Abstract
Protein disulfide oxidoreductases are redox enzymes that catalyze thiol–disulfide exchange reactions. These enzymes include thioredoxins, glutaredoxins, protein disulfide isomerases, disulfide bond formation A (DsbA) proteins, and Pyrococcus furiosus protein disulfide oxidoreductase (PfPDO) homologues. In the genome of a hyperthermophilic archaeon, Thermococcus onnurineus NA1, the genes encoding one PfPDO homologue (TON_0319, Pdo) and three more thioredoxin- or glutaredoxin-like proteins (TON_0470, TON_0472, TON_0834) were identified. All except TON_0470 were recombinantly expressed and purified. Three purified proteins were reduced by a thioredoxin reductase (TrxR), indicating that each protein can form redox complex with TrxR. SurR, a transcription factor involved in the sulfur response, was tested for a protein target of a TrxR-redoxin system and only Pdo was identified to be capable of catalyzing the reduction of SurR. Electromobility shift assay demonstrated that SurR reduced by the TrxR-Pdo system could bind to the DNA probe with the SurR-binding motif, GTTttgAAC. In this study, we present the TrxR-Pdo couple as a redox-regulator for SurR in T. onnurineus NA1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disulfide bonds in proteins usually function in stabilizing protein structures and also in various redox reactions as part of a catalytic or regulatory cycle (Betz 1993). The formation and reduction of disulfide bonds is catalyzed by specialized thiol–disulfide exchanging enzymes, i.e., protein disulfide oxidoreductases. The enzymes include the families of thioredoxin, glutaredoxin, protein disulfide isomerase, and disulfide bond formation A (DsbA) proteins and contain a sequence motif of CXXC (where C = cysteine, X = any amino acid) at their active sites (Kadokura et al. 2003).
Thioredoxins as the major ubiquitous protein disulfide oxidoreductase are generally responsible for maintaining proteins in their reduced state and receive electrons from NADPH through thioredoxin reductase (Holmgren 1985). Glutaredoxins exist in all prokaryotic and eukaryotic cells that contain glutathione and catalyze the reduction of intracellular disulfides in a coupled system with NADPH, glutathione, and glutathione reductase and keeps the inside of the cell reduced (Vlamis-Gardikas and Holmgren 2002). Thioredoxins and glutaredoxins generally have a molecular mass of 9–12 kDa and contain conserved active-site sequence of CGPC and CPYC, respectively. They are multifunctional playing an important role in maintenance of intracellular redox homeostasis and of the thiol/disulfide state of proteins for their structure and functions, and in regulation of vital cellular processes, such as regulation of cell cycle, inhibition of apoptosis, and control of transcription factor activities (Kalinina et al. 2008).
In the eukaryotic endoplasmic reticulum, protein disulfide isomerases catalyze the formation or rearrangement of disulfide bridges in the protein-folding process, thus playing physiological and pathophysiological roles including hemostasis, facilitation of pathogen entry, and reactive nitrogen and oxygen signaling (Marcus et al. 1996; Ali Khan and Mutus 2014). In bacteria, disulfide bonds are introduced in the periplasm by the Dsb proteins (Nakamoto and Bardwell 2004; Ito and Inaba 2008). DsbA with a thioredoxin domain is the primary disulfide bond donor in the periplasmic space and is reoxidized by the inner membrane protein DsbB. DsbC corrects incorrectly formed disulfide bonds and is regenerated as an active enzyme by the membrane protein DsbD. The system utilizes the oxidizing and the reducing equivalents of quinone and NADPH, respectively. Unlike thioredoxin, glutaredoxin, and DsbA, which possess only one thioredoxin-fold motif, protein disulfide isomerase contains two thioredoxin-like domains.
In contrast to the wealth of information on protein disulfide oxidoreductases of bacteria and eukaryotes, our knowledge concerning archaeal enzymes is very limited. Intriguingly, the small (12 kDa) redox protein of Methanothermobacter thermautotrophicus have the active site motif CPYC, which is conserved in all glutaredoxins, but can catalyze the reduction of insulin disulfides, although it did not react with either thioredoxin reductase or glutathione (McFarlan et al. 1992). The thioredoxin homologues of Methanocaldococcus jannaschii and an aerobic archaeon Aeropyrum pernix K1 were capable of reducing insulin and were reduced by thioredoxin reductases, but have unusual sequence CPHC, which is the same as that of Escherichia coli DsbA (Lee et al. 2000; Jeon and Ishikawa 2002). In Methanosarcina acetivorans, seven thioredoxin homologues with various active site sequences were identified, and a protein, which is encoded adjacent to a thioredoxin reductase gene, catalyzed the reduction of insulin disulfides together with thioredoxin reductase (McCarver and Lessner 2014).
Specific protein disulfide oxidoreductases have been recognized as a potential key player in intracellular disulfide-shuffling in archaea such as Pyrococcus furiosus, Pyrococcus horikoshii, and Sulfolobus solfataricus (Ladenstein and Ren 2006; Pedone et al. 2004, 2006; Kashima and Ishikawa 2003). These proteins display the unique features of having molecular masses of 25–27 kDa and two thioredoxin-folds with a distinct CXXC active site motif each. They resemble protein disulfide isomerases in having two thioredoxin-like motifs, but domain architectures are fairly different (Ren et al. 1998). The functional studies have revealed their multifunctional features. They exhibited not only isomerase activity, but also foldase/chaperone activity, which are related to protein disulfide isomerase (Pedone et al. 2004, 2006). They can utilize glutathione, glutathione reductase, and NADH to reduce disulfide substrates and thus showed thioltransferase activity, which is typical for glutaredoxin (Guagliardi et al. 1995). However, there is no report on the existence of glutathione or glutathione reductase in archaea. Above all, the fact that they are the substrate of thioredoxin reductase strongly suggests that they are involved in a thioredoxin-like system (Pedone et al. 2006; Kashima and Ishikawa 2003). The unusual structural features of these proteins suggest that they are new members of the protein disulfide oxidoreductase superfamily and are classified as a separate P. furiosus protein disulfide oxidoreductase (PfPDO)-like family.
Little is known about physiological roles of archaeal protein disulfide oxidoreductases. Proteomic analyses in a methanogen, M. jannaschii, revealed that a thioredoxin homologue targets 152 polypeptides which participate in fundamental processes such as methanogenesis, biosynthesis, transcription, translation, and oxidative response. Among them, two enzymes, an F420-dependent sulfite reductase and an F420-dependent methylenetetrahydromethanopterin dehydrogenase, were confirmed as thioredoxin targets by in vitro activation assay (Susanti et al. 2014). It has been reported that in S. solfataricus, protein disulfide oxidoreductase (SsPDO) was involved in a cell defense mechanism against reactive oxygen species (ROS) accumulation by connecting thioredoxin reductase system with peroxiredoxins, bacterioferritin comigratory protein (Bcp)1, Bcp3, and Bcp4 (Limauro et al. 2008, 2009, 2010, 2014). Our understanding of members of the PfPDO-like family has been limited to its biochemical and structural properties (Ladenstein and Ren 2006). Recently, one of PfPDO homologues, named as a Pdo, has exhibited that it works together with thioredoxin reductase to catalyze the reduction of cystine to cysteine, which then was utilized for reduction of extracellular dimethyl sulfoxide (DMSO) probably to dispose excess reducing power in a hyperthermophilic archaeon, Thermococcus onnurineus NA1 (Choi et al. 2016).
In this study, we identified a new protein target for Pdo in T. onnurineus NA1. The gene encoding Pdo is known as a regulon of the target protein and thus, we can establish a feedback loop of regulation between them.
Materials and methods
Strain, media, and culture conditions
T. onnurineus NA1 (KCTC 10859) was previously isolated from a deep-sea hydrothermal vent area in the Papua New Guinea–Australia–Canada–Manus field and YPS (yeast extract–peptone–sulfur) medium was used to culture the strain as previously reported (Bae et al. 2006). T. onnurineus NA1 cells were serially cultured in a 20-ml serum bottle and 1-liter anaerobic jar, the working volumes of which were 20 and 700 ml, respectively, at 80 °C for 20 h. E. coli DH5α was used for plasmid propagation and for nucleotide sequencing. E. coli Rosetta(DE3)pLysS (Stratagene, La Jolla, USA) were used for gene expression. E. coli strains were cultivated in Luria–Bertani (LB) medium at 37 °C. Kanamycin and chloramphenicol were added to the medium at a final concentration of 50 and 12.5 µg/ml, respectively.
In silico analysis
A homology search of deduced amino acid sequences of genes encoding Trx- or Grx-like proteins, TON_0319 (GenBank accession no. ACJ15804), TON_0470 (ACJ15956), TON_0472 (ACJ15958), and TON_0834 (ACJ16322), was performed using the Basic Local Alignment Search Tool (BLAST) program against the non-redundant protein database from the National Center for Biotechnology Information (NCBI, Bethesda, USA). Nucleotide sequence alignment was conducted using the LALIGN program on web server (http://www.ebi.ac.uk/Tools/psa/lalign/). Multiple sequence alignment for proteins was performed using the ClustalW program (Thompson et al. 1994) or Clustal Omega program on web server (http://www.ebi.ac.uk/Tools/msa/clustalo/). The phylogenetic tree was constructed using Molecular Evolutionary Genetics Analysis (MEGA 7) software (Kumar et al. 2016) via the neighbor-joining algorithm with a bootstrap support value (500 replicates).
Cloning, gene expression, and protein purification
Genes encoding Trx- or Grx-like proteins (TON_0470, TON_0472, and TON_0834) and SurR (TON_0318, GenBank accession no. ACJ15803) were amplified by PCR using genomic DNA isolated using a standard procedure (Ramakrishnan and Adams 1995). All the PCR primers used in this study are listed in Table S1. Cloning and expression of those genes were performed as described previously with some modifications (Choi et al. 2016). Briefly, the amplified DNA fragments were used to clone into the pET-28a(+) vector (Novagen, Madison, USA) via the one-step sequence- and ligation-independent cloning (SLIC) method (Jeong et al. 2012). After confirmation of the correct sequences, the resulting constructs were transformed into E. coli Rosetta(DE3)pLysS (Stratagene, La Jolla, USA). E. coli transformants were grown at 37 °C in LB medium containing 12.5 µg/ml of chloramphenicol and 50 µg/ml of kanamycin to an OD600nm of 0.6. Overexpression was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG) and further incubation at 37 °C for 3 h. Cells were harvested by centrifugation at 6000×g for 20 min and resuspended in 50 mM Tris–HCl (pH 8.5) containing 100 mM NaCl, 19 mM KCl, 10% glycerol, and a protease inhibitor cocktail (cOmplete ULTRA Tablet; Roche Diagnostics, Mannheim, Germany). In the case of TON_0834 protein, the protease inhibitor cocktail was excluded. The cells were disrupted by sonication and centrifuged at 25,000×g for 50 min. The proteins purified using a column containing TALON metal affinity resin (Clontech, Mountain View, USA) were examined via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using standard procedures (Laemmli 1970).
Two proteins, TrxR (GenBank accession no. ACJ17093) and Pdo (ACJ15804), had been purified in the previous study (Choi et al. 2016) and were used in this study.
Enzyme assay
Sulfhydryl groups of the proteins were oxidized by 200 µM diamide to form disulfide bonds. 50 nM TrxR was incubated with 1–2 µM Trx- or Grx-like proteins in 50 mM potassium phosphate buffer (pH 7.6) containing 2 mM EDTA and 100 µM NADH in the presence or absence of 4 µM SurR. The reaction was carried out at 70 °C for 15 min in a final volume of 100 µl. Sulfhydryl groups were visualized as their fluorescent monobromobimane (mBBr, Sigma-Aldrich, St. Louis, USA) derivatives. Aurothiomalate (Sigma-Aldrich, St. Louis, USA) was added up to 0.5 µM concentration to inhibit the reducing activity of TrxR. After incubation, 100 µM mBBr was added and the reaction was continued for another 15 min. mBBr-labeled protein samples were separated in 15% polyacrylamide gels. After electrophoresis, fluorescent images of protein spots were captured with a ChemiDOC MP Imaging System (Bio-Rad, Hercules, USA). Gels were then stained with Coomassie brilliant blue for visualization of all separated proteins.
Electromobility shift assay (EMSA)
The 95-base pair (bp) mbh promoter probe (genome coordinates 1,452,063–1,452,157) with the SurR binding motif, GTTttgAAC, was PCR amplified from T. onnurineus NA1 genomic DNA using primers (Table S1). EMSA reactions were carried out in 50 mM potassium phosphate buffer (pH 7.6) containing 2 mM EDTA. 30 nM DNA probe was added to the SurR and then incubated for 10 min at 70 °C. The EMSA reaction samples were treated with 20 µl of 50% glycerol prior to load onto 5% native polyacrylamide gel. The resulting gel was stained with ethidium bromide (EtBr) and analyzed to visualize the presence of proteins in the shifted bands.
Results
Trx- or Grx-like proteins in the T. onnurineus NA1 genome
SurR was identified as a transcription factor involved in the response of a hyperthermophilic archaeon P. furiosus to sulfur (S0) (Lipscomb et al. 2009). The activity of SurR was demonstrated to be modulated by the redox status of cysteine residues in a CXXC motif that constitutes a redox switch, in which the active form contains reduced thiols and the inactive form contains an intramolecular disulfide bond (Yang et al. 2010). Oxidation of the switch with S0 inhibits sequence-specific DNA binding by SurR, leading to deactivation of genes related to H2 production and derepression of genes involved in S0 metabolism. The pdo was also identified as a member of the SurR regulon (Lipscomb et al. 2009). The pdo was upregulated during the primary S0 response as shown in microarray expression profiling experiments (Schut et al. 2007) and was repressed by SurR which binds pdo promoter DNA in the absence of S0 (Lipscomb et al. 2009). It has been thought that SurR would be converted back to its reduced active state once oxidizing S0 species are depleted inside the cell, but the mechanism has not been demonstrated (Yang et al. 2010).
It is generally admitted that the thiol/disulfide redox balance is controlled by the glutathione and thioredoxin (Trx) pathways. Among thiol-based redox sensors that specifically sense ROS via thiol-based mechanisms, several have been shown to be redox regulated by Trx or glutaredoxin (Grx), with either their activation or inactivation dependent on Trx (or Grx)-catalyzed reduction (Hillion and Antelmann 2015). Therefore, we attempted to search for a Trx or Grx capable of catalyzing the reduction of SurR in T. onnurineus NA1, where surR gene product was identified to have considerable amino acid sequence homology (62% identity) with the SurR protein of P. furiosus.
In addition to the pdo, three other genes encoding Trx- or Grx-like proteins were detected in the T. onnurineus NA1 genome. These three genes, TON_0470, TON_0472, and TON_0834, were annotated as encoding Grx-related protein, Grx/Trx-like protein, and thiol reductase Trx, respectively. Each one contains an active site CXXC-motif, but in comparison with the conventional Trx (CGPC) or Grx (CPYC) active site motifs, only TON_0472 has the archetypical motif, CPYC (Fig. S2). TON_0834 has an unusual CPPC motif, distinct from any of the other known Trxs and Grxs. The CPPC motif was previously found in Staphylococcus aureus NrdH redoxin, which has been identified as a Grx-like protein with a Trx-like activity profile (Jordan et al. 1997; Rabinovitch et al. 2010). The motif CPHC detected in TON_0470 is identical to the ones identified in periplasmic protein disulfide oxidoreductases from Escherichia coli (DsbA), Vibrio cholera (TcpG), and Haemophilus influenza (Por) (Bardwell et al. 1991; Kamitani et al. 1992; Peek and Taylor 1992; Tomb 1992). Interestingly, the CPHC motif was also found in M. jannaschii MjTrx with a Grx-like fold and Trx-like activities, and in A. pernix ApTrx with biochemical activities similar to classical Trx (Lee et al. 2000; Jeon and Ishikawa 2002). In addition to the distinctive active site motif, multiple sequence alignment revealed remarkable sequence difference between these four proteins. They exhibited very low level of sequence identities between them, with a range from 12 to 20% only. We collected all the sequences of their homologues from the Thermococcales genomes available in the public databases and performed phylogenetic analysis using Molecular Evolutionary Genetics Analysis (MEGA 7) software (Kumar et al. 2016). We found that the homologues of each gene clustered tightly together into separate groups (Fig. S1). The pdo and TON_0470 genes are very highly conserved across all 24 the Thermococcales species, while others are relatively less conserved (Table S2). The homologue of TON_0472 and TON_0834 was missing in several Thermococcus and Pyrococcus species. While the pdo and TON_0472 genes exhibited high level of amino acid identity (70–94%) to their homologues, intriguingly, TON_0470 and TON_0834 genes showed relatively low level of identity (40–63%) to their homologues, with exceptions of two homologues in Thermococcus paralvinellae (87% identity) and Palaeococcus pacificus (80% identity).
Pdo as a SurR-reducing factor
To specify the system involved in the SurR disulfide switch, all four genes were cloned and expression was attempted in E. coli. Three genes, pdo, TON_0472, and TON_0834, were highly expressed and gene products were accumulated after induction, but TON_0470 gene product (23 kDa) was not detected under all tested conditions due to growth impairment. Two recombinant proteins, Pdo (26 kDa) and TON_0834 (12 kDa), were purified to homogeneity by affinity chromatography via N-terminal His-tag whereas TON_0472 proteins (13 kDa) were partially degraded and degraded products of different sizes were observed (Fig. S3). Heat treatment (15 min at 70 °C) or addition of a protease inhibitor cocktail could not prevent further degradation of the proteins during experiments.
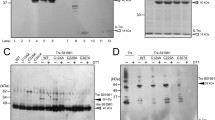
First of all, the ability of the Trx- or Grx-like proteins to serve as a substrate of thioredoxin reductase (TrxR) was examined in the presence of an electron donor NADH. Assay was performed by measuring the fluorescence of proteins labeled with monobromobimane (mBBr), a fluorescent thiol-specific probe, when they are reduced (Crawford et al. 1989). All three purified Trx- or Grx-like proteins could be reduced when incubated with TrxR (36 kDa) and NADH (Fig. 1). This result reveals that each Trx- or Grx-like protein could form a complete redoxin redox system with TrxR as a redox partner. Then, the capability of TrxR-dependent redoxin system was tested for the SurR reduction (Fig. 2). SurR was oxidized by diamide, a thiol-specific oxidant, before the addition of TrxR-redoxin system, so it was not labeled by mBBr and thus not fluorescent. When TrxR and each Trx- or Grx-like protein was added to SurR, fluorescent SurR bands (27 kDa) were detected, but not in the absence of any Trx- or Grx-like protein, indicating the flow of electrons from NADH to SurR by TrxR-redoxin catalysis process (Fig. 2). However, the fluorescence intensity of SurR reduced by Trx- or Grx-like proteins was the highest with Pdo than TON_0834 or TON_0472. When the SurR reduction was measured as a function of the Pdo concentration, the fluorescence intensity of mBBr-labeled SurR increased proportionally as the Pdo concentration increased (Fig. S4). The addition of aurothiomalate, a TrxR-specific inhibitor (Smith et al. 2001), led to the gradual decrease of the fluorescence intensity of the Pdo and SurR as the inhibitor concentration increased (Fig. 3). These results demonstrate that Pdo mediated the electron transfer between TrxR and SurR.
Reduction of Trx- or Grx-like proteins by TrxR. Disulfide bonds of 2 µM Pdo, TON_0834, or TON_0472 were reduced by incubating with 50 nM TrxR in the presence of 100 µM NADH. All proteins (oxidized and reduced) were detected by staining with Coomassie brilliant blue as a loading control (upper panel) and mBBr-labeled proteins were detected by fluorescence illumination (lower panel) on 15% SDS-PAGE gels. M Molecular mass marker, − No addition of TrxR, + addition of TrxR
Reduction of SurR by Trx- or Grx-like proteins. Disulfide bonds of 4 µM SurR were reduced by incubating with 50 nM TrxR and 1 µM Pdo, TON_0834, or TON_0472 in the presence of 100 µM NADH. All proteins (oxidized and reduced) were detected by staining with Coomassie brilliant blue as a loading control (upper panel) and mBBr-labeled proteins were detected by fluorescence illumination (lower panel) on 15% SDS-PAGE gels. M Molecular mass marker, Control Only SurR oxidized by diamide, − No addition of Trx- or Grx-like protein
Effect of TrxR-specific inhibitor, aurothiomalate, on the reduction of Pdo and SurR. Reduction of disulfide bonds of 2 µM Pdo (a) or 2 µM Pdo and 4 µM SurR (b) by 50 nM TrxR in the presence of 100 µΜ NADH was detected with increased concentration of aurothiomalate. All proteins (oxidized and reduced) were detected by staining with Coomassie brilliant blue as a loading control (each upper panel) and mBBr-labeled proteins were detected by fluorescence illumination (each lower panel) on 15% SDS-PAGE gels
Binding of SurR, reduced by TrxR-Pdo system, to DNA
It has been reported that aerobically purified SurR was present approximately 80% in the reduced state, so it was not necessary to reduce the protein to show DNA binding in electromobility shift assays (EMSAs) and fluorescence DNase I footprinting experiments (Lipscomb et al. 2009). However, once SurR was oxidized by S0, excess reductant DTT, but not cysteine, sodium dithionite, and sodium sulfide, could almost completely reverse the effect of oxidation by S0 by demonstrating that the protein was capable of DNA binding (Yang et al. 2010). We performed an EMSA to determine whether SurR can bind to DNA after its oxidized form is treated with TrxR-Pdo system. We identified a palindromic DNA sequence, GTTttgAAC, in the promoter region (−30 to −22 relative to the translation start site) of the open reading frame, mbh1 (TON_1582) (Fig. S5). The sequence was quite similar to the SurR DNA binding motif GTTn3AAC, which has been determined by footprinting experiment in P. furiosus (Lipscomb et al. 2009). Using the DNA probe covering this motif, we could detect SurR–DNA complex formation (Fig. 4a). SurR bound to the DNA probe in a concentration dependent manner so that DNA probe was completely shifted at 0.5 µM SurR while there was no shift with diamide-oxidized SurR. For DNA binding of SurR, DTT could be replaced by NADH and TrxR only in the absolute presence of Pdo (Fig. 4b). In the presence of a TrxR inhibitor, no shift of DNA probe was observed with SurR reacted with TrxR and Pdo. This result is consistent with the inhibition of SurR reduction by a TrxR inhibitor observed in an mBBr-labeling experiment (Fig. 3).
Binding of SurR reduced by TrxR and Pdo to DNA probe. a EMSA showing SurR–DNA complex formation with increased concentration of SurR, which was reduced (upper panel) or not (lower panel) by 50 nM TrxR and 2 µM Pdo in the presence of 100 µM NADH. b DNA binding of 0.5 µM SurR to 30 nM DNA probe, depending on the redox status of SurR under various conditions, such as the presence of the reducing agent, dithiothreitol (DTT) (100 µM) or the TrxR-specific inhibitor, aurothiomalate (ATM) (1 µM)
Discussion
Our results showed that TrxR-Pdo couple acted as a redox system to reduce the transcription factor SurR. SurR reduction by TrxR-Pdo redox system was only demonstrated in vitro, but the data have a special meaning that it can reverse the S0-response regulation by SurR in the absence of S0. For the in vivo testing to assess the indispensability of TrxR-Pdo system in SurR reduction, we tried to make TrxR or Pdo deletion mutants, but failed. It is worth noting that pdo is a member of the SurR regulon and plays a role in reducing SurR as well. The pdo expression is repressed by reduced form of SurR in the absence of S0. The pdo expression is derepressed when SurR is oxidized by S0 and then the expressed Pdo reduces SurR, thereby establishing a feedback loop of regulation. In this regard, Pdo may play a similar role to the Trx or Grx proteins which have been known to deactivate yeast Yap1 or E. coli OxyR. It has been shown that Yap1 and OxyR are activated through the formation of a disulfide bond and are deactivated by enzymatic reduction with Trx1/Trx2 and Grx1 and the genes encoding TRX2 and Grx1 are transcriptionally regulated by Yap1 and OxyR, respectively, demonstrating the autoregulatory feedback control (Kuge and Jones 1994; Zheng et al. 1998; Delaunay et al. 2000; Carmel-Harel et al. 2001; Toledano et al. 2004). In addition to pdo, surR was also found to be conserved in all investigated genomes of Thermococcales species (data not shown), reflecting their common sulfur-metabolizing activity to use S0 as an electron acceptor. It should be noticed that the genomic context for surR is identical in all known Thermnococcales genomes, i.e., surR and pdo are arranged adjacent to each other in the divergent organization. The genetic organization implicates that their action has to be tightly coordinated in vivo.
We previously reported that cystine is the substrate for TrxR-Pdo redox couple in T. onnurineus NA1 (Choi et al. 2016), but other Trx- or Grx-like proteins were not tested for cystine-reducing activity. Thus, it cannot be said that cystine is a specific substrate for Pdo. In this study, we present a protein substrate for the TrxR-Pdo couple in T. onnurineus NA1. At this moment, we cannot provide a conclusive answer to the possibility that TON_0470 and TON_0472 gene products can reduce SurR due to failure in expressing recombinant gene and obtaining stable proteins, respectively. More efforts will be required to conclude this issue. The redox regulation of SurR by the thioredoxin system might influence on the H2 and sulfur metabolism, which is involved with energy conservation. This finding is important in that the phenomenon might not seem to be restricted to T. onnurineus NA1, but extends to other members belonging to the order Thermococcales. An in vivo demonstration of the redox regulatory network by the TrxR-Pdo couple awaits further studies.
Abbreviations
- Trx:
-
Thioredoxin
- Grx:
-
Glutaredoxin
- TrxR:
-
Thioredoxin reductase
- SurR:
-
Sulfur-response regulator
- mBBr:
-
Monobromobimane
- ATM:
-
Aurothiomalate
References
Ali Khan H, Mutus B (2014) Protein disulfide isomerase a multifunctional protein with multiple physiological roles. Front Chem 2:70
Bae SS, Kim YJ, Yang SH et al (2006) Thermococcus onnurineus sp, nov., a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent area at the PACMANUS field. J Microbiol Biotechnol 16:1826–1831
Bardwell JC, McGovern K, Beckwith J (1991) Identification of a protein required for disulfide bond formation in vivo. Cell 67:581–589
Betz SF (1993) Disulfide bonds and the stability of globular proteins. Protein Sci 2:1551–1558. doi:10.1002/pro.5560021002
Carmel-Harel O, Stearman R, Gasch AP et al (2001) Role of thioredoxin reductase in the Yap1p-dependent response to oxidative stress in Saccharomyces cerevisiae. Mol Microbiol 39:595–605
Choi AR, Kim M-S, Kang SG, Lee HS (2016) Dimethyl sulfoxide reduction by a hyperhermophilic archaeon Thermococcus onnurineus NA1 via a cysteine-cystine redox shuttle. J Microbiol 54:31–38
Crawford NA, Droux M, Kosower NS, Buchanan BB (1989) Evidence for function of the ferredoxin/thioredoxin system in the reductive activation of target enzymes of isolated intact chloroplasts. Arch Biochem Biophys 271:223–239
Delaunay A, Isnard AD, Toledano MB (2000) H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J 19:5157–5166
Guagliardi A, de Pascale D, Cannio R et al (1995) The purification, cloning, and high level expression of a glutaredoxin-like protein from the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem 270:5748–5755
Hillion M, Antelmann H (2015) Thiol-based redox switches in prokaryotes. Biol Chem 396:415–444
Holmgren A (1985) Thioredoxin. Ann Rev Biochem 54:237–271
Ito K, Inaba K (2008) The disulfide bond formation (Dsb) system. Curr Opin Struct Biol 18:450–458
Jeon SJ, Ishikawa K (2002) Identification and characterization of thioredoxin and thioredoxin reductase from Aeropyrum pernix K1. Eur J Biochem 269:5423–5430
Jeong J-Y, Yim H-S, Ryu J-Y et al (2012) One-step sequence- and ligation-independent cloning as a rapid and versatile cloning method for functional genomics studies. Appl Environ Microbiol 78:5440–5443
Jordan A, Åslund F, Pontis E et al (1997) Characterization of Escherichia coli NrdH: A glutaredoxin-like protein with a thioredoxin-like activity profile. J Biol Chem 272:18044–18050
Kadokura H, Katzen F, Beckwith J (2003) Protein Disulfide Bond Formation in Prokaryotes. Annu Rev Biochem 72:111–135. doi:10.1146/annurev.biochem.72.121801.161459
Kalinina EV, Chernov NN, Saprin AN (2008) Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. BioChemistry 73:1493–1510
Kamitani S, Akiyama Y, Ito K (1992) Identification and characterization of an Escherichia coli gene required for the formation of correctly folded alkaline phosphatase, a periplasmic enzyme. EMBO J 11:57–62
Kashima Y, Ishikawa K (2003) A hyperthermostable novel protein-disulfide oxidoreductase is reduced by thioredoxin reductase from hyperthermophilic archaeon Pyrococcus horikoshii. Arch Biochem Biophys 418:179–185
Kuge S, Jones N (1994) YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J 13:655–664
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol 33:1870–1874
Ladenstein R, Ren B (2006) Protein disulfides and protein disulfide oxidoreductases in hyperthermophiles. FEBS J 273:4170–4185
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee DY, Ahn BY, Kim KS (2000) A thioredoxin from the hyperthermophilic archaeon Methanococcus jannaschii has a glutaredoxin-like fold but thioredoxin-like activities. BioChemistry 39:6652–6659
Limauro D, Pedone E, Galdi I, Bartolucci S (2008) Peroxiredoxins as cellular guardians in Sulfolobus solfataricus- characterization of Bcp1, Bcp3 and Bcp4. FEBS J 275:2067–2077. doi:10.1111/j.1742-4658.2008.06361.x
Limauro D, Saviano M, Galdi I et al (2009) Sulfolobus solfataricus protein disulphide oxidoreductase: insight into the roles of its redox sites. Protein Eng Des Sel 22:19–26. doi:10.1093/protein/gzn061
Limauro D, D’Ambrosio K, Langella E et al (2010) Exploring the catalytic mechanism of the first dimeric Bcp: Functional, structural and docking analyses of Bcp4 from Sulfolobus solfataricus. Biochimie 92:1435–1444. doi:10.1016/j.biochi.2010.07.006
Limauro D, De Simone G, Pirone L et al (2014) Sulfolobus solfataricus thiol redox puzzle: characterization of an atypical protein disulfide oxidoreductase. Extremophiles 18:219–228. doi:10.1007/s00792-013-0607-8
Lipscomb GL, Keese AM, Cowart DM et al (2009) SurR: a transcriptional activator and repressor controlling hydrogen and elemental sulphur metabolism in Pyrococcus furiosus. Mol Microbiol 71:332–349
Marcus N, Shaffer D, Farrar P, Green M (1996) Tissue distribution of three members of the murine protein disulfide isomerase (PDI) family. Biochim Biophys Acta Gene Struct Expr 1309:253–260
McCarver AC, Lessner DJ (2014) Molecular characterization of the thioredoxin system from Methanosarcina acetivorans. FEBS J 281:4598–4611
McFarlan SC, Terrell CA, Hogenkamp HP (1992) The purification, characterization, and primary structure of a small redox protein from Methanobacterium thermoautotrophicum, an archaebacterium. J Biol Chem 267:10561–10569
Nakamoto H, Bardwell JCA (2004) Catalysis of disulfide bond formation and isomerization in the Escherichia coli periplasm. Biochim. Biophys Acta Mol Cell Res 1694:111–119
Pedone E, Ren B, Ladenstein R et al (2004) Functional properties of the protein disulfide oxidoreductase from the archaeon Pyrococcus furiosus: a member of a novel protein family related to protein disulfide-isomerase. Eur J Biochem 271:3437–3448
Pedone E, Limauro D, D’Alterio R et al (2006) Characterization of a multifunctional protein disulfide oxidoreductase from Sulfolobus solfataricus. FEBS J 273:5407–5420
Peek JA, Taylor RK (1992) Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc Natl Acad Sci USA 89:6210–6214
Rabinovitch I, Yanku M, Yeheskel A et al (2010) Staphylococcus aureus NrdH redoxin is a reductant of the class Ib ribonucleotide reductase. J Bacteriol 192:4963–4972
Ramakrishnan V, Adams MWW (1995) Preparation of genomic DNA from sulfur-dependent hyperthermophilic Archaea. Robb, F T, Place, A R Archaea, pp 95–96
Ren B, Tibbelin G, de Pascale D et al (1998) A protein disulfide oxidoreductase from the archaeon Pyrococcus furiosus contains two thioredoxin fold units. Nat Struct Biol 5:602–611
Schut GJ, Bridger SL, Adams MW (2007) Insights into the metabolism of elemental sulfur by the hyperthermophilic archaeon Pyrococcus furiosus: characterization of a coenzyme A-dependent NAD(P)H sulfur oxidoreductase. J Bacteriol 189:4431–4441
Smith AD, South PK, Levander OA (2001) Effect of gold(I) compounds on the virulence of an amyocarditic strain of coxsackievirus B3. Biol Trace Elem Res 84:67–80
Susanti D, Wong JH, Vensel WH et al (2014) Thioredoxin targets fundamental processes in a methane-producing archaeon, Methanocaldococcus jannaschii. Proc Natl Acad Sci USA 111:2608–2613
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Toledano MB, Delaunay A, Monceau L, Tacnet F (2004) Microbial H2O2 sensors as archetypical redox signaling modules. Trends Biochem Sci 29:351–357
Tomb JF (1992) A periplasmic protein disulfide oxidoreductase is required for transformation of Haemophilus influenzae Rd. Proc Natl Acad Sci USA 89:10252–10256
Vlamis-Gardikas A, Holmgren A (2002) Thioredoxin and glutaredoxin isoforms. Methods Enzymol 347:286–296
Yang H, Lipscomb GL, Keese AM et al (2010) SurR regulates hydrogen production in Pyrococcus furiosus by a sulfur-dependent redox switch. Mol Microbiol 77:1111–1122
Zheng M, Aslund F, Storz G (1998) Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718–1721
Acknowledgements
We are grateful to Jeong Ho Jeon and Tae-Jun Yang for technical assistance. This work was supported by grant from the KIOST in-house program (PE99514) and the Development of Technology for Biohydrogen Production Using the Hyperthermophilic Archaea program of the Ministry of Oceans and Fisheries in the Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Albers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, J.K., Jung, HC., Kang, S.G. et al. Redox regulation of SurR by protein disulfide oxidoreductase in Thermococcus onnurineus NA1. Extremophiles 21, 491–498 (2017). https://doi.org/10.1007/s00792-017-0919-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-017-0919-1