Abstract
A hyperthermophilic and thermostable xylanase of 82 kDa (TtXynA) was purified from the culture supernatant of T. terrestris Co3Bag1, grown on carboxymethyl cellulose (CMC), and characterized biochemically. TtXynA showed optimal xylanolytic activity at pH 5.5 and at 85 °C, and retained more than 90% of its activity at a broad pH range (4.5–10). The enzyme is highly thermostable with a half-life of 23.1 days at 65 °C, and active in the presence of several metal ions. Circular dichroism spectra strongly suggest the enzyme gains secondary structures when temperature increases. TtXynA displayed higher substrate affinity and higher catalytic efficiency towards beechwood xylan than towards birchwood xylan, oat-spelt xylan, and CMC. According to its final hydrolysis products, TtXynA displays endo-/exo-activity, yielded xylobiose, an unknown oligosaccharide containing about five residues of xylose and a small amount of xylose on beechwood xylan. Finally, this report represents the description of the first fungal hyperthermophilic xylanase which is produced by T. terrestris Co3Bag1. Since TtXynA displays relevant biochemical properties, it may be a suitable candidate for biotechnological applications carried out at high temperatures, like the enzymatic pretreatment of plant biomass for the production of bioethanol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
After cellulose, hemicellulose is the most abundant polysaccharide comprising about 25–35% of most plant materials, including forest and agricultural residues (Deutschmann and Dekker 2012). This polysaccharide is a branched heteropolymer consisting of pentose (d-xylose and d-arabinose) and hexose (d-mannose, d-glucose and d-galactose) sugars (Juturu and Wu 2012). Xylan is the major component of hemicellulose and is comprised of a backbone of xylose residues joined by β-1,4 glycosidic bonds (Ravanal et al. 2013). Because of its heterogeneous structure, xylan degradation requires an enzyme complex, but the key enzymes involved are endo-β-1,4-xylanases (EC. 3.2.1.8) and β-xylosidases (EC 3.2.1.37) (Polizeli et al. 2005).
Filamentous fungi are particularly remarkable producers of xylanases from an industrial point of view, owing to the fact that they secrete xylan-degrading enzymes into the medium, thus avoiding cell disruption. Furthermore, the xylanase levels obtained from fungal cultures are typically much higher than those obtained from yeast or bacteria. In addition, fungi typically produce several auxiliary enzymes that are necessary for debranching of the substituted xylans (Haltrich et al. 1996).
The mesophilic fungi belonging to the genera Aspergillus and Trichoderma are preeminent in xylanase production (Polizeli et al. 2005; Goluguri et al. 2012). It is well known that carbohydrate-active enzymes secreted by these fungi exhibit N-linked and O-linked glycosylation, which can impart stability against protein aggregation and enhance thermal stability (Beckham et al. 2012).
In recent years, thermophilic enzymes are gaining wide industrial and biotechnological interest, because they generally are better suited for processes carried out under harsh conditions and because they have numerous advantages, including rapid kinetics, better mass transfer, reduced risk of contamination, and increased opportunity for enzyme recycling (Turner et al. 2007; Viikari et al. 2007). Thus, xylanases from thermophilic fungi that are active at higher temperatures are receiving considerable attention for industrial applications (Maheshwari et al. 2000; Polizeli et al. 2005; Lee et al. 2009; Maalej et al. 2009).
In this context, the thermophilic ascomycete fungus Thielavia terrestris is of great interest, since it breaks down lignocellulosic biomass and is a source of thermostable enzymes (Langston et al. 2012). The genome sequence of T. terrestris NRRL 8126 was reported and compared to that of Myceliophthora thermophila. Both fungi were grown on alfalfa straw, barley straw, or glucose as the sole carbon sources; then, the identity and expression levels of a number of enzymes in the fungal secretomes were reported for each substrate (Berka et al. 2011). Furthermore, it has been reported that T. terrestris 255B expresses two major xylanases (xylanase I and II) when it is grown on oat-spelt xylan as carbon source; where xylanase II displayed optimal activity at pH 3.6–4.0 and at 60–65 °C (Gilbert et al. 1992). However, little is known about the xylanolytic activity of T. terrestris Co3Bag1 and about the biochemical characteristics of enzymes involved in the xylan breakdown by this fungus.
Hence, the general aim of this work was to purify and biochemically characterize a xylanase from T. terrestris Co3Bag1.
Materials and methods
Chemicals
Culture media were obtained from JT Baker (USA). Carboxymethyl cellulose (CMC), Avicel (crystalline cellulose), beechwood xylan, oat-spelt xylan, birchwood xylan, and Bradford reagent were purchased from Sigma-Aldrich (USA). Chromatographic media and all the chemicals used in the SDS-PAGE analysis, including the protein molecular weight (MW) markers, were purchased from Bio-Rad (USA). Xylose was obtained from Difco™ (USA). Xylobiose, xylotetraose, and xylohexose were purchased from Megazyme (Ireland). Silica gel 60 plates were purchased from Merck (Germany). All other used chemicals were of analytical grade and were purchased from Sigma-Aldrich (USA) and JT Baker (USA), unless otherwise specified.
Microorganisms and growth conditions
Thielavia terrestris Co3Bag1 was obtained from the CDBB Culture Collection, CINVESTAV, México (Accession number CDBB-H-1938). The Co3Bag1 strain used in this study was isolated from sugar-cane bagasse compost by the research group of Dr. Sergio R. Trejo-Estrada, and taxonomically identified as Thielavia terrestris by Charles River Laboratories International, Inc. (USA). Spores were obtained by culturing the fungus at 45 °C in solid medium described by Tien and Kirk (1988). Growth kinetics and xylanolytic activity measurements were carried out by inoculating 1 × 106 spores in 125 mL Erlenmeyer flasks with 50 mL of the basal medium as described (Zouari-Mechichi et al. 2006), containing: 5 g/L peptone, 1 g/L yeast extract, 2 g/L ammonium tartrate, 1 g/L KH2PO4, 0.5 g/L MgSO4·7H2O, 0.5 g/L KCl, and 1 mL of trace elements solution. Basal medium was supplemented with 1% (w/v) xylan or 1% (w/v) CMC as carbon source. Flasks were incubated at 45 °C while shaking at 120 rpm for 10 days. Every 24 h, each flask was assayed for biomass (dry weight) and xylanase activity. The results presented are expressed as mean ± standard deviation of three replicates.
Xylanase assay and zymogram analysis
Xylanase activity was routinely determined, unless otherwise stated, as follows: 0.1 mL of enzyme preparation was added to 0.9 mL substrate solution, containing 0.3% (w/v) beechwood xylan in 50 mM citrate buffer, pH 5.5. After 15 min incubation at 85 °C, the amount of reducing sugars released was measured by the 3,5-dinitrosalicylic acid (DNS) method (Miller 1959) using xylose as standard. The results are expressed as the mean ± standard deviation of three independent experiments. One unit of xylanase activity was defined as the amount of enzyme liberating 1 μmol of reducing sugars per minute. Zymogram analyses were carried out on polyacrylamide gels co-polymerized with 0.1% (w/v) Remazol Brilliant Blue-Xylan as previously described (Schwarz et al. 1987).
SDS–polyacrylamide gel electrophoresis and protein assay
Proteins were analyzed on 10% SDS-PAGE gels, according to the method of Laemmli (1970). Proteins in the gel were visualized by Coomassie Brilliant Blue R-250 staining. Protein molecular weight (MW) was estimated using broad range MW protein standards as reference. Images of gels were recorded and analyzed using a gel documentation system (DigiDoc-It Imaging System, UVP, USA). Protein concentration was determined as described (Bradford 1976), using bovine serum albumin (Pierce, USA) as standard.
Xylanase purification
For xylanase production, a total of twenty 125 mL Erlenmeyer flasks containing 50 mL of the basal medium as described (Zouari-Mechichi et al. 2006), was supplemented with 1% (w/v) CMC as carbon source, where each inoculated with 1 × 106 spores. Cultures were incubated at 45 °C for 10 days and shaken at 120 rpm. The mycelium was removed by vacuum filtration and the culture supernatant, named crude extract, was used as a source of xylanase activity. The total protein from the crude extract was precipitated at 80% (NH4)2SO4 saturation overnight at 4 °C. Then, the pellet was recovered by centrifugation (8800×g, 4 °C, 20 min), suspended in 5 mL of 50 mM citrate buffer, pH 5.5, and dialyzed against Buffer A (25 mM KCl, 50 mM Tris–HCl, pH 7.5). The dialyzed protein was loaded onto an anionic exchange chromatography column (UNOsphere Q, Bio-Rad, USA), previously equilibrated with Buffer A. Proteins bound to the column were eluted by applying a linear gradient of KCl (0.25 mM–1 M) in Buffer A, at a constant flow rate of 2 mL/min, and 2.0 mL fractions were collected. Fractions with the highest xylanase activity were pooled and concentrated threefold using a filtration device with a 10 kDa cutoff (Pall Corporation, USA). This xylanase preparation was loaded onto a gel filtration column (Bio-Gel P-100, Bio-Rad, USA), equilibrated with Buffer A. The gel filtration chromatography was carried out at a constant flow rate of 0.25 mL/min, and 1.0 mL fractions were collected. Fractions manifesting xylanase activity were analyzed by 10% SDS-PAGE; subsequently, fractions of purified xylanase were stored at 4 °C for further study.
Glycosylation of TtXynA
The glycosylated nature of TtXynA was determined by periodic acid-Schiff staining, following the method previously described (Segrest and Jackson 1972), and its carbohydrate content was assessed by anthrone assay (Dimler et al. 1952).
Optimal pH and pH stability assays
The optimal pH was determined by measuring the xylanase activity of TtXynA at different pH values, ranging from 3 to 10.5, using 50 mM citrate–phosphate buffer (pH 3–7); 50 mM citrate buffer (pH 4–6), 50 mM phosphate buffer (pH 6–8), and 50 mM glycine-NaOH buffer (pH 8.5–10.5). Each buffer contained 0.3% beechwood xylan. Reaction samples were incubated at 70 °C for 15 min.
For pH stability, samples of the purified enzyme were incubated at 25 °C for 2 h at different pH values, ranging from 4.0 to 10.5, using 50 mM citrate buffer (pH 4–6), 50 mM phosphate buffer (pH 7–8), 50 mM Tris–HCl buffer (8.5–9), and 50 mM glycine-NaOH buffer (pH 9.5–10.5). Then, the residual xylanase activity was measured under standard conditions.
Optimal temperature and thermal stability assay
The optimal temperature was determined by measuring the xylanase activity of TtXynA in 50 mM citrate buffer, pH 5.5, containing 0.3% beechwood xylan, over a temperature range of 25–90 °C for 15 min. To evaluate thermal stability, TtXynA was incubated at 85, 75, and 65 °C without substrate. Aliquots were withdrawn at different time intervals, and the residual activity was measured under standard conditions. The half-life (t 1/2), corresponding to the 50% of the original activity, for each temperature, was calculated.
Circular dichroism (CD) analysis
Far-UV CD spectra were obtained using a Jasco J-815 Spectropolarimeter (Jasco Inc., Easton, MD). Scans were taken between 210 and 250 nm using a 0.1 cm path-length cuvette at a temperature range of 25–85 °C. Temperature was regulated using a Peltier system. All samples were prepared at a protein concentration of 0.18 mg/mL in 5 mM citrate buffer (pH 5.5). CD data are reported as mean residue ellipticity [θ]MRW. Each spectrum presented is an average of five scans. Thermal denaturation curves were obtained by continuously monitoring the CD signal at 230 nm for TtXynA and at 220 nm for Xyn11AAOX1 as control protein, while the temperature of the samples was increased at a constant heating rate of 2 °C/min. Xyn11AAOX1 corresponds to the xylanase Xyn11A from Cellulomonas uda expressed in Pichia pastoris under the control of AOX1 promoter (Cayetano-Cruz et al. 2016).
Kinetic parameters and substrate specificity
The effect of the concentration of beechwood xylan, birchwood xylan, oat-spelt xylan, CMC, and Avicel at concentrations ranging from 0.25 to 6.0 mg/mL on xylanase activity was evaluated under optimal assay conditions (85 °C, pH 5.5, and 15 min). The maximum reaction velocity (V max) and affinity constant (K M) were estimated by linear regression from Lineweaver–Burk (or double reciprocal) plots, and the turnover number (k cat) for each substrate was calculated using the data obtained from the corresponding enzymatic kinetic measurements.
Effect of metal ions and EDTA
The effect of different metal ions (Mn2+, Cu2+, Ca2+, Co2+, Zn2+, Hg2+, Ni2+, Mg2+, and Fe2+) and EDTA was examined by incubating TtXynA with each of the compounds at concentrations of 1 and 5 mM under optimal assay conditions (85 °C, pH 5.5, and 15 min). The activity was expressed as the percentage of the activity observed in the absence of any compound.
Analysis of hydrolysis products
To analyze the hydrolysis products of xylan, a reaction mixture containing TtXynA (3.2 U), 1% (w/v) beechwood xylan, and 50 mM citrate buffer pH 5.5 was incubated at 65 °C for 72 h. Aliquots were withdrawn at different time intervals and were then analyzed by thin-layer chromatography (TLC). Aliquots (2 µL) of each sample were loaded on silica gel plates and then fractioned in a solvent system containing butanol:ethanol:water (50:30:20, v/v.). The plate was sprayed with 15% (v/v) H2SO4 and heated at 90 °C until spots became visible. Xylose, xylobiose, xylotetraose, and xylohexose, at a final concentration of 3 mg/mL, were used as standards. For the analysis of the hydrolysis products on CMC, a reaction mixture containing TtXynA (3.2 U), 2% (w/v) CMC, and 50 mM acetate buffer pH 5.5 was incubated at 65 °C for 24 h. Hydrolysis products were fractioned in a solvent system containing ethyl acetate:water:methanol (8:3:4, v/v.). Glucose and cellobiose at a final concentration of 3 mg/mL were used as standards.
Identity of TtXynA
Purified TtXynA was partially sequenced by tandem mass spectrometry (MS/MS) at the Proteomics Unit, National Institute of Genomic Medicine (INMEGEN, Mexico). The amino-acid sequence of peptides obtained was analyzed for similarity using tools available at Expasy Bioinformatic Resource Portal (https://www.expasy.org/) and NCBI server (https://www.ncbi.nlm.nih.gov/).
Results
Kinetics of growth and xylanolytic activity of T. terrestris Co3Bag1
The ability of T. terrestris Co3Bag1 to exhibit xylanolytic activity was evaluated by growing the fungus in the liquid media described by Zouari-Mechichi et al. 2006, and using 1% (w/v) beechwood xylan or CMC as carbon source. The growth of the cell mass of T. terrestris Co3Bag1 was similar for both substrates (Fig. 1). Xylanase activity was detected in both carbon sources; however, higher activity was obtained when T. terrestris Co3Bag1 was grown on CMC (1.6 U/mL) than on xylan (0.7 U/mL), after 96 h of culture at 45 °C (Fig. 1).
a Growth and xylanolytic activity kinetics of T. terrestris Co3Bag1. Cell mass growth of T. terrestris Co3Bag1 in CMC (filled circle) and xylan (line with triangle). Dotted lines xylanolytic activity produced on 1% (w/v) CMC (filled circle) and on 1% (w/v) beechwood xylan as carbon source (line with triangle). b Zymographic analysis of xylanase activity in culture supernatant at 96 h
Because of the xylanolytic activity was about 2.2-fold higher on CMC than on beechwood xylan, CMC was chosen as the carbon source to study the kinetic growth of T. terrestris Co3Bag1. The onset of xylanolytic activity was at 48 h of culture, while the highest activity (1.56 U/mL) was reached at 96 h of culture. After this time, the activity remained at about the same level for the next 6 days (Fig. 1a). The xylanolytic activity in the culture supernatant of T. terrestris Co3Bag1, grown on CMC at 96 h of culture, was assessed by zymogram analysis using 1% (w/v) beechwood xylan as the substrate, and at least three bands of xylanolytic activity were detected (Fig. 1b).
Purification of TtXynA from T. terrestris Co3Bag1
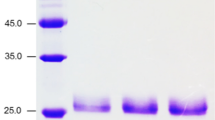
A xylanase was purified from the culture supernatant of T. terrestris Co3Bag1, grown on CMC as the only carbon source. All the purification steps are summarized in Table 1. The xylanase was purified to homogeneity with a 9.4-fold purification factor and a recovery yield of 10.8%. SDS-PAGE analysis of the purified enzyme showed a single band with an estimated MW of 82 kDa (Fig. 2a) that displayed xylanolytic activity by zymogram analysis (Fig. 2b).
Glycosylation of TtXynA
The glycosylated nature of TtXynA was assessed by staining a semipurified preparation of the enzyme with periodic acid-Schiff reagent (Fig. 3). A band with an estimated MW of 82 kDa was positively stained, indicating that the enzyme is a glycoprotein. The percentage of glycosylated enzyme, determined by the Anthrone assay, was 20% ± 0.36 of its total mass.
Optimal pH and pH stability
TtXynA showed maximal activity at pH 5.5, and exhibited about 80% of its maximal activity at different pH values ranging from 5 to 8, and more than 60% from pH 4–9 (Fig. 4). The pH stability assays showed that purified enzyme retains more than 90% of its activity at a broad pH range (4.5–10) after 2 h of incubation at 25 °C (Fig. 4B).
Optimal temperature and thermal stability
An increase in the xylanolytic activity of TtXynA was observed as the temperature rises in the temperature range of 25–85 °C (Fig. 5). The enzyme shows optimal activity at 85 °C and displays 70–80% of its maximum activity in a temperature range of 65–80 °C and at 90 °C (Fig. 5a).
Thermal stability of TtXynA at 85, 75 (Fig. 5b), and 65 °C (Fig. 5c) was assessed. To determine the t 1/2 at 85 and 75 °C, the natural logarithm of percent remaining activity at the temperature tested was plotted versus time, as described (Atkins and de Paula 2006). The data fitted first-order kinetics. The thermal inactivation function of TtXynA at 85 °C was: Y = −0.022x + 4.5455, R = 0.9444, and at 75 °C was: Y = −0.0029x + 4.5517, R = 0.9810. The rate constant of thermal inactivation at 85 and 75 °C is 0.022 and 0.0029 min−1, respectively. Therefore, the t1/2 of the enzyme at 85 and 75 °C are 31.5 and 238.9 min (3.9 h), respectively. At 65 °C, the data fitted second-order kinetics; therefore, the data were linearized by plotting the inverse of the activities versus time, as described (Atkins and de Paula 2006). The thermal inactivation function at this temperature was Y = 0.0752x + 1.8089, R = 0.9656. The rate constant of thermal inactivation and t 1/2 at 65 °C are 0.0772 (U/ml)−1 day−1 and 23.1 days, respectively.
Changes on the TtXynA secondary structure
The effect of raising the temperature in increments on the secondary structure of TtXynA was explored by CD spectroscopy. Far-UV CD spectra of the enzyme at different temperatures ranging from 25 to 85 °C are shown in Fig. 6a. The spectrum of the enzyme at 25 °C showed a negative peak centered around 228 nm, and when the temperature of the sample was increased, this peak became more negative, indicating that the xylanase gains secondary structures, probably β sheets. In contrast, there was no change in the CD signal for the control protein, the moderately thermostable xylanase Xyn11AAOX1, when temperature was increased (inner graphic in Fig. 6a). These data were substantiated in subsequent experiments where thermal transition profiles at 230 nm of TtXynA and Xyn11AAOX1 (Fig. 6b) were in agreement to those previously observed for both enzymes.
a Far-UV CD spectra of the purified TtXynA and Xyn11AAOX1 as control protein (inner a), at different temperatures, as indicated. Spectra were recorded employing 0.1 cm path-length cells, averaging 4.0 s at 0.5 nm intervals in the range of 250–210 nm, and 1 nm bandwidth was used. Buffer baselines were subtracted from all spectra. b Thermal transition profiles of TtXynA (black line) and Xyn11AAOX1 (red dashed line). CD signal was recorded at 220 and 230 nm, respectively. The samples were heated at constant rate of 2 °C/min
Effect of metal ions and EDTA on TtXynA activity
The effect of metal ions and EDTA, at 1 and 5 mM each, on the activity of TtXynA was evaluated, and a summary of the results is presented in Table 2. The ions Ca2+, Fe2+, Co2+, and Zn2+ (1 mM) had no effect on the enzyme activity, whereas the presence of Co2+ and Zn2+ (5 mM each) leads to a reduction in the xylanolytic activity by 24 and 28%, respectively. The ions Cu2+, Mn2+, and Hg2+ (1 and 5 mM) showed the highest negative effect on xylanolytic activity, reducing the enzyme activity by more than 50%. The enzyme was completely inactivated by the Hg2+ ion (5 mM).
Kinetic parameters and substrate specificity
The kinetic parameters of TtXynA were assayed under optimal pH and temperature conditions against several substrates, including beechwood xylan, birchwood xylan, oat-spelt xylan, CMC, and Avicel, at different concentrations ranging from 0.25 to 6.0 mg/mL, and the results are shown in Table 3. The enzyme shows the highest affinity towards beechwood xylan (K M of 0.41 mg/mL), and the V max for this substrate was 21.52 U/mg.
With respect to substrate affinity, TtXynA shows higher affinity towards all of the tested xylan-rich substrates than to CMC, while no activity was detected in the presence of Avicel. In particular, the enzyme displayed the highest affinity towards beechwood xylan, and this affinity was 39-fold higher than that observed for CMC (Table 3).
Analysis of the hydrolysis products
The hydrolysis products of beechwood xylan and CMC yielded by TtXynA at different times of incubation were analyzed by TLC. With regard to xylan, after 72 h of incubation, the hydrolysis products released were mainly xylobiose, xylopentose, and a small amount of xylose (Fig. 7a); whereas on the case of CMC, the enzyme released mainly high MW cellooligomers (Fig. 7b). In other hand, the hydrolysis products released by the action of TtXynA on CMC were analyzed by TLC in acetate buffer, as described above, due to no hydrolysis, products were detected when the assay was carried out with citrate buffer.
a TLC analysis of beechwood xylan hydrolysis by TtXynA. Lane 1 Xylose; lane 2 xylobiose; lane 3 xylotetraose; lane 4 xylohexose; lane 5 beechwood xylan; lanes 6–9 hydrolysis products at 24, 48, 72 and 144 h, respectively. b TLC analysis of the CMC hydrolysis by TtXynA. Lane 1 glucose; lane 2 cellobiose; lane 3 CMC; lane 4–8 hydrolysis products at 1, 3, 5, 8, and 12 h, respectively
Identity of TtXynA
TtXynA was identified by partial amino-acid sequencing, and the following peptides were obtained: AWDVVNEAIADDGTWR and TCQKLNDWYYQCL (Fig. 8). Bioinformatic analysis of the amino-acid sequence of the two peptides obtained showed 100% identity to β-xylanase from T. terrestris NRRL8126 (UniProtKB accession number G2R5G6), belonging to the glycoside hydrolase family 10 (GH10). Furthermore, the peptide AWDVVNEAIADDGTWR showed 94% identity with xylanase from Achaetomium sp. Xz-8 (UniProtKB accession number V9XZ28); while the peptide TCQKLNDWYYQCL showed 92% identity with β-xylanase from the fungus Scedosporium apiospermum (Accession number A0A084GH03). Interestingly, the peptide TCQKLNDWYYQCL corresponds to the C-terminal end of β-xylanase (UniProtKB accession number G2R5G6) (Fig. 8), and Cel7A (UniProtKB accession number G2QZD9) from T. terrestris NRRL8126; in addition, this peptide forms part of the fungal-type carbohydrate-binding domain (CBM1).
Discussion
Due to the importance that thermophilic and thermostable xylanases have gained in recent years, we focused on the identification of at least one of the enzymes involved in the xylanolytic activity from the thermophilic ascomycete T. terrestris Co3Bag1. First, we assessed the xylanolytic activity produced by this fungus in the presence of beechwood xylan or CMC, finding that the enzymatic activity was higher in the presence of the latter substrate. This indicates that CMC is a better inducer of xylanase activity than beechwood xylan, under the conditions tested. Interestingly, in agreement to these results, it has been reported that when Neurospora crassa was cultured with Avicel as the only carbon source, both cellulase and hemicellulases genes were induced, and the expression levels of some hemicellulases genes were much higher than those observed when N. crassa was cultured with xylan (Sun et al. 2012; Amore et al. 2013). Moreover, the production of xylanases by fungi grown on cellulose as the only carbon source has been previously reported. The fungus T. reesei exhibits xylanolytic and cellulolytic activities in the presence of cellulose, xylan, or mixtures of plant polymers (Amore et al. 2013). Likewise, it has been reported that cellulose, cellobiose, and even by heterodisaccharide consisting of glucose and xylose induce the production of xylanolytic enzymes in Aspergillus terreus (Hrmova et al. 1989; Amore et al. 2013).
TtXynA was purified from the supernatant culture of T. terrestris Co3Bag1. Fungal xylanases with MW ranging from 8.5 (Silva et al. 1999) to 253 kDa (Anthony et al. 2003) have been reported. TtXynA is a glycoprotein, with a carbohydrate content of 20%. Most fungal xylanases are glycoproteins with a diverse carbohydrate content (Wong et al. 1988; Kulkarni et al. 1999), e.g., the carbohydrate decoration on β-xylosidases contributes 10–30% of their MW (Juturu and Wu 2012). In addition, it has been found that extent and heterogeneity of glycosylation depend on growth conditions and the presence of glycan trimming enzymes in the secretomes of the producer microorganism (Beckham et al. 2012).
Some biochemical characteristics of fungal xylanases are compared with those of TtXynA in Supplementary Table S1. The optimal pH assay showed that the enzyme had maximal activity at 5.5, which is within the range of optimal pH values for several fungal xylanases. An optimal pH value of 5.5 was reported for a purified xylanase from H. brevis var. thermoidea (Masui et al. 2012). Moreover, the enzyme exhibited more than 60% of its maximal activity at pH values ranging from 4 to 9. Likewise, xylanases from Chaetomium sp., which has more than 50% of its maximal activity at pH values ranging from 4.5 to 11 (Jiang et al. 2010), and Paecilomyces thermophile, which has 70% of its maximal activity at pH values ranging from 5.0 to 9.5 (Li et al. 2006), have been described. However, TtXynA was twofold more active at pH 7 than a xylanase produced by A. ficuum AF-98, which has 30% of its maximal activity at this pH value (Lu et al. 2008). The pH stability data of TtXynA were similar to those reported for xylanases from Chaetomium sp. (Jiang et al. 2010) and Paecilomyces thermophile (Li et al. 2006), which were stable in a pH range of 4–11 and 6.5–10.5, respectively; whereas a xylanase from T. lanuginosus (Li et al. 2005) was stable only in a pH range of 5–6.
Depending on their optimal temperature, enzymes can be classified as mesophilic, thermophilic, and hyperthermophilic, with the last group of enzymes working optimally at temperatures >80 °C (Vieille and Zeikus 1996; Polizeli et al. 2005; Lee et al. 2009). According to these criteria, TtXynA can be classified as a hyperthermophilic, being the most thermophilic fungal xylanolytic activity reported so far. To the best of our knowledge, the highest optimal temperature for fungal xylanases is registered at 80 °C for P. thermophile (Li et al. 2006) and L. sulphureus (Lee et al. 2009) xylanases (Supplementary Table S1), highlighting the significance of our current findings. Xylanases with optimal temperatures higher than 85 °C have been reported for other non-fungal microorganisms, such as Dictyoglomus thermophilum (Li et al. 2013) and Thermotoga maritima (Jia et al. 2012).
The high thermal stability of TtXynA at 75 and 65 °C is similar to those reported for xylanases isolated from T. lanuginosus (Li et al. 2005), P. thermophile (Li et al. 2006), and L. sulphureus (Lee et al. 2009). Furthermore, the purified enzyme is notably more stable (t 1/2 of 23.1 days at 65 °C) than a xylanase produced by Chaetomium sp. (t 1/2 of 14.9 min at 65 °C) (Jiang et al. 2010), and a xylanase produced by A. japonicus, which lost all its activity after 30 min of incubation at 55 °C (Wakiyama et al. 2010). Based on the observations that TtXynA is highly active at temperatures >75 °C and highly stable at 65 and 75 °C, this enzyme could be very useful in biotechnological processes that are carried out at high temperatures. Circular dichroism spectra strongly suggest that TtXynA gains secondary structures when temperature increases. The changes in secondary structure were mainly observed at temperatures ranging from 50 to 80 °C, reaching the highest degree of ellipticity around 85 °C, which coincides with the temperature at which the enzyme displays optimal activity. The temperature-induced structural changes observed can be attributed to conformational rearrangements involving mainly β-sheet structures. Some other proteins have been shown to gain ellipticity due to altering physicochemical conditions. For example, decreasing pH is responsible for a strong increase in the negative ellipticity of xylanase A from the hyperthermophilic bacterium T. maritima; in addition, the cellulose-binding domain of this enzyme also gains ellipticity as temperature increases (Wassenberg et al. 1997). On the contrary, some other proteins, such as β-1,3-1,4-glucanase (Lichenase) from P. thermophile, lose their regular secondary structures as the temperature increases (Yuan et al. 2008).
It has been reported that some metal ions and reagents significantly affect xylanase activities (Juturu and Wu 2012). Therefore, the effect of metal ions and EDTA on the xylanolytic activity of TtXynA was evaluated. Interestingly, the enzyme displayed 40–100% of its original activity in the presence of most of metal ions (Cu2+, Ca2+, Fe2+, Mg2+, Ni2+, Mn2+, Co2+, and Zn2+) used in this study, at concentrations of 1 and 5 mM each. However, the enzymatic activity was partially (46%) and completely inhibited by Hg+2 1 and 5 mM, respectively; thus suggesting that the thiol groups of cysteine residues are present in the vicinity of the active site, as has been pointed out previously (Bastawde 1992; Knob and Carmona 2010). On the other hand, the enzyme retained more than 70% of its activity in the presence of EDTA (1 and 5 mM), suggesting that no metal ions are required for its activity. Although xylanases do not behave in the same way in the presence of most metallic ions, findings here are similar to those previously reported for purified and characterized xylanases from L. sulphureus (Lee et al. 2009), P. sclerotiorum (Knob and Carmona 2010), A. gemina (Dhiman et al. 2013), Achaetomium sp. (Zhao et al. 2013), Penicillium funiculosum, and P. occitanis (Driss et al. 2013).
The kinetic parameters of TtXynA, K M, and V max values, determined for each of xylan substrates used in this study, are in agreement with those obtained for other fungal xylanases with K M values ranging from 0.15 to 49.5 mg/mL and V max values ranging from 0.106 to 6300 U/mg (Beg et al. 2001; Knob and Carmona 2010). It has been reported that over 90% of birchwood and beechwood xylan are composed of xylose. In birchwood xylan, xyloses are joined mainly by 1,4-linkages, whereas in beechwood xylan, xyloses are mainly joined by both 2,4- and 1,4-linkages. Most of the oat-spelt xylans contain xylose and arabinose, as well as small amounts of glucose and galactose (Li et al. 2000; Lee et al. 2009). Hence, findings here suggest that TtXynA has a higher affinity towards 1,4-linkages between xyloses present in beechwood and birchwood xylans. As the amount of xylose decreases, as in the case of oat-spelt xylan, the affinity of the enzyme also decreases. On the other hand, TtXynA showed a 16-fold lower affinity to CMC than to beechwood xylan, which indicates that the enzyme has a lower affinity for 1,4-linkages between glucoses than for 1,4-linkages between xyloses. This behavior is characteristic of many xylanases that are active on CMC and β-glucans (Kulkarni et al. 1999; Grishutin et al. 2004; Viikari et al. 2007).
Since TtXynA, a putative member of GH10 family, is able to hydrolyze xylan and CMC, its final hydrolysis products on both substrates were analyzed. Interestingly, in the presence of xylan as the substrate, the enzyme shows exo- and endo-xylanase activities due to its ability to release xylopentose, xylobiose, and xylose from beechwood xylan. Endo-xylanases that release xylose as one of their main hydrolysis products have been previously reported. In accordance with our findings, it has been reported that endo-xylanases from Cryptococcus albidus and Streptomyces lividans, belonging to GH10 family, display several catalytic activities, which are compatible with β-xylosidase enzymes (Biely et al. 1997). Furthermore, the endo-xylanase XYNIV from T. reesei, belonging to GH30 family, shows exo- and endo-xylanase activities, because its main hydrolysis products were xylose, xylobiose, aldotetraouronic acid, and longer acidic xylo-oligosaccharides from xylan of different sources (Tenkanen et al. 2013). While on CMC, the major final product of TtXynA-mediated hydrolysis of CMC was an unknown high MW oligosaccharide, suggesting that the enzyme has endo-activity on β-1,4-glucanes. In agreement to our data, a bifunctional xylanase/endoglucanase (RuCelA) from yak rumen microorganisms, active against xylan and CMC, has been described (Chang et al. 2011).
Hence, this work may represent the description of TtXynA from T. terrestris Co3Bag1 as the first fungal highly thermophilic xylanase, which displays activity towards cellulose, as well. Its biochemical characteristics, including the ability to release xylo-oligosaccharides and cello-oligosaccharides, for instance, during enzymatic pretreatment of plant biomass for the production of bioethanol, could be advantageous over glycoside hydrolases with a single activity. Moreover, the hyperthermophilic nature of TtXynA may make it suitable for biotechnological processes carried out at high temperatures.
References
Amore A, Giacobbe S, Faraco V (2013) Regulation of cellulase and hemicellulase gene expression in fungi. Curr Genom 14:230–249. doi:10.2174/1389202911314040002
Anthony T, Chandra Raj K, Rajendran A, Gunasekaran P (2003) High molecular weight cellulase-free xylanase from alkali-tolerant Aspergillus fumigatus AR1. Enzyme Microb Technol 32:647–654. doi:10.1016/S0141-0229(03)00050-4
Atkins P, de Paula J (2006) Physical chemistry for the life sciences, second. Oxford University Press
Bastawde KB (1992) Xylan structure, microbial xylanases, and their mode of action. World J Microbiol Biotechnol 8:353–368. doi:10.1007/BF01198746
Beckham GT, Dai Z, Matthews JF, Momany M, Payne CM, Adney WS, Baker SE, Himmel ME (2012) Harnessing glycosylation to improve cellulase activity. Curr Opin Biotechnol 23:338–345. doi:10.1016/j.copbio.2011.11.030
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338. doi:10.1007/s002530100704
Berka RM, Grigoriev IV, Otillar R, Salamov A, Grimwood J, Reid I, Ishmael N, John T, Darmond C, Moisan M-C, Henrissat B, Coutinho PM, Lombard V, Natvig DO, Lindquist E, Schmutz J, Lucas S, Harris P, Powlowski J, Bellemare A, Taylor D, Butler G, de Vries RP, Allijn IE, van den Brink J, Ushinsky S, Storms R, Powell AJ, Paulsen IT, Elbourne LDH, Baker SE, Magnuson J, LaBoissiere S, Clutterbuck AJ, Martinez D, Wogulis M, de Leon AL, Rey MW, Tsang A (2011) Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat Biotechnol 29:922–927. doi:10.1038/nbt.1976
Biely P, Vrsanska M, Tenkanan M, Kluepfel D (1997) Endo-β-1,4-xylanase families: differences in catalytic properties. J Biotechnol 57:151–166. doi:10.1016/S0168-1656(97)00096-5
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Cayetano-Cruz M, Santos PDL, Itzel A, García-Huante Y, Santiago-Hernández A, Pavón-Orozco P, Eric V, Hidalgo-Lara ME (2016) High level expression of a recombinant xylanase by Pichia pastoris cultured in a bioreactor with methanol as the sole carbon source: purification and biochemical characterization of the enzyme. Biochem Eng J 112:161–169. doi:10.1016/j.bej.2016.04.014
Chang L, Ding M, Bao L, Chen Y, Zhou J, Lu H (2011) Characterization of a bifunctional xylanase/endoglucanase from yak rumen microorganisms. Appl Microbiol Biotechnol 90:1933–1942. doi:10.1007/s00253-011-3182-x
Deutschmann R, Dekker RFH (2012) From plant biomass to bio-based chemicals: latest developments in xylan research. Biotechnol Adv 30:1627–1640. doi:10.1016/j.biotechadv.2012.07.001
Dhiman SS, Kalyani D, Jagtap SS, Haw JR, Kang YC, Lee JK (2013) Characterization of a novel xylanase from Armillaria gemina and its immobilization onto SiO2 nanoparticles. Appl Microbiol Biotechnol 97:1081–1091. doi:10.1007/s00253-012-4381-9
Dimler RJ, Schaefer WC, Wise CS, Rist CE (1952) Quantitative paper chromatography of d-glucose and its oligosaccharides. Anal Chem 24:1411–1414
Driss D, Berrin JG, Juge N, Bhiri F, Ghorbel R, Chaabouni SE (2013) Functional characterization of Penicillium occitanis Pol6 and Penicillium funiculosum GH11 xylanases. Protein Expr Purif 90:195–201. doi:10.1016/j.pep.2013.06.007
Gilbert M, Breuil C, Yaguchi M, Saddler JN (1992) Purification and characterization of a xylanase from the thermophilic Ascomycete Thielavia terrestris 255b. Appl Biochem Biotechnol 34–5:247–259. doi:10.1007/BF02920549
Goluguri B, Thulluri C, Cherupally M, Nidadavolu N, Achuthananda D, Mangamuri L, Addepally U (2012) Potential of thermo and alkali stable xylanases from Thielaviopsis basicola (MTCC-1467) in biobleaching of wood kraft pulp. Appl Biochem Biotechnol 167:2369–2380. doi:10.1007/s12010-012-9765-x
Grishutin SG, Gusakov AV, Markov AV, Ustinov BB, Semenova MV, Sinitsyn AP (2004) Specific xyloglucanases as a new class of polysaccharide-degrading enzymes. Biochim Biophys Acta Gen Subj 1674:268–281. doi:10.1016/j.bbagen.2004.07.001
Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Zupancic S (1996) Production of fungal xylanases. Bioresour Technol 58:137–161. doi:10.1016/S0960-8524(96)00094-6
Hrmova M, Biely P, Vrsanska M (1989) Cellulose- and xylan-degrading enzymes of Aspergillus terreus and Aspergillus niger. Enzyme Microb Technol 11:610–616. doi:10.1016/0141-0229(89)90090-2
Jia H, Fan G, Yan Q, Liu Y, Yan Y, Jiang Z (2012) High-level expression of a hyperthermostable Thermotoga maritima xylanase in Pichia pastoris by codon optimization. J Mol Catal B Enzym 78:72–77. doi:10.1016/j.molcatb.2012.02.009
Jiang Z, Cong Q, Yan Q, Kumar N, Du X (2010) Characterisation of a thermostable xylanase from Chaetomium sp. and its application in Chinese steamed bread. Food Chem 120:457–462. doi:10.1016/j.foodchem.2009.10.038
Juturu V, Wu JC (2012) Microbial xylanases: engineering, production and industrial applications. Biotechnol Adv 30:1219–1227. doi:10.1016/j.biotechadv.2011.11.006
Knob A, Carmona EC (2010) Purification and characterization of two extracellular xylanases from Penicillium sclerotiorum: a novel acidophilic xylanase. Appl Biochem Biotechnol 162:429–443. doi:10.1007/s12010-009-8731-8
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456. doi:10.1016/S0168-6445(99)00006-6
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Langston JA, Brown K, Xu F, Borch K, Garner A, Sweeney MD (2012) Cloning, expression, and characterization of a cellobiose dehydrogenase from Thielavia terrestris induced under cellulose growth conditions. Biochim Biophys Acta Proteins Proteom 1824:802–812. doi:10.1016/j.bbapap.2012.03.009
Lee JW, Park JY, Kwon M, Choi IG (2009) Purification and characterization of a thermostable xylanase from the brown-rot fungus Laetiporus sulphureus. J Biosci Bioeng 107:33–37. doi:10.1016/j.jbiosc.2008.09.006
Li K, Azadi P, Collins R, Tolan J, Kim JS, Eriksson KEL (2000) Relationships between activities of xylanases and xylan structures. Enzyme Microb Technol 27:89–94. doi:10.1016/S0141-0229(00)00190-3
Li X, Jiang Z, Lil Yang S, Feng W, Fan J, Kusakabe I (2005) Characterization of a cellulase-free, neutral xylanase from Thermomyces lanuginosus CBS 288.54 and its biobleaching effect on wheat straw pulp. Bioresour Technol 96:1370–1379. doi:10.1016/j.biortech.2004.11.006
Li L, Tian H, Cheng Y, Jiang Z, Yang S (2006) Purification and characterization of a thermostable cellulase-free xylanase from the newly isolated Paecilomyces themophila. Enzyme Microb Technol 38:780–787. doi:10.1016/j.enzmictec.2005.08.007
Li H, Kankaanpää A, Xiong H, Hummel M, Sixta H, Ojamo H, Turunen O (2013) Thermostabilization of extremophilic Dictyoglomus thermophilum GH11 xylanase by an N-terminal disulfide bridge and the effect of ionic liquid [emim]OAc on the enzymatic performance. Enzyme Microb Technol 53:414–419. doi:10.1016/j.enzmictec.2013.09.004
Lu F, Lu M, Lu Z, Bie X, Zhao H, Wang Y (2008) Purification and characterization of xylanase from Aspergillus ficuum AF-98. Bioresour Technol 99:5938–5941. doi:10.1016/j.biortech.2007.10.051
Maalej I, Belhaj I, Masmoudi N, Belghith H (2009) Highly thermostable xylanase of the thermophilic fungus Talaromyces thermophilus: purification and characterization. Appl Biochem Biotechnol 158:200–212. doi:10.1007/s12010-008-8317-x
Maheshwari R, Bharadwaj G, Bhat MK (2000) Thermophilic fungi: their physiology and enzymes. Microbiol Mol Biol Rev 64:461–488. doi:10.1128/MMBR.64.3.461-488.2000
Masui DC, Zimbardi ALRL, Souza FHM, Guimarães LHS, Furriel RPM, Jorge JA (2012) Production of a xylose-stimulated β-glucosidase and a cellulase-free thermostable xylanase by the thermophilic fungus Humicola brevis var. thermoidea under solid state fermentation. World J Microbiol Biotechnol 28:2689–2701. doi:10.1007/s11274-012-1079-1
Miller GL (1959) Use of dinitrosalicylic acid reagent. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67:577–591. doi:10.1007/s00253-005-1904-7
Ravanal MC, Alegría-Arcos M, Gonzalez-Nilo FD, Eyzaguirre J (2013) Penicillium purpurogenum produces two GH family 43 enzymes with β-xylosidase activity, one monofunctional and the other bifunctional: biochemical and structural analyses explain the difference. Arch Biochem Biophys 540:117–124. doi:10.1016/j.abb.2013.10.017
Schwarz WH, Bronnenmeier K, Gräbnitz F, Staudenbauer WL (1987) Activity staining of cellulases in polyacrylamide gels containing mixed linkage beta-glucans. Anal Biochem 164:72–77. doi:10.1016/0003-2697(87)90369-1
Segrest JP, Jackson RL (1972) Molecular weight determination of glycoproteins by polyacrylamide gel electrophoresis in sodium dodecyl sulfate. Methods Enzymol 28:54–63. doi:10.1016/0076-6879(72)28007-7
Silva CHC, Puls J, Sousa MV, Filho EXF (1999) Purification and characterization of a low molecular weight xylanase from solid-state cultures of Aspergillus fumigatus Fresenius. Rev Microbiol 30:114–119. doi:10.1590/S0001-37141999000200005
Sun J, Tian C, Diamond S, Louise Glassa N (2012) Deciphering transcriptional regulatory mechanisms associated with hemicellulose degradation in Neurospora crassa. Eukaryot Cell 11:482–493. doi:10.1128/EC.05327-11
Tenkanen M, Vršanská M, Siika-Aho M et al (2013) Xylanase XYN IV from Trichoderma reesei showing exo- and endo-xylanase activity. FEBS J 280:285–301. doi:10.1111/febs.12069
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. Method Enzymol 161:238–249. doi:10.1016/0076-6879(88)61025-1
Turner P, Mamo G, Karlsson E (2007) Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact 6:9. doi:10.1186/1475-2859-6-9
Vieille C, Zeikus JG (1996) Thermozymes: identifying molecular determinants of protein structural and functional stability. Trends Biotechnol 14:183–190. doi:10.1016/0167-7799(96)10026-3
Viikari L, Alapuranen M, Puranen T, Vehmaanperä J, Siika-Aho M (2007) Thermostable enzymes in lignocellulose hydrolysis. Adv Biochem Eng Biotechnol 108:121–145. doi:10.1007/10_2007_065
Wakiyama M, Yoshihara K, Hayashi S, Ohta K (2010) An extracellular endo-1,4-beta-xylanase from Aspergillus japonicus: purification, properties, and characterization of the encoding gene. J Biosci Bioeng 109:227–229. doi:10.1016/j.jbiosc.2009.09.005
Wassenberg D, Schurig H, Liebl W, Jaenicke R (1997) Xylanase XynA from the hyperthermophilic bacteriaThermotoga maritima: structure and stability of the recombinant enzyme and its isolated cellulose binding-domain. Protein Sci 6:1718–1726. doi:10.1002/pro.5560060812
Wong KK, Tan LU, Saddler JN (1988) Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev 52:305–317
Yuan SF, Wu TH, Lee HL, Hsieh HY, Lin WL, Yang B, Chang CK, Li Q, Gao J, Huang CH, Ho MC, Guo RT, Liang PH (2008) Biochemical characterization of a novel thermostable beta-1,3-1,4-glucanase (lichenase) from Paecilomyces thermophila. J Agric Food Chem 56:5345–5351. doi:10.1021/jf800303b
Zhao Y, Meng K, Luo H, Huang H, Yuan T, Yang P, Yao B (2013) Molecular and biochemical characterization of a new alkaline active multidomain xylanase from alkaline wastewater sludge. World J Microbiol Biotechnol 29:327–334. doi:10.1007/s11274-012-1186-z
Zouari-Mechichi H, Mechichi T, Dhouib A, Sayadi S, Martínez AT, Martínez MJ (2006) Laccase purification and characterization from Trametes trogii isolated in Tunisia: decolorization of textile dyes by the purified enzyme. Enzyme Microb Technol 39:141–148. doi:10.1016/j.enzmictec.2005.11.027
Acknowledgements
The project was funded by CINVESTAV-IPN. Y. G. G-H was recipient of a doctoral fellowship (203140) from the Consejo Nacional de Ciencia y Tecnología, México.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by F. Robb.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
García-Huante, Y., Cayetano-Cruz, M., Santiago-Hernández, A. et al. The thermophilic biomass-degrading fungus Thielavia terrestris Co3Bag1 produces a hyperthermophilic and thermostable β-1,4-xylanase with exo- and endo-activity. Extremophiles 21, 175–186 (2017). https://doi.org/10.1007/s00792-016-0893-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-016-0893-z