Abstract
The occurrence of cultivable fungi was investigated along the water column (25–2500 m depth) of four off-shore stations in the Mediterranean basin. An unexpected high abundance of fungi, accompanied by a scarce biodiversity, was observed up to 2500 m depth. The black yeast Hortaea werneckii, known to be one of the most salt tolerant eukaryotic organisms, was isolated for the first time from the Mediterranean Sea, and it was the dominant fungus present in seawater in almost all stations and depths, suggesting its ubiquitous distribution. Isolation of cultivable strains allowed their phylogenetic and taxonomic characterization, and demonstrated that almost all the retrieved fungal species should be considered of terrestrial origin, but well adapted to survive and reproduce at temperature and salinity conditions of the Mediterranean seawater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fungal biodiversity in the marine environment has been not entirely explored (Nagano and Nagahama 2012; Richards et al. 2012). Regarding the terminology of “marine fungi”, it has been very much disputed and different interpretations have arisen (Kohlmeyer and Volkmann-Kohlmeyer 2003; Raghukumar 2008; Li and Wang 2009). Recently, Bonugli-Santos et al. (2015) used the term “marine-derived fungi” that includes both groups of fungi. Jones (2011, 2015) estimated that more than 10,000 marine fungi are unknown and suggested that the lack of information is mainly due to the fact that some habitats or marine substrates such as sediments, sand, water, plankton, and deep sea need further investigations. In addition, unculturable marine fungi and cryptic species in all the above habitats are very difficult to identify, increasing the number of unclassified species.
To date, very few studies have been carried out on the occurrence of fungi in the Mediterranean Sea (Cuomo et al. 1988; Garzoli et al. 2012). Hofrichter (2002) associated the presence of approximately 140 fungal saprophytic species to lignin debris in the Mediterranean Basin. Some authors (Alexander et al. 2009; Stock et al. 2012; Richards et al. 2015) explored the fungal biodiversity in deep-sea anoxic hypersaline basins (characterized by extreme conditions in terms of salinity and pressure) in the Mediterranean Sea using metagenomics approaches on DNA and/or RNA samples. In particular, Stock et al. (2012), applying culture-independent methods on Mediterranean hypersaline brines (eastern Mediterranean deep-sea basin Thetis), observed the predominance of fungi within the micro-eukaryotic community.
In this context, this study was aimed at exploring the presence of cultivable fungi, along the off-shore water column (25–2500 m) in the Mediterranean basin. Fungal isolates were also tested under laboratory conditions for their ability to grow at high salt concentrations (up to 10% NaCl) in combination with low temperatures (4 and 10 °C).
Materials and methods
Sampling area
Sampling campaign was carried out in the framework of the DEEP-PRESSURE Cruise on board of the R/V Urania (December 2013) in different water columns of Mediterranean Sea. There, three types of stratified water masses occur: the uppermost layer (0–250 m depth), in which there is the Modified Atlantic Water (MAW), coming from the Atlantic Ocean water; the levantine intermediate water masses (LIW) characterize the mesopelagic layer (250–700 m depth). The mixture of highly oligotrophic LIW with Western mediterranean dense water (WMDW) forms the tyrrhenian deep water (TDW) present in the bathypelagic layer (700–3300 m depth) (Kress et al. 2003; 2014).
A total of 14 water samples (as specified below) and reported in Table 1 were collected along the water column in four stations in the central and south-eastern Mediterranean basin, as follows:
-
(a)
Station vector (DP1) in the Tyrrhenian Sea located in the central Tyrrhenian Sea. It presents three distinct water masses at 25, 500 and 3000 m which have a crucial role in the water circulation in the Mediterranean Sea. As reported by Smedile et al. (2015), the main hydrogeological characteristics of the site are winter stratification, oligotrophic conditions, and presence of homogeneous warm (≥ 13 °C) water masses down to bathyal and abyssal (> 4000 m) depths.
-
(b)
Station KM3 (DP6) in the Ionian Sea, located about 100 km SE off Capo Passero in the open Ionian Sea. It is the place of a submarine deep observatory (NEutrino Marine Observatory; NEMO) for studying mammal migrations (Sciacca et al. 2015).
-
(c)
Stations DP2 and DP5 above the anoxic hypersaline lakes (DHAB) L’Atalante and Medee, respectively, at the depth > 3000 m in the Ionian Sea. The anoxic lakes are characterized by strong phosphorus limitation and very low primary and secondary productivity (Alexander et al. 2009; Bortoluzzi et al. 2011; Yakimov et al. 2013).
Samplings were performed using 12-L Niskin bottles (General Oceanics Inc., Miami, FL, USA), pre-treated with a HCl 10 N solution, and mounted on a rosette system. Coordinates, sampling depths, and depth of Levantine intermediate Water (LIW), where the maximum values of salinity and temperature were observed, are summarized in Table 1 and shown in Fig. 1.
Cultural analyses
Immediately after sampling, 1 L of each sample was divided in three aliquots of 200, 300, and 500 ml, which were individually filtered through sterile 1.2-μm pore-size Millipore membranes (Millipore, S.A. 67120, Molsheim, France) under vacuum (< 50 ml min−1).
Each membrane was placed on the surface of a 60 mm diameter Petri plate-containing the Whickeram agarized medium, YMPGA (3 g of yeast extract, 3 g of malt extract, 5 g of peptone, 10 g of glucose, 20 g of agar in 1000 ml of seawater; 200 mg/l chloramphenicol was added to inhibit the growth of bacteria and pH was adjusted to 5.5) specific for the isolation of marine fungi as reported by Kutty and Philip (2008). The plates were incubated at temperature of 25 °C up to 1 month. Counts were done at regular interval of time (7–15–30 days). Results are expressed as propagules or cells/L.
Identification
Due to the high number of isolates, a preliminary grouping of the strains was carried out on the basis of micro- and macro-morphological characteristics have been carried out. To this purpose, strains were cultivated on different cultural media (Potato Dextrose Agar, PDA, Malt Extract Agar, MEA, Oatmeal Agar, OA, Czapek-Dox CZ, Oxoid) and macro-morphology of the colonies (diameter, color of the front and side back) and micro-morphology of the reproduction structures, hyphal maturation, and conidiogenesis were studied by setting up of cultures on slides on MEA and observed under Light Microscopy (LEICA DMR 100) at intervals of time of 2 days (Ellis 1971; 1976; Domsh et al. 1980; Fassatiovà 1986; de Hoog 2000; Kurtzman et al. 2011). This approach allowed us in most cases to identify the strains at genus level.
One-to-three representative isolates (where possible), showing similar micro- and macro-morphological characteristics, were randomly selected for the sequence analysis of the rDNA internal transcribed spacer (ITS1 and ITS2 and 5.8S rDNA) rDNA that is the primary DNA barcode for the fungal kingdom (Stielow et al. 2015) and the subsequent phylogenetic identification by BLAST search comparison sequences obtained as follows.
Strains are kept in the Mycological Collection (MC) of the Department of Chemical, Biological, Pharmacological and Environmental Sciences at the University of Messina (Italy).
DNA extraction and PCR amplification
Cells were collected by centrifugation of 2 ml culture at 12,000 rev min−1 in an Eppendorf centrifuge (Eppendorf 5417 R). The pellet was washed twice with sterile demineralized water. DNA was extracted following the protocol of Möller et al. (1992) with some modifications: the pellet was dissolved in 0.5 ml of TES buffer (0.1 mM Tris-HCl, pH 5.8; 10 mM EDTA; 2% SDS) containing 400 μl of glass beads (0.45–0.50 mm diam.) and vortexed several times for 1 min. for at least 10 min. For protein degradation, Proteinase K (Promega, Italy) (10 mg/ml) was added at the mixture that was incubated at the temperature of 58 °C for 30 min. Then, the salt concentration was adjusted to 1.4 M with 5 M NaCl 1/10 vol and 10% CTAB (cetyltrimethylammoniumbromide) was added to the mixture and it was incubated at 65 °C for 10 min. DNA precipitation was carried out by adding one vol. SEVAG (chloroform:isoamyl alcohol, 24:1, v/v), and the mixture was incubated for 30 min at 0 °C. After centrifugation for 10 min at 48 °C 12,000 rev min−1, 225 ml 5 M ammonium acetate were added to the supernatant, placed on ice for 30 min, and then centrifuged at 48 °C at 12,000 rev min−1 for 30 min. DNA was precipitated with 2.5 vol of ethanol and 0.3 M sodium acetate, 1 h at 80 °C, then was centrifuged for 20 min at 12,000 rev min−1. The pellet was washed with 70% cold ethanol, dried and dissolved in 50 μl TE (10 mM Tris-HCl, pH 7.4; 1 mM EDTA, pH 8.0).
The genomic DNA was PCR-amplified with the universal primers ITS1/ITS4 which are specific for fungal interspacers ITS1, ITS2, and 5.8S rRNA genes (White et al. 1990) The reaction mixture contained 0.48 μM of each primer, 25 μl of MyTaq™ Mix 2× (Bioline, London, UK), and 2 μl of template in the total reaction volume of 50 μl. PCR was performed with the following thermocycling program: 5 min denaturation at 95 °C, followed by 35 cycles of 1 min at 94 °C, 1 min 55 °C, and 1 min and 30 s at 72 °C, and final extension was run at 72 °C for 10 min in the T-Personal Thermal Cycler (Biometra, Germany). The PCR products were visualized on 1.5% (w/v) agarose gel in TAE buffer (0.04 M Tris-acetate, 0.02 M acetic acid, and 0.001 M EDTA), containing 1 µg/ml−1 of ethidium bromide and visualized under UV lamp.
Sequencing and analysis of ITS
After the verification of the successful amplification on agarose gel electrophoresis, the PCR products were sent to a commercial sequencing facility (Biofab, Rome, Italy) that proceeded to purification and sequencing. BLAST search comparison of rDNA gene sequences present in the NCBI GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and EMBL-EBI databases (http://www.ebi.ac.uk/fasta33/nucleotide.html) were used to obtain the closest species. The phylogenetic tree of aligned sequences was generated using the neighbor-joining reconstruction method and Kimura2-parameter model with 1000 bootstrap replications in MEGA 7 software. All sequences obtained were deposited in the NCBI database under GenBank accession numbers from KX427190 to KX427206 and from KX442762 to KX442764 (Table 2).
Tolerance to low temperature and high salt concentration
Representative strains were inoculated in duplicate in YMPGA agar medium prepared with seawater (3.8% NaCl, v/w) as described in par. 2.2 and with the addition of NaCl up to 10% (w/v). Incubation was carried out at the temperatures of 4, 10, and 25 °C for 1 month. At the end of the incubation time, the diameter of the colonies was measured to evaluate the optimum conditions for growth.
Results and discussion
Fungal colonies were found in all the samples taken from the four stations and at all depths. The number of cultivable fungi was very high in all samples and estimated as more than 500 propagules or cells/L (data not shown). However, it was impossible to count due to the diffuse growth of black small colonies (an example is given in Fig. 2). Kutty and Philip (2008) reported that the low organic deep-sea oceanic regions contain 10 or less cells of fungi/L, with some exceptions in polluted areas. The Mediterranean Sea is considered an oligotrophic sea, and thus, one could expect a similar low number of fungi for deep-sea regions, but our research showed a surprisingly very high number of fungi present in all water columns in the different stations and without significant differences at different depths.
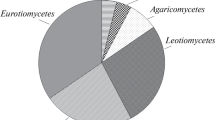
One hundred and ten colonies were randomly isolated from all samples and clustered in 12 morphotypes on the basis of micro- and macro-morphological characteristics. Twenty representative strains (listed in Table 2) were further characterized by molecular and physiological analyses. Based on the phylogenetic analysis (Fig. 3), the isolates mostly belonged to the phylum Ascomycota within the classes Dothideomycetes (species H. werneckii, Cladosporium sphaerospermum, and C. cladosporioides), Eurotiomycetes (species Aspergillus sydowii and Penicillium chrysogenum), Sordariomycetes (species Purpureocillium lilacinum (Luangsa-ard, Houbraken, Hywel-Jones and Samson, comb. Nov., 2011) ex Paecilomyces lilacinus (Thom, Samson 1974), Sarocladium strictum and Corallomycetella repens), and Mitosporic fungi (species Engyodontium album and Engyodontium sp.). Basidiomycota were represented only by the species Rhodotorula mucilaginosa. Fungal isolate classification was mainly based on morphological characteristics and ITS sequence analysis. It could be noted that, although this DNA barcode is discriminant at species level for most of the fungal genera (Stielow et al. 2015), such analysis could be not exhaustive for species belonging to the genera Cladosporium and Aspergillus/Penicillium, even if important indications are achieved. For these species, the morphological analyses were discriminant for genus assignment.
Regarding the distribution and abundance of fungal isolates, H. werneckii and P. lilacinum occurred in all samples until down to 2500 m except for DP6 station (KM3) in which they were found at the maximum depth of 200 m. The frequency of isolation of H. werneckii was very high in almost all samples with percentages of isolation ranging from 40 to 80%, while some exceptions were found in the samples collected from 25 m in DP5 station (10%; Fig. 4c), and from 55 m in DP6 station (5%; Fig. 4d).
In contrast, C. repens (ex Nectria mauritiicola) and S. strictum were isolated only from the station DP5 (Medee) at 25 m and 2500 m, (Fig. 4c), while E. album and Engyodontium sp. were isolated from 2500 m from DP1 and DP6 stations, respectively. In this latter station this genus was isolated at very high percentages (75%) (Fig. 4a–d). Remaining species (i.e., Rh. mucilaginosa, C. cladosporioides, C. sphaerospermum, Acremonium sp., P. chrysogenum, and A. sydowii) were isolated at variable frequency and distribution along the water column and down to 2500 m depth except for Acremonium sp. and Rh. mucilaginosa which have were never been isolated below 200 m depth (Fig. 4). As previously reported by different authors (Bianchi et al. 2012; Coll et al. 2010; Rédou et al. 2015), our results highlighted a lower number of fungal species than in samples near the coast, with a similar distribution along the water column off-shore. All fungal taxa retrieved in this study are typically isolated from terrestrial environments and most of them are saprobes and occasional pathogens for humans, plants or animals. Some species, such as A. sydowii and C. repens, were seldom found in the marine environment associated with corals and/or deep sediments (e.g., E. album, Penicillium, C. sphaerospermum, and Acremonium sp.), and in the deep seawater, such as Rh. mucilaginosa isolated from Pacific Ocean at 10,000 m depth (Nagahama et al. 2001; Li and Wang 2009; Rédou et al. 2015).
The ability to grow at different salt concentrations and temperatures demonstrated that our isolates were able to grow and reproduce at the salt concentration of seawater, and even at higher salt concentrations and lower temperatures (Table 3). At 4 °C the majority of the strains grew only at seawater salt concentration. On the contrary, some fungal strains such as H. werneckii (strains V25c, M94a and AT25f) grew better at 25 °C at NaCl 10% (Table 3).
Due to the high frequency of isolation and distribution in the seawater even at 2500 m depth, the species of H. werneckii, P. lilacinum and Engyodontium sp., should be considered associated with deep-sea off-shore environments. Molecular and physiological studies of comparison with strains from different environmental sources gave a better understanding of their taxonomy and eco-physiological characteristics (Marchetta et al. 2018). H. werneckii, in particular, is the dominant species in almost all the stations and depths and it is interesting to note that this species has never been isolated from the Mediterranean Sea, even if its presence was hypothesized in the brine of an anoxic basin, but it not was demonstrated (Stock et al. 2012). H. werneckii has been previously detected in non-Mediterranean deep-sea environments. Two isolates of H. werneckii were obtained by Le Calvez et al. (2009) from a deep-sea hydrothermal ecosystem in the Pacific Ocean, whereas Singh et al. (2012) reported on the occurrence of such black yeast at 5000 m depth sediment in the Central Indian Basin, as observed by both culture-dependent and culture-independent methods. H. werneckii is a ubiquitous black yeast, isolated from both terrestrial (e.g., food, rock, and sandy beach) and marine environments (Gunde-Cimerman et al. 2000; Zalar et al. 2005; Gunde-Cimerman and Plemenitaš 2006). It is also known as etiological agent of human skin infection “Tinea Nigra” occurring in tropical and subtropical regions of the world and reported as a cause of occasional marine fish disease (Todaro et al. 1983; Bonifaz et al. 2008). It is currently considered as the globally dominant fungal species in high salt environments and hypersaline waters (> 25% w/v NaCl) (Gunde-Cimerman and Zalar 2014). For the above-described reasons and for the yeast-like form and fast growth in the common fungal media, this fungus is used as a model-organism for studying extremo-tolerance mechanisms in salty conditions, and it is also considered as an excellent candidate for the discovery of new molecules (Gunde-Cimerman et al. 2018).
Conclusion
The few studies regarding the occurrence of fungi in deep marine Mediterranean Sea were based only on metagenomics (Alexander et al. 2009; Stock et al. 2012).
The application of modern culture-independent molecular methods to study the microbial diversity in different marine habitats is doubtless useful to give a wider knowledge on fungal biodiversity by discovering a huge number of fungal taxa. On the other hand, in the absence of cultures, the identification at species level is almost impossible due to insufficient sequence information in the databases. Thus, data arisen from culture-dependent methods accomplish not only the taxonomic identification of the strains, but also their deep eco-physiological characterization.
The present research reports the first survey on cultivable fungi present in off-shore Mediterranean seawater and to a depth of 2500 m, also demonstrating the small number of fungal species that are well adapted to survive and reproduce at conditions reported for the Mediterranean basin. H. werneckii was isolated for the first time from the Mediterranean Sea, and it was the dominant fungus present in seawater in almost all stations and depths, suggesting its ubiquitous distribution.
Cultivation methods, further, allowed the phylogenetic and taxonomic characterization of fungal isolates. These strains are under investigation for the production of enzymes and secondary metabolites useful for biotechnological application.
References
Alexander E, Stock A, Breiner HW, Behnke A, Bunge J, Yakimov MM, Stoeck T (2009) Microbial eukaryotes in the hypersaline anoxic L’Atalante deep-sea basin. Environ Microbiol 11:360–381
Bianchi CN, Morri C, Chiantore M, Montefalcone M, Parravicini V, Rovere A (2012) Mediterranean Sea Biodiversity Between the Legacy From the Past and a Future of Change. In: Stambler N (ed) Life in the Mediterranean Sea: A Look at Habitat Changes. Nova Science Publishers, New York, pp 1–55
Bonifaz A, Badali H, de Hoog GS, Cruz M, Araiza J, Cruz MA, Fierro L, Ponce RM (2008) Tinea nigra by Hortaea werneckii, a report of 22 cases from Mexico. Stud Mycol 61:77–82
Bonugli-Santos RC, Vasconcelos MRDS, Passarini MRZ, Vieira GAL, Lopes VCP, Mainardi PH, Santos JAD, Duarte LDA, Otero IVR, Yoshida AMDS, Feitosa VA, Pessoa A, Sette L (2015) Marine-derived fungi: diversity of enzymes and biotechnological applications. Front Microbiol 6:269. https://doi.org/10.3389/fmicb.2015.00269
Bortoluzzi G, Polonia A, Marozzi G, Yakimov MM, Borghini M, Genovese L, Riminucci F, La Cono V, Foraci F (2011) Marine research at CNR. In: CNR-Dipartimento Terra e Ambiente, Roma (ed) The exploration of deep hypersaline anoxic basins of the Eastern Mediterranean Sea, vol 6, pp 95–108
Coll M, Piroddi C, Steenbeek J, Kaschner K et al (2010) The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE 5(8):e11842. https://doi.org/10.1371/journal.pone.0011842
Cuomo V, Jones EBG, Grasso S (1988) Occurrence and distribution of marine fungi along the coast of Mediterranena sea. Prog Oceanogr 21:189–200
de Hoog GS (2000) Atlas of Clinical Fungi, 2nd edn. American Society for Microbiology, New York
Domsh KH, Gams W, Anderson TH (1980) Compendium of soil fungi 1. Academic Press, London
Ellis MB (1971) Dematiaceous hyphomycetes. C.A.B. International Mycological Institute, Kew, Surrey
Ellis MB (1976) More Dematiaceous hyphomycetes. C.A.B. International Mycological Institute, Kew, Surrey
Fassatiovà O (1986) Moulds and filamentous fungi in technical microbiology. In: Bushel ME (ed) Progress in industrial microbiology 22. Elsevier, Amsterdam
Garzoli L, Tosi S, Picco AM (2012) Marine fungi: a preliminary screening to detect new promising strains for biotechnological applications. Environ Eng Manag J 11(3):S147
Gunde-Cimerman N, Plemenitaš A (2006) Ecology and molecular adaptations of the halophilic black yeast Hortaea werneckii. Rev Environ Sci Biotechnol 5:323–331
Gunde-Cimerman N, Zalar P (2014) Extremely halotolerant and halophilic fungi inhabit brine in solar salterns around the globe. Food Technol Biotech (FTB) 52:170–179
Gunde-Cimerman N, Zalar P, de Hoog S, Plemenitaš A (2000) Hypersaline waters in salterns—Natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol 32:235–240
Gunde-Cimerman N, Plemenitaš A, Oren A (2018) Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol Rev 42(3):353–375
Hofrichter RC (2002) El Mar Mediterraneo. Fauna, Flora, Ecologıa. II/1. Guıda Sistematica y de Identificacion. Ediciones Omega, Barcelona
Jones EBG (2011) Are there more marine fungi to be described? Bot Mar 54:391–402
Jones EBG, Suetrong S, Sakayaroj J, Bahkali AH, Abdel-Wahab MA, Boekhout T, Pang KL (2015) Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers 73:1–72
Kohlmeyer J, Volkmann-Kohlmeyer B (2003) Marine Ascomycetes from algae and animal hosts. Bot Mar 46:285–306
Kress N, Manca BB, Klein B, Deponte D (2003) Continuing influence of the changed thermohaline circulation in the eastern Mediterranean on the distribution of dissolved oxygen and nutrients: physical and chemical characterization of the water masses. J Geophys Res 108(C9):8109. https://doi.org/10.1029/2002JC001397
Kress N, Gertman I, Herut B (2014) Temporal evolution of physical and chemical characteristics of the water column in the Easternmost Levantine basin (Eastern Mediterranean Sea) from 2002 to 2010. J Marine Syst 135:6–13
Kurtzman C, Fell JW, Boekhout T (2011) The Yeasts: A Taxonomic study, vol 1, 5th edn. Elsevier, Amsterdam
Kutty SN, Philip R (2008) Marine yeasts—a review. Yeast 25:465–483
Le Calvez T, Burgaud G, Mahe S, Barbier G, Vandenkoornhuyse P (2009) Fungal diversity in deep-sea hydrothermal ecosystems. Appl Environ Microb 75:6415–6421
Li Q, Wang G (2009) Diversity of fungal isolates from three Hawaiian marine sponges. Microbiol Res 164:233–241
Marchetta A, Gerrits van den Ende B, Al-Hatmi AMS, Hagen F, Zalar P, Sudhadham M, Gunde-Cimerman N, Urzì C, de Hoog S, De Leo F (2018) Global molecular diversity of the halotolerant fungus Hortaea werneckii. Life 8(3):31. https://doi.org/10.3390/life8030031
Möller EM, Bahnweg G, Sandermann H, Geiger H (1992) A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res 20:6115–6116
Nagahama T, Hamamoto M, Nakase T, Takami H, Horikoshi K (2001) Distribution and identification of red yeasts in deep-sea environments around the northwest Pacific Ocean. A Van Leeuw J Microb 80:101–110
Nagano Y, Nagahama T (2012) Fungal diversity in deep-sea extreme environments. Fungal Ecol 5:463–471
Raghukumar C (2008) Marine fungal biotechnology: an ecological perspective. Fungal Divers 31:19–35
Rédou V, Navarri M, Meslet-Cladière L, Barbier G, Burgaud G (2015) Species richness and adaptation of marine fungi from deep-subseafloor sediments. Appl Environ Microb 81:3571–3583
Richards TA, Jones MDM, Leonard G, Bass D (2012) Marine Fungi: their Ecology and Molecular Diversity. Annu Rev Mar Sci 4:495–522
Richards TA, Leonard G, Mahé F, del Campo J, Romac S, Jones MDM, Maguire F, Dunthorn M, De Vargas C, Massana M, Chambouvet A (2015) Molecular diversity and distribution of marine fungi across 130 European environmental samples. Proc R Soc B 282:20152243. https://doi.org/10.1098/rspb.2015.2243
Sciacca V, Caruso F, Beranzoli L, Chierici F, De Domenico E, Embriaco D, Favali P, Giovanetti G, Larosa G, Marinaro G, Papale E, Pavan G, Pellegrino C, Pulvirenti S, Simeone F, Viola S, Riccobene G (2015) Annual acoustic presence of fin whale (Balaenoptera physalus) offshore Eastern Sicily, central Mediterranean Sea. PLoS ONE. https://doi.org/10.1371/journal.pone.0141838
Singh P, Raghukumar C, Meena RM, Verma P, Shouche Y (2012) Fungal diversity in deep-sea sediments revealed by culture-dependent and culture-independent approaches. Fungal Ecol 5:543–553
Smedile F, Scarfi S, De Domenico E, Garel MH, Gentile G, La Cono V, Tamburini C, Giuliano L, Yakimov MM (2015) Variations in microbial community structure through the stratified water column in the Tyrrhenian Sea (Central Mediterranean). J Mar Sci Eng 3:845–865. https://doi.org/10.3390/jmse3030845
Stielow JB, Lavèsque CA, Seifert KA, Meyer W et al (2015) One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35:242–263
Stock A, Breiner HW, Pachiadaki M, Edgcomb V, Filker S, La Cono V, Yakimov MM, Stoeck T (2012) Microbial eukaryote life in the new hypersaline deep-sea basin Thetis. Extremophiles 16:21–34
Todaro F, Berdan A, Cavaliere A, Criseo G, Pernice L (1983) Gasophthalmus in black sea bream (Spondyliosoma cantharus) caused by Sarcinomyces crustaceus Lindner. Mycopathologia 81:95–97
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White T (eds) PCR protocols—A guide to methods and application. Innis Academic Press Inc, New York
Yakimov MM, La Cono V, Slepak VZ, La Spada G, Arcadi E, Messina E, Borghini M, Monticelli LS, Rojo D, Barbas C, Golyshina OV, Ferrer M, Golyshin PN, Giuliano L (2013) Microbial life in the Lake Medee, the largest deep-sea salt-saturated formation. Sci Rep UK 3:3554. https://doi.org/10.1038/srep03554
Zalar P, Kocuvan MA, Plemenitaš A, Gunde-Cimerman N (2005) Halophilic black yeasts colonize wood immersed in hypersaline water. Bot Mar 48:323–326
Acknowledgements
The Cruise and IAMC-CNR-related research were supported by research funds from the Italian Ministry of University and Research (MIUR) under RITMARE Flagship Project (2012–2016). The authors are grateful to M. M. Yakimov, V. La Cono, and M. Borghini for the seawater samples. We thank Mrs. Sherron Collins for her revision of English text.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
Dedicated to Luigi Michaud (Messina, Italy 5 October 1974—Antarctica 17 January 2014) whose enthusiasm, professional skills, and friendship will be always in our hearts.
Rights and permissions
About this article
Cite this article
De Leo, F., Lo Giudice, A., Alaimo, C. et al. Occurrence of the black yeast Hortaea werneckii in the Mediterranean Sea. Extremophiles 23, 9–17 (2019). https://doi.org/10.1007/s00792-018-1056-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-018-1056-1