415324.004
Péter M, Glatz A, Gudmann P et al (2017) Metabolic crosstalk between membrane and storage lipids facilitates heat stress management in Schizosaccharomyces pombe. PLoS One 12:e0173739. doi:10.1371/journal.pone.0173739
Piper PW (1993) Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 11:339–355
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ravindran C, Naveenan T (2011) Adaptation of marine derived fungus Chaetomium globosum (NIOCC 36) to alkaline stress using antioxidant properties. Process Biochem 46:847–857. doi:10.1016/j.procbio.2010.12.005
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40:253–266. doi:10.1016/j.molcel.2010.10.006
Rollins JA (2003) The Sclerotinia sclerotiorum pac1 gene is required for sclerotial development and virulence. Mol Plant Microbe Interact 16:785–795. doi:10.1094/MPMI.2003.16.9.785
Sanders D, Slayman CL (1982) Control of intracellular pH. Predominant role of oxidative metabolism, not proton transport, in the eukaryotic microorganism Neurospora. J Gen Physiol 80:377–402. doi:10.1085/jgp.80.3.377
Simons K, Sampaio JL (2011) Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol 3:a004697. doi:10.1101/cshperspect.a004697
Sinensky M (1974) Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA 71:522–525
Siskind LJ (2005) Mitochondrial ceramide and the induction of apoptosis. J Bioenerg Biomembr 37:143–153. doi:10.1007/s10863-005-6567-7
Smolyanyuk EV, Bilanenko EN, Tereshina VM et al (2013) Effect of sodium chloride concentration in the medium on the composition of the membrane lipids and carbohydrates in the cytosol of the fungus Fusarium sp. Microbiology 82:600–608. doi:10.1134/S0026261713050111
Somogyi M (1945) Determination of blood sugar. J Biol Chem 160:69–73
Sorokin DY, Berben T, Melton ED et al (2014) Microbial diversity and biogeochemical cycling in soda lakes. Extremophiles 18:791–809. doi:10.1007/s00792-014-0670-9
Steiman R, Ford L, Ducros V et al (2004) First survey of fungi in hypersaline soil and water of Mono Lake area (California). Antonie Van Leeuwenhoek 85:69–83
Tereshina VM, Memorskaya AS, Kotlova ER (2011) The effect of different heat influences on composition of membrane lipids and cytosol carbohydrates in mycelial fungi. Microbiology 80:455–460. doi:10.1134/S0026261711040199
Vigh L, Escribá PV, Sonnleitner A et al (2005) The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res 44:303–344. doi:10.1016/j.plipres.2005.08.001
Yanutsevich EA, Memorskaya AS, Groza NV et al (2014) Heat shock response in the thermophilic fungus Rhizomucor miehei. Microbiology 83:498–504. doi:10.1134/S0026261714050282
Yu RK, Koerner TA, Scarsdale JN, Prestegard JH (1986) Elucidation of glycolipid structure by proton nuclear magnetic resonance spectroscopy. Chem Phys Lipids 42:27–48
Zajc J, Kogej T, Galinski EA et al (2014) Osmoadaptation strategy of the most halophilic fungus, Wallemia ichthyophaga, growing optimally at salinities above 15% NaCl. Appl Environ Microbiol 80:247–256. doi:10.1128/AEM.02702-13
Zavarzin GA, Zhilina TN, Kevbrin VV (1999) The alkaliphilic microbial community and its functional diversity. Microbiology 68:503–521
Zhang T, Sun X, Xu Q et al (2013) The pH signaling transcription factor PacC is required for full virulence in Penicillium digitatum. Appl Microbiol Biotechnol 97:9087–9098. doi:10.1007/s00253-013-5129-x
Zou CG, Tu HH, Liu XY et al (2010) PacC in the nematophagous fungus Clonostachys rosea controls virulence to nematodes. Environ Microbiol 12:1868–1877. doi:10.1111/j.1462-2920.2010.02191.x
Acknowledgements
This work was partially supported by the grant 15-04-06975 of the Russian Foundation for Basic Research, RSF GRANT No. 14-50-00029 (the identification of fungi, Bilanenko). The work on collecting samples of soda soils was supported by the Program of Government jobs, parts 2 no. 01 10 (№ AAAA-A16-116021660088-9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Albers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bondarenko, S.A., Ianutsevich, E.A., Danilova, O.A. et al. Membrane lipids and soluble sugars dynamics of the alkaliphilic fungus Sodiomyces tronii in response to ambient pH. Extremophiles 21, 743–754 (2017). https://doi.org/10.1007/s00792-017-0940-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-017-0940-4