Abstract
A recently discovered thermophilic isolate, Geobacillus sp. R7, was shown to produce a thermostable cellulase with a high hydrolytic potential when grown on extrusion-pretreated agricultural residues such corn stover and prairie cord grass. At 70°C and 15–20% solids, the thermostable cellulase was able to partially liquefy solid biomass only after 36 h of hydrolysis time. The hydrolytic capabilities of Geobacillus sp. R7 cellulase were comparable to those of a commercial cellulase. Fermentation of the enzymatic hydrolyzates with Saccharomyces cerevisiae ATCC 24860 produced ethanol yields of 0.45–0.50 g ethanol/g glucose with more than 99% glucose utilization. It was further demonstrated that Geobacillus sp. R7 can ferment the lignocellulosic substrates to ethanol in a single step that could facilitate the development of a consolidated bioprocessing as an alternative approach for bioethanol production with outstanding potential for cost reductions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global trend for production of cellulosic bioethanol from renewable resources is currently driven by three important factors: (1) increasing demand and prices of petroleum-derived fuel; (2) increasing food needs; and (3) increasing greenhouse gas emissions. As reported, nearly 1.3 billion dry tons of biomass could be available for large-scale bioenergy and biorefinery industries, enough to displace 30% or more of the nation’s current consumption of liquid transportation fuels (Perlack et al. 2005). In 2009, the US produced 10.75 billion gallons of ethanol (mainly corn-based), and together with Brazil, both countries accounted for 89% of the world’s production. The new US Renewable Fuels Standard of 2008 calls for the production of 36 billion gallons of biofuels, mainly ethanol and biodiesel, annually by 2022, with 21 billion gallons coming from “advanced biofuels,” of which 16 billion gallons is expected as “cellulosic biofuels” derived from lignocellulosic biomass. Cellulosic biofuels as a carbon–neutral technology have to achieve at least 60% lower emissions than petroleum fuel based on the lifecycle studies that include all emissions resulting from making the fuel from the field to the tank. Although significant progress has recently been made towards commercialization of cellulosic ethanol, there are still economic, social and environmental challenges that need to be addressed.
The bioethanol strategies need to be based on a thorough assessment of opportunities and costs associated with the upward pressure on food prices, intensified competition for land and water, and deforestation. As the feedstock costs comprise more than 20% of the production costs, it has now been recognized that biomass waste such as agricultural waste can provide a cost-effective alternative to improve the economic viability of bioethanol production (Nwabueze and Otunwa 2006). However, among the bottlenecks in the development of a cost-effective process for cellulosic ethanol production are the slow rates of enzyme hydrolysis of lignocellulose, the relatively high enzyme production costs and low ethanol yields. It has been shown that consolidated bioprocessing (CBP) has the potential to provide the lowest cost route for biological conversion of biomass to ethanol by combining cellulase production, cellulose hydrolysis and fermentation in one step (Lynd et al. 2005). From this perspective, the search for thermophilic cellulolytic organisms and ethanologens and the discovery of novel thermostable enzymes with enhanced capabilities for cellulose degradation may lead to significant improvements in the bioethanol process (Buckley and Wall 2006). Owing to the improved substrate solubility and mass transfer rates, thermostable cellulases provide greater stability and reaction rates in the enzymatic hydrolysis of cellulose thereby decreasing the amount of enzyme needed (Blumer-Schuette et al. 2008). Added benefits are the decreased risk of contamination, improved substrate accessibility to cellulases and reduced viscosity of feedstock allowing the use of higher solid loadings (Kumar and Wyman 2008). Biomass suspensions become viscous and difficult to mix and transport at slurry concentrations above 10% and large volumes of water needed to reduce viscosity and provide a flowable slurry that limits the ethanol titers to 3–5% (Hahn-Hägerdal et al. 2005). Biomass solids loadings of at least 15% are necessary to minimize the downstream distillation costs for product recovery that would provide the advantages of (1) increased productivity and product concentration resulting in lower downstream processing costs, (2) lower energy requirements for heating and cooling resulting in lower operating costs, (3) lower water usage due to reduced volume resulting in reduced disposal, treatment and capital costs (Hodge et al. 2009).
The objective of this work was to examine the potential of a recently discovered cellulolytic extremophile, Geobacillus R7 (Rastogi et al. 2009), for its ability to hydrolyze and ferment agricultural residues of corn stover and prairie cord grass to ethanol. Geobacillus sp. R7, a facultative anaerobic bacterium that showed a high 16S rDNA sequence homology (99%) to the genus Geobacillus, was isolated from soil samples collected from the 4,850 ft level below the surface of the Homestake gold mine, lead, South Dakota (now known as the NSF Deep Underground Science and Engineering Laboratory, DUSEL). We reported that the crude cellulase of this isolate had a temperature and pH optimum of 75–80°C and 5.0, respectively, and exhibited a remarkable thermostability retaining 50% of its initial cellulase activity after incubation at 70°C for 7 days (Rastogi et al. 2009). In this study, the hydrolytic potential of Geobacillus R7 thermostable cellulase was evaluated at solids loadings of up to 20%. The glucose recovery and ethanol yields were compared with those produced by a commercial cellulase in ethanol fermentation studies using Saccharomyces cerevisiae ATCC 24860.
Materials and methods
Pretreatment and analysis of feedstock
Corn stover (CS) and prairie cord grass (PCG) were thermo-mechanically pretreated using a single screw extruder (Brabender Plasti-corder Extruder Model PL2000, Hackensack, NJ). During extrusion, the screw speed of the extruder and the barrel temperature were maintained at 100 rpm and 100°C, respectively. The chemical composition of both substrates was analyzed by Olson Biochemistry Laboratories, South Dakota State University, Brookings, SD, USA using standard chromatography methods.
Glucose recovery from feedstock
Pretreated corn stover (PCS) and pretreated prairie cord grass (PPCG) were acid hydrolyzed as described in TAPPI Test Methods (1984). Samples of 0.35 g (dry weight) were treated with 3 ml of 72% sulfuric acid (v/v), stirred and placed in a 30°C water bath for 1 h with occasional stirring. Thereafter, each sample was washed with 84 mL of water in a 250 mL capacity flask and the whole content was autoclaved at 103°C (15 psi) for 1 h. After autoclaving, samples were cooled to room temperature using an ice bath and neutralized with alkali. The filtered hydrolyzates were then analyzed for glucose on a 2700 Biochemistry Analyzer (YSI Life Sciences, Yellow Spring, OH, USA) according to the manufacturer’s instructions. The acid hydrolysis of PCS and PPCG produced glucose yields of 0.258 and 0.176 g glucose/g dry matter, respectively. These glucose yields, obtained as described above, served as control of 100% glucose recovery from PCS and PPCG. The efficiency of enzymatic hydrolysis was evaluated based on the comparison of the respective glucose recovery yields to the PCS and PPCG controls and expressed as percentage of these controls.
Cellulase production
Geobacillus sp. R7 was maintained on minimal salt (MS) agar slants at 4°C and as glycerol stock at −20°C. Cellulase was produced under aerobic conditions at 60°C, pH 7.0 and 150 rpm for 6 days in a MS medium containing (per liter): 0.5 g microcrystalline cellulose (MCC) (or 0.5 g CS, PCS, PCG or PPCG), 0.1 g nitrilotriacetic acid, 1 ml FeCl3 solution (0.03%), 0.05 g CaCl2·2H2O, 0.1 g MgSO4·7H2O, 0.01 g NaCl, 0.01 g KCl, 0.3 g NH4Cl, 1.8 g H3PO4 (85%), 0.005 g methionine, 0.05 g yeast extract, 0.01 g casamino acids and 1 mL of Nitsch’s trace element solution (Rastogi et al. 2009). Before use in enzyme assays and enzymatic hydrolysis, the crude enzyme was partially purified by ammonium sulfate precipitation (80% saturation) followed by dialysis against 100 mM acetate buffer (pH 6).
Cellulase assays
The cellulase activity was assayed on carboxymethyl cellulose (CMC) as a substrate. A suitably diluted enzyme (0.1 mL) was incubated with 0.5 mL of CMC solution (1%, w/v) in acetate buffer (0.1 M, pH 6) at 70°C for 20 min. The reaction was stopped by addition of 1.0 mL of 3,5-dinitrosalicylic acid (DNS). The mixture was boiled for 10 min and allowed to cool to room temperature. Reducing sugars (glucose) were determined by measuring the absorbance at 540 nm (Miller 1959). One unit (U) of enzyme activity was defined as the amount of enzyme that released 1 μmol of glucose per min under the assay conditions. Likewise, FPase activity was measured with 0.1 g of Whatman filter paper No. 1 as a substrate (Ghose 1987). One filter paper unit (FPU) was defined as the amount of enzyme that released 1 μmol of glucose per min under the assay conditions.
The β-glucosidase activity was determined using a modified method of Deshpande et al. (1988). p-Nitrophenol-β-d-glucopyranoside (pNPG) (2.5 mg/mL) dissolved in 0.1 M acetate buffer (pH 6) was used as a substrate for the β-glucosidase assay. The assay mixture contained 80 μL of pNPG in 100 μl 0.1 M acetate buffer (pH 6), and 20 μL of enzyme solution. The control contained 20 μL of distilled water in place of the enzyme solution. The reaction was carried out at 60°C for 30 min and then stopped by the addition of 100 μL of sodium carbonate (2%, w/v). The p-nitrophenol released was determined by measuring the absorbance of each sample at 410 nm against the control using a Spectronic GENESYS 10 UV–visible spectrophotometer (Thermo Electron Corporation, Madison, WI, USA). One unit of enzyme activity was defined as the amount of enzyme that liberated 1 μmol of p-nitrophenol per min under the assay conditions.
Electrophoresis
The crude and partially purified enzymes were subjected to denaturation using sodium-dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) on 10% (w/v) gels according to Holt and Hartman (1994). After electrophoresis, gels were silver-stained for protein (Chevallet et al. 2006) using protein molecular weight markers of 10–225 kDa purchased from Promega Corporation, Madison, WI, USA.
Enzymatic hydrolysis of feedstock
PCS and PPCG were hydrolyzed with the Geobacillus sp. R7 partially purified cellulase (19.4 FPU/mL) and a commercial cellulase complex NS50013 (58.3 FPU/mL) obtained from Novozymes (Bagdsvaerd, Denmark). The enzyme hydrolysis was carried out using a dosage of 15 FPU/g glucan at 70°C (Geobacillus cellulase) and 50°C (NS50013), 5–20% solids loadings, pH 6.0 (0.1 M acetate buffer) and 150 rpm. Two sets of cellulase hydrolysis experiments were performed: short hydrolysis (36 h) and long hydrolysis (96 h). During hydrolysis, samples were withdrawn at 12-h intervals and analyzed for glucose as described above.
Ethanol fermentation
S. cervisiae ATCC 24860, grown in yeast and mold (YM) broth, was used to ferment the enzyme-derived hydrolyzates of PCS and PPCG to ethanol. Yeast extract (0.3%), peptone (0.3%) and inoculum (1 mL) of S. cerevisiae were added to 50 mL screw cap bottles containing 25 mL of enzymatic hydrolyzate. The bottles were incubated at 30°C and 150 rpm for 72 h. Following incubation, ethanol yields and residual glucose were measured using 2700 Biochemistry Analyzer (YSI Life Sciences, Yellow Spring, OH, USA) as per manufacturer’s instructions.
Consolidated bioprocessing
CBP of PCS and PPCG to ethanol was carried out with Geobacillus sp. R7. 1 mL of R7 inoculum, produced in a MS medium (pH 7) at 60°C for 48 h, was added to serum bottles containing 50 mL MS medium with 2% (w/v) PCS or PPCG as sole carbon source. All bottles were incubated at 60°C and 150 rpm for up to 216 h. Samples were withdrawn at regular time intervals of 24 h and analyzed for cellulase activity and ethanol concentration as described above. The morphology of PCS and PPCG before and after enzymatic hydrolysis was observed using an inverted microscope (Olympus Model 1X70, Melville, NY, USA) with epifluorescence capability, phase contrast optics and Nowarski optics.
Scanning electron microscopy
The morphology and cellulose complex of Geobacillus sp. R7 on MCC was observed using a scanning electron microscope (SEM) model SUPRA40VP (Zeiss, Thornwood, NY, USA) equipped with an SE2 detector. The samples for SEM analysis were prepared according to Deflaun et al. (2006).
Results and discussion
Pretreatment
The thermomechanical extrusion of CS and PCG appeared to have a positive effect on the cellulase production by Geobacillus sp. R7 (data not shown). For instance, pretreatment of CS resulted in a 6.3-fold increase in the cellulase activity (from 22 U/L, on CS, to 138 U/L, on PCS). This represented a 2.1-fold increase over the maximum cellulase activity of 65 U/L produced when R7 was grown on MCC. Pretreatment of PCG had no significant effect on the cellulase activity (data not shown). Trichoderma reesei is the most commonly used fungus for cellulase production. Juhasz et al. (2005) produced 1.5 U/mL endoglucanase, 1.2 FPU/mL FPase and 0.75 U/mL β-glucosidase activities after 7 days of incubation of T. reesei RUT-C30 on steam-pretreated CS. A recent review of pretreatment methods claimed that the positive effect on biomass bioprocessing was due to the partial removal of lignin (Kumar et al. 2009). However, in our work, the pretreatment effect could not be explained by the change of the chemical composition of PCS (32.3% cellulose, 23.8% hemicellulose and 18.3% lignin) and PPCG (30, 24.3 and 18%, respectively) which remained relatively unchanged following extrusion (data not shown).
Consolidated bioprocessing
As shown in Fig. 1, under limited oxygen (microaerophilic) conditions, in addition to cellulase, Geobacillus sp. R7 produced minimal amounts of ethanol: up to 0.023 g/L (on PCG) and 0.035 g/L (on PPCG). The cellulase production was found to be growth-dependent and maximum growth was observed with PPCG. This is a very interesting observation as it indicates that R7 has the ability to grow on PCS and PPCG and is potent in the simultaneous production of cellulase, enzymatic hydrolysis and ethanol fermentation in a single step—a prerequisite for the establishment of a CBP as an alternative approach for bioethanol production with outstanding potential for cost reductions. Geobacillus sp. has been described to ferment glucose and xylose to 0.1 g/L of ethanol (Riyanti and Rogers 2009). Furthermore, Geobacillus thermoglucosidasius was metabolically engineered to produce a high ethanol yield of 0.47 g ethanol/g cellobiose substrate (Cripps et al. 2009). However, to the best of our knowledge, this work reports for first time in literature on the CBP capabilities of Geobacillus. Only a few anaerobic bacteria, such as Clostridium thermocellum and C. thermohydrosulfuricum have been previously described as capable of directly converting cellulosic substrates to ethanol (Ng et al. 1981). Because of that CBP has been associated with the genomic structure of Clostridium cultures containing a cellulosome complex. In literature, SEM confirmed the presence of extracellular protuberant structures (cellulosome-resembling structures) in a novel thermophilic cellulolytic bacterium Brevibacillus sp. JXL isolated from swine waste (Liang et al. 2009). However, in our study, a cellulosome complex could not be seen from the SEM image (Fig. 2). Thus, Geobacillus sp. R7 may be operating under a different metabolic pathway for CBP. Under limited oxygen conditions, Geobacillus sp. showed growth and secreted cellulase enzymes. At a later stage, when anaerobic conditions developed following oxygen depletion, the pyruvate decarboxylase and alcohol dehydrogenase enzymes, responsible for ethanol fermentation, may have been activated (Anthony et al. 2010).
Electrophoresis and substrate specificities
The sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) pattern of the crude and partially purified cellulase enzyme (5 µg) revealed that dialysis has led to the removal of low molecular weight proteins (10–30 kDa) from the partially purified sample (Fig. 3). The substrate specificity of the dialyzed enzyme was as follows: 331.9 ± 5.3 U CMCase/L on CMC as substrate, 51.2 ± 3.3 U FPase/L, on Whatmann filter paper, and 15.8 ± 0.1 U β-glucosidase/L, on β-d-glucopyronoside. The FPase is believed to represent exoglucanase activity (Shafique et al. 2004) while CMCase and β-glucosidases exhibit endoglucanase activities (Onyike et al. 2008; De Castro et al. 2010; Bahrin et al. 2011).
Enzymatic hydrolysis
Following enzymatic hydrolysis with Geobacillus R7 cellulase for up to 96 h, a higher glucose recovery from PCS was observed at 5 and 10% solids loadings than 15 and 20%. For instance, at 10% solids, the glucose recovery was 26.7% (36 h) and 31% (96 h). This translated into glucose concentrations in the resultant enzymatic hydrolyzates of 6.9 and 8.0 g/L (Table 1).
In the case of PPCG, the impact of dry matter was less pronounced and after 96 h of hydrolysis the glucose recovery was between 44.8 and 48.7% in all instances (Table 1). As expected, the highest glucose concentrations were detected in the hydrolyzates of 20% solids (Table 1). It is noteworthy that at 15 and 20% solids, a partial liquefaction of biomass was observed after 36 h of enzymatic hydrolysis with both substrates. The partial liquefaction transformed the solid biomass into a flowable slurry which was confirmed by a microscopic observation of the biomass before and after hydrolysis (Fig. 4). The glucose recovery yields ranged from 27.8 to 48.7% after enzymatic hydrolysis for 96 h (Table 1). Extension of hydrolysis time from 36 to 96 h increased the glucose recovery yields in both hydrolyzates by 3.5–10%. For instance, the glucose recovery in the PCS hydrolyzate at 15% solids increased by 3.5% resulting in a net increase in the glucose content of 0.6 g/L, whereas that difference was 3.9% and 1.1 g/L, respectively, for the same hydrolyzate at 20% solids. Comparison of our results to literature is impaired by the lack of information on the use of thermophilic cellulases for high solid hydrolysis of cellulose as most of the work has been carried out with commercial mesophilic enzymes at 45–50°C (Lu et al. 2010; Byung-Hwan and Hanley 2008; Jørgensen et al. 2010).
Ethanol fermentation
Fermentation of the PCS and PPCG hydrolyzates with S. cerevisiae produced ethanol yields in the range of 0.45 to 0.50 g ethanol/g glucose. In all instances, glucose was utilized more than 99%. It is interesting to note that the maximum yields of 0.50 g ethanol/g glucose were obtained at 15% solids with both substrates (Table 1). In comparison, Saha and Cotta (2008) reported 0.49 g ethanol/g sugar from pretreated rice husk at 15% solids by a recombinant E. coli. The ethanol yields reported here are in agreement with those obtained by other researchers using S. cerevisiae strains in fermentation of pretreated agro-waste such as wheat straw (Alfani et al. 2000), corn stover (Ohgren et al. 2007) and palm kernel press cake (Jørgensen et al. 2010).
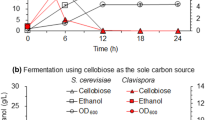
The simultaneous saccharification and fermentation (SSF) process, which combines enzymatic hydrolysis with ethanol fermentation, is recognized as a leading strategy to keep the glucose levels low and avoid enzyme inhibition (Lin and Tanaka 2006). Strain improvement through genetic engineering of yeast to provide pentose-fermenting capabilities for co-fermentation of glucose and xylose is another strategy to improve ethanol yields (Pejo et al. 2008). Also, alcohol production can be further increased by initial adaptation and acclimatization of the fermenting yeast to the specific feedstock (Yu and Zhang 2004). In our study, the glucose and ethanol concentrations, obtained with the Geobacillus sp. R7 cellulase at 15 and 20% PPCG solids, were comparable to those obtained with a commercial enzyme, NS50013 (Fig. 5). This was especially evident after enzymatic hydrolysis for 36 h and is indicative for the enhanced hydrolytic potential that the thermostable cellulase had at elevated temperatures.
Glucose and ethanol production following enzymatic hydrolysis of pretreated prairie cord grass with Geobacillus sp. R7 cellulase and NS50013 (Novozymes) at 15% (a) and 20% (b) solids loadings. Hydrolysis conditions: 15 FPU/g glucan, pH 6, 70°C (Geobacillus sp. R7 cellulase) and 50°C (NS50013). Fermentation conditions: S. cerevisiae ATCC 24860, 30°C, pH 6, 150 rpm, 72 h. Error bars represent the mean of triplicate analysis ± standard deviation
Conclusions
A newly isolated thermophile, Geobacillus sp. R7, was able to grow on lignocellulosic agricultural residues, such as pretreated corn stover or prairie cord grass as sole carbon source and produce a thermostable cellulase with a high hydrolytic potential. At high solid loadings of 15 and 20%, the hydrolytic capabilities of Geobacillus sp. R7 cellulase were comparable, especially in short-time (36 h) enzymatic hydrolysis of biomass to those of a commercial cellulase. This may present an opportunity to significantly reduce the enzymatic hydrolysis time with important implications for reduced size of reactor vessels, increased throughput, reduced water usage, and therefore reduced capital and operating costs of bioethanol production. It was further demonstrated that Geobacillus sp. R7 can ferment lignocellulosic agro-waste to ethanol in a single step which could facilitate the development of a consolidated bioprocessing (CBP) as an alternative approach for bioethanol production with outstanding potential for cost reductions.
References
Alfani A, Gallifuoco F, Saporosi A, Spera A, Cantarella M (2000) Comparison of SHF and SSF process for the bioconversion of steam-exploded wheat straw. J Ind Microbiol Biotechnol 25:184–192
Anthony A, Roger C, Brian R, Martin EK, Milner S, Claire PM (2010) Thermophilic microorganisms for ethanol production. US Patent No. 20100173373:1–23
Bahrin EK, Seng PY, Abd-Aziz S (2011) Effect of oil palm empty fruit bunch particle size on cellulase production by Botryosphaeria sp. under solid state fermentation. Aust J Basic Appl Sci 5:276–280
Blumer-Schuette SE, Kataeva I, Westpheling J, Adams MW, Kelly RM (2008) Extremely thermophilic microorganisms for biomass conversion: status and prospects. Curr Opin Biotechnol 19:210–217
Buckley M, Wall J (2006) Microbial energy conversion. American Society of Microbiology, Washington
Byung-Hwan U, Hanley TR (2008) High-solid enzymatic hydrolysis and fermentation of solka floc into ethanol. J Microbiol Biotechnol 18:1257–1265
Chevallet M, Luche S, Rabilloud T (2006) Silver staining of proteins in polyacrylamide gels. Nat Protoc 1:1852–1858
Cripps RE, Eley K, Leak DJ, Rudd B, Taylor M, Todd M, Boakes S, Martin S, Atkinson T (2009) Metabolic engineering of Geobacillus thermoglucosidasius for high yield ethanol production. Metab Eng 11:398–408
De Castro AM, Ferreira MC, da Cruz JC, Pedro KCNR, Carvalho DF, Leite SGF, Pereira N (2010) High-yield endoglucanase production by Trichoderma harzianum IOC-3844 cultivated in pretreated sugarcane mill byproduct. Enzyme Res 2010:1–8
Deflaun MF, Fredrickson JK, Dong H, Pfiffner SM, Onstott TC, Balkwill DL, Streger SH, Stackebrandt E, Knoessen S, van Heerden E (2006) Isolation and characterization of a Geobacillus thermoleovorans strain from an ultra-deep South African gold mine. Syst Appl Microbiol 30:152–164
Deshpande MV, Pettersson LG, Erikssom (1988) Selective assay for exo-1, 4-β-glucosidases. Methods Enzymol 110:126–130
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:267–268
Hahn-Hägerdal B, Karhumaa K, Larsson CU, Gorwa-Grauslund MF, Görgens J, van Zyl WH (2005) Role of cultivation media in the development of yeast strains for large scale industrial use. Microb Cell Factor 4:31
Hodge DB, Karim MN, Schell DJ, McMillan JD (2009) Model-based fed-batch for high-solids enzymatic cellulose hydrolysis. Appl Biochem Biotechnol 152:88–107
Holt SM, Hartman PA (1994) A zymogram method to detect endoglucanases from Bacillus subtilis, Myrothecium verrucaria and Trichoderma reesei. J Ind Microbiol Biotechnol 13:2–4
Jørgensen H, Sanadi AR, Felby C, Lange NEK, Fischer M, Ernst S (2010) Production of ethanol and feed by high dry matter hydrolysis and fermentation of palm kernel press cake. Appl Biochem Biotechnol 161:318–332
Juhasz T, Szengyel Z, Reczey K, Siika-Aho M, Viikari L (2005) Characterization of cellulases and hemicellulases produced by Trichoderma reesei on various carbon sources. Process Biochem 40:3519–3525
Kumar R, Wyman CE (2008) Effect of Enzyme supplementation at moderate cellulase loadings on initial glucose and xylose release from corn stover solids pretreated by leading technologies. Biotechnol Bioeng 102:457–467
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Liang Y, Yesuf J, Schmitt S, Bender K, Bozzola J (2009) Study of cellulases from a newly isolated thermophilic and cellulolytic Brevibacillus sp. strain JXL. J Ind Microbiol Biotechnol 36:961–970
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642
Lu Y, Wang Y, Xu G, Chu J, Zhuang Y, Zhang S (2010) Influence of high solid concentration on enzymatic hydrolysis and fermentation of steam-exploded corn stover biomass. Appl Biochem Biotechnol 160:360–369
Lynd LR, Van Zyl WH, McBride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16:577–583
Miller GL (1959) Use of dinitro salicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Ng TK, Bassat AB, Zeikus JG (1981) Ethanol production by thermophilic bacteria: fermentation of cellulosic substrates by cocultures of Clostridium thermocellum and Clostridium thermohydrosulfuricum. Appl Environ Microbiol 41:1337–1343
Nwabueze TU, Otunwa U (2006) Effect of supplementation of African breadfruit (Treculia africana) hulls with organic wastes on growth characteristics of Saccharomyces cerevisiae. Afr J Biotechnol 5:1494–1498
Ohgren K, Bura R, Lesnicki G, Saddler J, Zacchi G (2007) A comparison between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using steam-pretreated corn stover. Process Biochem 42:834–839
Onyike E, Auta R, Nok AJ (2008) Isolation, partial purification and characterization of endoglucanase (EC.3.2.1.4) from Aspergillus niger S.L.1 using corn cobs as carbon source. Nigerian J Biochem Mol Biol 23(2):1–11
Pejo ET, Oliva JM, Ballesteros M, Olsson L (2008) Comparison of SHF and SSF processes from steam-exploded wheat straw for ethanol production by xylose-fermenting and robust glucose-germinating Saccharomyces cerevisiae strains. Biotechnol Bioeng 100:1112–1131
Perlack RD, Wright LL, Turhollow AF, Graham RL, Stokes BJ, Erbach DC (2005) Biomass as feedstock for a bioenergy and bioproducts industry: the technical feasibility of a billion-ton annual supply. Oak Ridge National Laboratory, USDA
Rastogi G, Muppidi GL, Gurram RN, Adhikari A, Bischoff KM, Hughes SR, Apel WA, Bang SS, Dixon DJ, Sani RK (2009) Isolation and characterization of cellulose-degrading bacteria from the deep subsurface of Homestake Mine, Lead, South Dakota, USA. J Ind Microbiol Biotechnol 36:585–598
Riyanti EI, Rogers PL (2009) Kinetic evaluation of ethanol-tolerant thermophile Geobacillus thermoglucosidasius M10EXG for ethanol production. Indonesian J Agric Sci 10:34–41
Saha BC, Cotta MA (2008) Lime pretreatment, enzymatic saccharification and fermentation of rice hulls to ethanol. Biomass Bioenergy 32:971–977
Shafique S, Asgher M, Sheikh MA, Asad MJ (2004) Solid state fermentation of banana stalk for exoglucanase production. Int J Agric Biol 6:488–491
TAPPI Test Methods (1984) Carbohydrate composition of extractive-free wood and wood pulp by gas liquid chromatography. Technical Association of the Pulp Paper Industry, T249cm-85:1–8
Yu Z, Zhang H (2004) Ethanol fermentation of acid-hydrolyzed cellulosic pyrolysate with Saccharomyces cerevisiae. Bioresour Technol 93:199–204
Acknowledgments
Financial support by the Center for Bioprocessing R&D (CBRD) at the South Dakota School of Mines and Technology (SDSM&T), the South Dakota Board of Reagents (SD BOR) and the Governor’s Office for Economic Development (GOED) of South Dakota, USA, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Huang.
Rights and permissions
About this article
Cite this article
Zambare, V.P., Bhalla, A., Muthukumarappan, K. et al. Bioprocessing of agricultural residues to ethanol utilizing a cellulolytic extremophile. Extremophiles 15, 611 (2011). https://doi.org/10.1007/s00792-011-0391-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00792-011-0391-2