Abstract
Three thermophilic strains of chemolithoautotrophic Fe(III)-reducers were isolated from mixed sediment and water samples (JW/KA-1 and JW/KA-2T: Calcite Spring, Yellowstone N.P., WY, USA; JW/JH-Fiji-2: Savusavu, Vanu Levu, Fiji). All were Gram stain positive rods (∼0.5 × 1.8 μm). Cells occurred singly or in V-shaped pairs, and they formed long chains in complex media. All utilized H2 to reduce amorphous iron (III) oxide/hydroxide to magnetite at temperatures from 50 to 75°C (opt. ∼73°C). Growth occurred within the pH60C range of 6.5–8.5 (opt. pH60C 7.1–7.3). Magnetite production by resting cells occurred at pH60C 5.5–10.3 (opt. 7.3). The iron (III) reduction rate was 1.3 μmol Fe(II) produced × h-1 × ml−1 in a culture with 3 × 107 cells, one of the highest rates reported. In the presence or absence of H2, JW/KA-2T did not utilize CO. The G + C content of the genomic DNA of the type strain is 52.7 ± 0.3 mol%. Strains JW/KA-1 and JW/KA-2T each contain two different 16S rRNA gene sequences. The 16S rRNA gene sequences from JW/KA-1, JW/KA-2T, or JW/JH-Fiji-2 possessed >99% similarity to each other but also 99% similarity to the 16S rRNA gene sequence from the anaerobic, thermophilic, hydrogenogenic CO-oxidizing bacterium ‘Carboxydothermus restrictus’ R1. DNA–DNA hybridization between strain JW/KA-2T and strain R1T yielded 35% similarity. Physiological characteristics and the 16S rRNA gene sequence analysis indicated that the strains represent two novel species and are placed into the novel genus Thermolithobacter within the phylum ‘Firmicutes’. In addition, the levels of 16S rRNA gene sequence similarity between the lineage containing the Thermolithobacter and well-established members of the three existing classes of the ‘Firmicutes’ is less than 85%. Therefore, Thermolithobacter is proposed to constitute the first genus within a novel class of the ‘Firmicutes’, Thermolithobacteria. The Fe(III)-reducing Thermolithobacter ferrireducens gen. nov., sp. nov. is designated as the type species with strain JW/KA-2T (ATCC 700985T, DSM 13639T) as its type strain. Strain R1T is the type strain for the hydrogenogenic, CO-oxidizing Thermolithobacter carboxydivorans sp. nov. (DSM 7242T, VKM 2359T).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaerobic, microbially-mediated processes, such as carbon monoxide oxidation and autotrophic iron (III) reduction, may have been important on primitive Earth, which contained a reduced atmosphere and very low levels of oxygen (Lovley 1991; Gold 1992). Increasingly biotic dissimilatory iron reduction has become a topic of interest due to its possible role in biogeochemical cycling and potential importance in the evolution of microbial life (Lovley 1991).
Iron is the third most abundant element in the Earth’s crust (McGeary and Plummer 1997), and it is generally believed that iron-reducing bacteria are more abundant in nature than their representation in culture collections. Of particular interest are thermophilic, iron-reducing, chemolithoautotrophs, which could have been involved in the precipitation of ancient Banded Iron Formations. Bacteria from a variety of phylogenetic groups are capable of iron reduction (Boone et al. 1995; Lonergan et al. 1996; Slobodkin et al. 1997, 1999). Three out of eight published thermophilic, iron-reducing bacteria (Table 1) belong to the Gram-type positive ‘Firmicutes’ (Gibbons and Murray 1978; Garrity et al. 2002). Others belong to the Gram-type negative Flexistipes, Deferribacter thermophilus (Greene et al. 1997); Proteobacteria, ‘Geothermobacterium ferrireducens’ (Kashefi et al. 2002b) and Geothermobacter ehrlichii (Kashefi et al. 2003); Thermatogales, Thermotoga maritima (Huber et al. 1986; Vargas et al. 1998); and the Deinococcus-Thermus clade, Thermus scotoductus (Balkwill et al. 2004). In addition, two archaeal, thermophilic iron-reducers are known: Geoglobus ahangari (Kashefi et al. 2002a), and Pyrobaculum islandicum (Kashefi and Lovley 2000).
Autotrophic, dissimilatory iron reduction was first demonstrated for Thermoterrabacterium ferrireducens and Thermoanaerobacter siderophilus (Slobodkin et al. 1997, 1999). Moreover, Slobodkin and Wiegel (1997) showed that several different Fe(III)-reducing microorganisms must exist growing at temperatures up to 90°C. Since that time, the autotrophic, dissimilatory iron-reducers Geoglobus ahangari (Kashefi et al. 2002a) and ‘Geothermobacterium ferrireducens’ (Kashefi et al. 2002b) have been isolated, and Pyrobaculum islandicum has been shown to grow autotrophically (Kashefi and Lovley 2000).
Another type of chemolithotrophic growth that often presumably exists in tandem with dissimilatory, chemolithotrophic iron-reduction is hydrogenogenic, CO-oxidation. Members of both groups have been found to inhabit unique microbial communities and both often rely on the energy of reduced inorganic compounds (Slobodkin and Wiegel 1997; Slobodkin et al. 1999). Hydrogenogenic, CO-oxidizing anaerobic prokaryotes base their metabolism on the anaerobic oxidation of carbon monoxide to equimolar hydrogen gas and carbon dioxide production, according to the reaction CO + H2O → CO2 + H2 (ΔG 0 = −20 kJ). The name “hydrogenogens” has been proposed for this physiological group because hydrogen is the only reduced product of their metabolism, and physiological groups of anaerobic prokaryotes are frequently named after the major reduced product of their metabolism (Svetlitchnyi et al. 2001). CO-oxidizing hydrogenogenic prokaryotes belong to the bacterial genera Carboxydothermus (Svetlichnyi et al. 1991, 1994), Caldanaerobacter (Sokolova et al. 2001), Carboxydocella (Sokolova et al. 2002), Thermosinus (Sokolova et al. 2004a) and Thermincola (Sokolova et al. 2005) and to the hyperthermophilic archael genus Thermococcus (Sokolova et al. 2004b). Hydrogenogenic CO-oxidizing prokaryotes have been shown to grow obligately dependent on CO (Sokolova et al. 2002), fermentatively (Sokolova et al. 2001, 2004a), and employing the reduction of Fe(III) and various other electron acceptors (Sokolova et al. 2004a, b; Henstra and Stams 2004).
Three strains of thermophilic iron-reducers were isolated from hot springs in Yellowstone National Park and Fiji and have been partly described (J. Hanel, 2000, thesis, The University of Georgia; Wiegel et al. 2003). Based on their physiological features and 16S rRNA gene sequence analysis they were proposed as ‘Ferribacter thermautotrophicus’ gen. nov., sp. nov. (Wiegel et al. 2003). However, their 16S rRNA gene sequences were similar to the sequence of the hydrogenogenic CO-oxidizing strain R1T, which was described previously as ‘Carboxydothermus restrictus’ sp. nov. (Svetlichnyi et al. 1994). Currently neither name has been validated. The similarity of the above-mentioned strains 16S rRNA gene sequences, and their lack of similarity with other described ‘Firmicutes’ indicate that they should be (1) assigned as two novel species within a novel genus and (2) proposed as a novel lineage (class) within the ‘Firmicutes’. Because the previously suggested names would exclude the specific properties of the other species, we propose to assign them to the novel genus Thermolithobacter, a name which includes the key properties of both species.
Materials and methods
Environmental samples
A combined sample of water, organic filamentous material, and sediment containing 10–15 ppm Fe from a runoff of a hot spring close to the Yellowstone river at the Calcite Spring area from Yellowstone National Park (44°54.291′ N, 110°24.242′ W) contained white and black bacterial filaments. The thermal spring sampling point had a temperature gradient from 60 to 85°C with a pH of 7.6. The sample from Fiji contained water and sediment (Na/K ratio was 25.7, 1,158 ppm Na, 1,606 ppm Ca, 5,250 ppm Cl, 228 ppm SO4, and 0.06 ppm Fe) (Cox 1981; B.K.C. Patel, 1985, thesis, Waikato University, Hamilton, New Zealand) from Nakama springs (main spring; used by local residents for cooking) south of the soccer field in Savusavu on Vanu Levu. The estimated temperature for its underground origin is around 170°C with a pH ∼7.5. Samples were collected in sterile, N2-flushed 100 ml Pyrex jars, capped with butyl rubber stoppers and brought to the laboratory in Athens, GA, where they were stored at 4–7°C for several weeks before the enrichments were started.
Strain R1T was isolated from a terrestrial hot spring at the Raoul Island, Archipelago Kermadeck (New Zealand).
Media and cultivation
Mineral medium was prepared anaerobically by the Hungate technique (Ljungdahl and Wiegel 1986) under an atmosphere of hydrogen and carbon dioxide gases (80:20 v/v), or under 100% CO. It contained (per liter of de-ionized water): 0.33 g KH2PO4, 0.33 g NH4Cl, 0.33 g KCl, 0.33 g MgCl2·2H2O, 0.33 g CaCl2·2H2O, 2.0 g NaHCO3, 1 ml vitamin solution (Wolin et al. 1963), and 1.2 ml trace element solution (Slobodkin et al. 1997). The pH25C was adjusted to 7.0 with 10% (w/v) NaOH under an atmosphere of either hydrogen and carbon dioxide gases (80:20 v/v) or 100% CO. To this mineral medium, 90 mM amorphous Fe(III) oxide/hydroxide (mineral Fe medium; prepared as described by Slobodkin et al. 1997) or 20 mM 9,10-anthraquinone 2,6-disulfonic acid (AQDS) were typically added as electron acceptors. Medium was routinely sterilized by autoclaving at 121°C for 1 h due to the possible presence of heat stable spores (Byrer et al. 2000).
JW/KA-1, JW/KA-2T, and JW/JH-Fiji-2 were isolated from mineral Fe-medium enrichments positive for Fe reduction. Briefly, positive enrichments were used to inoculate (2% v/v) mineral AQDS medium. Agar shake roll tubes using AQDS medium supplemented with 2.5% Bacto agar were inoculated and incubated at 60°C. After 24 h, isolated colonies were picked in an anaerobic chamber (Coy Products) under a N2/H2 atmosphere (95:5 v/v) and used to inoculate AQDS medium. Cultures were incubated for 48 h at 60°C. Those tubes displaying AQDS reduction and cell growth were used to inoculate (2% v/v) mineral Fe medium. After 48 h, tubes displaying black magnetic precipitation and cell growth were repeatedly used for subsequent (nine times for JW/KA-2T) picking of colonies from agar shake- roll-tube-dilution rows to ensure obtaining axenic cultures. The picked colonies were re-suspended in 0.5 ml of pre-reduced media and used to inoculate the subsequent round of agar shake-roll-tube-dilution rows. The cell suspensions were examined microscopically to ensure that they contained mainly single cells and no clumps of cells. Stock cultures were stored in 50% v/v glycerol in AQDS and mineral Fe media at −75°C. For routine use, cultures were maintained in both iron oxyhydroxide and AQDS media.
The enrichments and pure cultures of JW/KA-1, JW/KA-2T, and JW/JH-Fiji-2 were typically grown in Hungate or Balch tubes at 60°C, and all transfers were carried out with disposable syringes and hypodermic needles. No reducing agents were added because the reducing agents dithionite, cysteine, Ti(III) citrate (Gaspard et al. 1998) and HCl-cysteine reduce Fe(III). Strain R1T was grown at 70–72°C in 50 ml serum flasks containing 10 ml of the liquid mineral medium described above, except that AQDS and Fe(III) oxide/hydroxide were omitted (Svetlichnyi et al. 1994). The medium was reduced with 0.5 g/l Na2S 9 H2O under 100% of CO.
Determination of growth
Growth was determined by direct cell counts and visually by determinations of reduced iron (black magnetic precipitation). Besides cell counting and measuring the change in OD600nm, the growth of the CO-utilizing strain was additionally measured by following CO utilization and H2 production employing gas chromatography (Sokolova et al. 2004a).
Microscopy
Light microscopic observations were performed using slides coated by 2% (w/v) ultra pure agar and an Olympus VANOX equipped with phase-contrast objectives. Electron microscopy of negatively stained cells was performed with a JEOL CX-100 transmission electron microscope (Valentine et al. 1968). Light and electron microscopy of strain R1T were performed as described by Svetlichnyi et al. (1994).
Quantification of Fe(II)
Fe(II) was determined employing the 2,2-dipyridyl iron assay of Balashova and Zavarzin (1980). In this assay, all species of iron are dissolved in 0.5 ml of 0.6 M HCl, but only Fe(II) reacts with the added 2,2-dipyridyl. Iron was quantified using an Fe(II) (FeCl2) standard curve.
Temperature and pH ranges for growth
The temperature range for the iron-reducers was determined in both mineral Fe- and AQDS-media at pH25C 7.3 using a temperature gradient incubator (Scientific Industries, Inc., Bohemia, NY) with shaking (∼20 strokes/min). To compensate for the effect of temperature on pH measurements, pH60C was determined with a pH meter calibrated with standards and the electrode equilibrated at 60°C (Wiegel 1998). Sterile anaerobic 1 M NaOH and 3 M HCl were used to adjust the pH of the medium prior to inoculation. The temperature and pH25C ranges for the growth of strain R1T were determined as described by Svetlichnyi et al. (1994) without shaking.
Carbon sources (substrate utilization)
Growth on potential carbon sources was assessed using Hungate tubes containing mineral medium and Fe(III) in which the autotrophic carbon source, CO2, was omitted and various other substrates were supplied. In the case of strain R1T potential carbon sources which could be utilized during growth on H2 were assessed using 50 ml serum flasks containing 10 ml of the same mineral medium, except 20 mM AQDS was supplied instead of Fe(III). Cell numbers and iron or AQDS reduction were used to determine growth. Negative controls contained identical media and carbon supplements but were inoculated with autoclaved suspensions of cells. This control tested the possibility of abiotic Fe(III) or AQDS reduction, which is common with carbohydrates at high temperatures (Slobodkin et al. 1997). To avoid false positive results, cultures were transferred into the same media using 1% (v/v) inocula before being scored as positive.
Electron acceptors
The ability to utilize various electron acceptors was tested in mineral medium without the addition of Fe(III). Utilization was assumed when cell counts increased from 1.0 × 106 to 2.0 × 107 in the second and third subsequent subcultures, and when the reduction of ferric citrate and AQDS was accompanied by changes in color from black to clear (reduced form) and yellow to brown or black (reduced form), respectively. When nitrate, thiosulfate, sulfate or elemental sulfur was added as the potential electron acceptor, the medium was reduced with sodium sulfide. The inoculum was 1% (v/v). A culture without addition of an electron acceptor was used as a control.
Lipid extraction and fractionation
Duplicate samples of strain JW/JH-Fiji-2 (0.5 g/ml) were extracted using a modified Bligh and Dyer (1959) extraction method (White et al. 2005). Lipids were separated on a silicic acid column with chloroform, acetone, and methanol into neutral lipid, glycolipid, and polar lipid fractions (Guckert et al. 1985). Solvents were removed under a gentle stream of nitrogen, and the fractions were stored at −20°C. The neutral lipid fraction was resolved in methanol and analyzed for respiratory quinones by HPLC-tandem mass spectrometry (Geyer et al. 2004). The phospholipid fatty acids (PLFAs) in the polar lipid fraction were derivatized with trimethylchlorosilane in methanol to produce fatty acid methylesters (FAMEs) and analyzed on a GC-MS system after the addition of FAME 19:0 as an internal standard (Geyer et al. 2005).
G + C content
The G + C mol% was determined by the HPLC method of Mesbah et al. (1989). DNA for the analysis was extracted using the mini-BeadBeater DNA extraction system (Biospec Products). DNA from strain R1T was isolated as described by Marmur (1961).
DNA extraction, sequencing, and 16S rRNA gene sequence phylogenetic analysis
Genomic DNA was extracted from cell pellets obtained from cultures of JW/KA-2T, JW/KA-1 and JW/JH-Fiji-2 (after multiple rounds of isolating single cell colonies) using the DNeasy Tissue kit (Qiagen). Purifed DNA was used to amplify the small-subunit rRNA gene by PCR as previously described (Götz et al. 2002). PCR products (approximately 1,500 base pairs) were purified using the PCRpure Spin Kit (Intermountain Scientific, Kaysville, Utah) and cloned into pCR2.1 vector using the TOPO-TA cloning kit (Invitrogen). Plasmid DNA was prepared using an alkaline-lysis method (Sambrook et al. 1989). Sequencing reactions were performed using the ABI PRISM BigDyeTerminator Cycle Sequencing Kit and an ABI 310 Genetic Analyzer (PE Biosystems) according to the manufacturer’s protocol. DNA was extracted, amplified, cloned, and sequenced three different times to note any discrepancies that might arise during these procedures. Three clones were sequenced of each strain to ensure the cultures were pure, and to again note any discrepancies that might exist within the sequences. Additionally, PCR products were sequenced directly to determine whether or not a single product was present. Both strands of clones JW/KA-1, JW/KA-2T, and JW/JH-Fiji- 2 were completely sequenced using a suite of 16S rRNA-specific primers (Vetriani et al. 1999) to generate an overlapping set of sequences which were assembled into one contiguous sequence. Sequence alignments were performed using the software suite ARB (Ludwig et al. 2004). Phylogenetic analysis was performed using 1,307 homologous nucleotides. Using a subset of sequences, and based on manual sequence alignments and secondary structure comparisons, all nucleotides were used to construct distance matrices by pairwise analysis with the Jukes and Cantor correction (Jukes and Cantor 1969). Maximum-likelihood, maximum-parsimony and neighbor-joining analyses were done as previously described (Götz et al. 2002). The FastDNAml (Olsen et al. 1994) tree is presented, and the T value was optimized to T = 1.2. Bootstrap values were based on 1,000 trials.
DNA–DNA hybridization
DNA–DNA hybridization was performed by the German culture collection (DSMZ). DNA was isolated using a French pressure cell (Thermo Spectronic) and purified by chromatography on hydroxyapatite as described by Cashion et al. (1977). DNA–DNA hybridization was carried out as described by De Ley et al. (1970), with the modifications described by Huss et al. (1983), using a model Cary 100 Bio UV/VIS spectrophotometer equipped with a Peltier-thermostatted 6 × 6 multicell changer and a temperature controller with in situ temperature probe (Varian).
Nucleotide sequence accession numbers of novel isolates
The 16S rRNA gene sequences of strain JW/KA-2T sequences A and B and JW/JH-Fiji-2 were deposited into GenBank under the accession numbers AF282253, AF282254, and AF282252, respectively. The sequence of strain R1T was deposited as DQ095862.
Results
Morphology
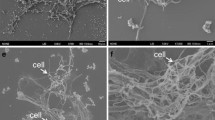
As examined by phase contrast microscopy, the iron-reducing strains JW/KA-1, JW/JH-Fiji-2 and JW/KA-2T cells were slightly curved rods, approximately 1.8 by 0.5 μm. They were usually found in V-shaped pairs, although chains were not uncommon. In mineral Fe-media, tumbling motility was observed during light microscopic imaging of cultures incubated under optimal growth conditions. Electron microscopy revealed the presence of 2–3 peritrichously located flagella (Fig. 1). Spores were never observed by phase contrast or electron microscopy. Cultures were unable to withstand 5 min. of heat treatment at 100°C, thus, heat resistant spores appeared to be absent. Cells stained Gram positive. Colonies formed on the agar surface in roll tubes (mineral medium with AQDS as electron acceptor) and were typically small (1–2 mm in diameter), flat, round, and white in color after 24 h.
The CO-oxidizing strain R1T cells were short, straight rods, motile with peritrichious flagella. They divided by binary fusion, and ultra-thin sections revealed a Gram-type positive cell wall (Wiegel 1981; Svetlichnyi et al. 1994).
Chemolithoautotrophic growth
The iron-reducing strain JW/KA-2T and the CO-oxidizing strain R1T were obligately anaerobic as O2 concentrations of 0.2% in the gas phase were bactericidal. Although the relationship was not strictly proportional, an increase in cell number of strain JW/KA-2T was observed with a concomitant increase in magnetite formation (Fig. 2a) during growth on CO2/H2 with Fe(III) as the electron acceptor. The amount of Fe(II) produced per cell in a growing culture of strain JW/KA-2T was initially high, but steadily decreased as the cells entered the later phases of growth. Both resting cells and growing cells in the culture may have been responsible for Fe(III) reduction. Strain JW/KA-2T utilized CO2, yeast extract, casamino acids, fumarate, and crotonate in the presence of H2. In the absence of H2, JW/KA-2T used formate as an electron donor and Fe(III) oxide/hydroxide, 9,10-anthraquinone 2,6-disulfonic acid, fumarate, and thiosulfate as terminal electron acceptors. None of the iron-reducing strains were able to grow on CO or a CO/H2 mixture. In contrast, strain R1T grew chemolithoautotrophically on CO (Fig. 2b) and produced equimolar quantities of H2 and CO2. The minimal generation time was 1.3 h. However, strain R1T did not grow with either Fe(III) citrate or Fe(III) oxide/hydroxide in mineral media supplied with HCO −3 , acetate, lactate, yeast extract, formate, or citrate under 100% H2 or 100% CO atmosphere. Strain R1T did not grow in media supplemented with AQDS, HCO −3 , acetate, lactate, yeast extract, formate, or citrate under 100% H2, and did not reduce AQDS during growth on CO. Strain R1T did not grow on yeast extract, formate, citrate, lactate or a H2:CO2 mixture in media supplemented with SO 2−4 , S2O 2−3 , or elemental sulfur.
Growth and a Fe(II) formation by JW/KA-2T and b CO utilization/ H2 production by strain R1T. a Cell growth of strain JW/KA-2T (diamond) paralleled Fe(II) accumulation (circle) when cells were grown in a mineral Fe medium at pH25C 7.2 and 60°C as compared to a sterile control (triangle). Gas atmosphere was 80:20 H2–CO2 (v/v). b Cell growth of strain R1T (triangle) was accompanied by CO utilization (diamond) and H2 production (square) when cells were grown in a mineral medium at pH25C 7.2 and 70–72°C. Gas atmosphere was 100% CO
The temperature range for growth of the iron-reducing strain JW/KA-2T at pH60C 7.0 was 50–75°C (opt. 73–74°C) (Fig. 3a); no growth occurred below 47°C or above 77°C. The pH60C range for growth of JW/KA-2T was between 6.5 and 8.5 (opt. ∼7.1–7.3) (Fig. 3b); no growth occurred below pH60C 5.5 or above 9.0. Using a temperature of 72°C and a pH60C of 7.3, the shortest doubling time observed was 46 min.
Influence of incubation a temperature and b pH on growth of JW/KA-2T. Influence of incubation c temperature and d pH on Fe(III) reduction by resting cells of JW/KA-2T. a Fe-medium with a pH60C 7.3 was inoculated with 1 × 106 cells/ml. After 24 h incubation with shaking (20 rpm), cells were counted using a Petroff–Hausser counting chamber. b Fe-medium was inoculated with 1 × 106 cells/ml and incubated at 72°C. After 24 h incubation with shaking (20 rpm), cells were counted using a Petroff–Hausser counting chamber. c Resting cells were inoculated to a concentration of 5 × 107 cells/ml, and incubated for 24 h in mineral media with a pH60C of 7.2 under an 80:20 H2–CO2 (v/v) atmosphere. Fe(II) accumulation (square) was monitored and compared to a sterile control (triangle). A pH meter calibrated at 60°C was used to determine pH of the media. d Resting cells (square) were inoculated to a concentration of 5 × 107 cells/ml, incubated at 60°C with shaking (20 rpm). Fe(II) accumulation was monitored (square) as compared to a sterile control (triangle)
The temperature and pH ranges for magnetite production by resting cells of JW/KA-2T were broader than those for growth. The temperature range (Fig. 3c) was from 50 to 90°C (opt. 74°C) and pH60C range from 5.5 to 10.3 (opt. 7.3) (Fig. 3d).
Experiments to determine an optimal headspace to liquid medium ratio revealed a requirement for ∼1.5 volumes of headspace for each volume of liquid. Large headspace volumes (100:1 and 200:1) resulted in no growth or iron reduction (data not shown). Experiments with varying levels of atmospheric H2 indicated that 100% H2 was optimal for both cell yields and Fe(III) reduction. However, growth was observed at H2 concentrations as low as 0.1% (v/v) in the headspace.
The observed stoichiometry of iron reduction to H2 uptake was 1.3 mol of Fe(II) produced per mol of H2 utilized. The theoretical ratio is 2 mol of Fe(II) produced per 1 mol of hydrogen utilized, but some of the hydrogen was used in reduction of CO2 to biomass (0.35 mol CO2 reduced/mol Fe(III) reduced).
By refeeding the JW/KA-2T cultures at 24 h intervals with H2/CO2 gas mixtures, a maximal cell yield of 108 cells ml-1 was obtained. The bacteria exhibited a high rate of Fe(III) reduction to Fe(II). For example, Thermoterrabacterium ferrireducens (Slobodkin et al. 1997), reduces Fe(III) to Fe(II) at a maximal rate of 0.6 μmol h−1 ml−1 in cultures containing 1.2 × 109 cells. Under optimal growth conditions, strain JW/KA−2T reduced approximately 1.3 μmol of Fe(III) to Fe(II) h−1 ml−1 in cultures containing 3.0 × 107 cells.
Substrate utilization
In the presence of Fe(III) as an electron acceptor and H2 as an electron donor, the iron-reducing strain JW/KA−2T utilized CO2, fumarate (20 mM), yeast extract (1%), casamino acids (1%), and crotonate (20 mM), and (without the addition of H2) formate (20 mM) as carbon sources, but no growth (with or without Fe(III)) on tryptone (1.0%), glucose (20 mM), galactose (20 mM), xylose (20 mM), sucrose (20 mM), propionate (20 mM), starch (5 g/l), sodium acetate (30 mM), succinate (20 mM), lactic acid (20 mM), glycerol (130 mM), cellobiose (20 mM), ethanol (20 mM), 1-butanol (20 mM), 2-propanol (20 mM), acetone (20 mM), phenol (10 mM), ethylene glycol (20 mM), 1,3 propanediol (20 mM), catechol (20 mM) or olive oil (10 ml/l) as carbon and energy sources. In the controls, glucose, sucrose, xylose, cellobiose, galactose, and starch reduced Fe(III) to Fe(II) abiotically at rates similar to those of a growing culture. However, no increase in cell numbers was observed with these substrates; i.e., growth was not observed with these particular substrates.
Electron acceptors
The oxidation of hydrogen gas by the iron-reducing strain JW/KA-2T was coupled to the reduction of Fe(III) to magnetite and siderite, as determined by X-ray diffraction analysis (data not shown). Strain JW/KA-2T also used AQDS (20 mM), thiosulfate (20 mM) and fumarate (20 mM) as electron acceptors but not ferric citrate, nitrate, sulfate (all at 20 mM), elemental sulfur (sublimated, 0.1% w/v), elemental sulfur (precipitated, 0.1% w/v), crystalline iron (III) oxide, 15 mM manganese (IV) added as MnO2 (Kostka and Nealson 1998), 100 μM selenium (VI) added as selenium acetate, 10 μM uranium (VI) added as uranyl acetate, or 100 μM arsenic (V) added as potassium arsenate.
The CO-oxidizing strain R1T did not use either Fe(III) or AQDS as electron acceptors under 100% H2 and it did not reduce AQDS during growth on CO. Strain R1T did not grow on CO in the presence of NO −3 , Fe(III) oxide/hydroxide, Fe(III) citrate, or SO 2−3 . Strain R1T grew on CO in the presence of SO 2−4 , S2O 2−3 , or fumarate, though the presence of the previously mentioned electron acceptors did not stimulate the growth and no indication of the reduction of either SO 2−4 , S2O 2−3 , or elemental sulfur in media supplemented with yeast extract, formate, acetate, pyruvate, citrate, succinate, lactate or H2:CO2, respectively, was observed. Strain R1T also did not use either NO −3 , SO 2−3 , or fumarate as electron acceptor while growing on H2:CO2 (66:34).
Antibiotic susceptibility
During growth at 60°C in mineral iron medium, strain JW/KA-2T was resistant to tetracycline and ampicillin at 100 μg ml-1 (higher concentrations not tested). It was resistant to 10 μg ml−1 rifampicin, erythromycin, streptomycin and chloramphenicol but not at 100 μg ml−1. The latter four were bactericidal at 100 μg ml−1. It should be noted that tetracycline and ampicillin lose their effectiveness rapidly at this incubation temperature (Peteranderl et al. 1990), so the exact concentration of these antibiotics JW/KA−2T is resistant to is unclear. However, due to their bactericidal action they could still be used to grant some selective advantage to strain JW/KA-2T in enrichment cultures. Strain R1T grows with antibiotics as previously described (Svetlichnyi et al. 1994).
Lipid extraction and fractionation
The phospholipid fatty acid (PLFA) profile of strain JW/JH-Fiji-2, which showed 99% 16S rRNA gene sequence similarity to JW/KA-1 and JW/KA-2T, contained the following fatty acids (mol%): i15:0 (32.2 ± 6.4), a15:0 (4.1 ± 0.9), i16:0 (0.8 ± 0.1), 16:0 (15.9 ± 1.0), i17:0 (35.0 ± 4.5), a17:0 (11.2 ± 1.5), and 18:0 (0.8 ± 0.2). The neutral lipid fraction of strain JW/JH-Fiji-2 contained the following respiratory quinones (mol%): demethylmenaquinone-9 (82.7) as the major compound, menaquinone (MK)-4 (2.2), MK-5 (0.7), MK-6 (2.2) and ubiquinone (UQ)-6 (9.0), UQ-7 (0.6), UQ-9 (1.4), UQ-10 (1.2) as minor compounds. The minor quinones, except UQ-9, were also found in the control (culture media) and could derive from passive accumulation in the membrane (R. Geyer, unpublished results).
G + C content
The mean (± SD from three measurements) guanosine (G) plus cytosine (C) content of the genomic DNA of strain JW/KA-2T was 52.7 ± 0.3 mol% G + C as determined by HPLC (Mesbah et al. 1989). Strain R1T DNA G + C content was 52 ± 1 mol% and was determined by melting-point analysis (Marmur and Doty 1962), using Escherichia coli K12 as a reference.
Phylogeny
DNA was extracted, amplified, cloned, and sequenced three different times from strains JW/KA-1, JW/KA-2T, and JW/JH-Fiji- 2. Three clones were sequenced for each of the above three listed strains to ensure that the cultures were pure, and to note any discrepancies that may exist within the sequences. Additionally, PCR products were sequenced directly to determine whether or not a single product was present. Sequencing of the PCR products indicated that there were two 16S rRNA gene sequences, designated A and B, present in both JW/KA-1 and JW/KA-2T, i.e. each strain has two 16S rRNA gene sequences. The two strains differed slightly in the cell and colony morphology at the time of isolation. When comparing the two 16S rRNA gene sequences from one strain, a similarity of 98.9% was observed with the changes distributed in the variable regions throughout the 16S rRNA gene sequence. The sequences were checked from three different colony isolations and three different DNA isolations of each strain after the strains were isolated nine times as a single colony in subsequent agar roll tube cultures. It should be noted here that comparable situations have been reported before, i.e. single-cell-derived pure cultures contained two different 16S rRNA gene sequences with up to 5% inferred differences in substitutions and bracketing different species (Amann et al. 2000; Onyenwoke et al. 2006). For strain JW/JH-Fiji-2, only one 16S rRNA gene sequence was observed. Further isolates need to be obtained and analyzed to test whether the occurrence of two 16S rRNA gene sequences is common among strains of this novel species.
The 16S rRNA gene sequences of strain R1T and strains JW/KA-2T, JW/KA-1 and JW/JH-Fiji-2 possessed 99% sequence similarity. The 16S rRNA gene sequences of JW/KA-2T, JW/KA-1 and JW/JH-Fiji-2 possessed only low similarity to any other previously described thermophilic or mesophilic iron-reducers. The closest neighbors on the 16S rRNA gene sequence-based phylogenetic tree is Thermanaeromonas toyohensis (Fig. 4), as well as the Desulfotomaculum species, several of which are also capable of chemolithoautotrophic growth, and the acetogenic Moorella species, of which two are also able to grow chemolithoautotrophically with CO (Menon and Ragsdale 1996; J. Wiegel, unpublished results). The chemolithoautotrophic, iron-reducing strains do not produce acetate although they share with Moorella species a high (52–56) mol% G + C content.
Phylogenetic tree. Unrooted phylogenetic tree showing the position of Thermolithobacter ferrireducens strains JW/KA-2T, with sequences A and B, and JW/JH-Fiji-2, and T. carboxydivorans strain R1T. Two 16S rRNA gene sequences were also observed for strain JW/KA-1. The tree was generated by maximum-likelihood. Reliability values at each internal branch are percentages obtained from 1,000 trials. The scale bar represents the expected number of changes per sequence position. Thermus aquaticus was used as outgroup. The superscript T denotes that the strain is the type strain of the species
DNA–DNA hybridization
DNA–DNA hybridization between strains R1T and JW/KA-2T was 35%.
Discussion
Iron-reducing strains. Physiologically, the three iron-reducing strains JW/KA-1, JW/KA-2T, and JW/JH-Fiji-2 were different from other iron-reducing, thermophilic bacteria. They required H2 or formate as their electron donor. They were not able to grow chemoorganotrophically. One of the distinguishing features of Thermolithobacter ferrireducens was that it only grew to low cell densities. However, the specific rates of Fe(III) reduction per biomass unit were approximately 10 times higher than those which have been reported for other iron-reducers (Table 2). The 16S rRNA gene sequence analysis has distinguished these strains from other thermophilic iron-reducers, such as Bacillus infernus (which is only capable of iron reduction under heterotrophic conditions) (Boone et al. 1995), Thermoterrabacterium ferrireducens (which utilizes glycerol, and grows to higher densities in similar media) (Slobodkin et al. 1997), and Thermoanaerobacter siderophilus (which also utilizes sulfite and elemental sulfur as an electron acceptor) (Slobodkin et al. 1999). In agreement with the 16S rRNA gene sequence analyses, we propose that the strains JW/KA-1, JW/JH-Fiji-2 and JW/KA-2T represent a novel genus and species, Thermolithobacter ferrireducens.
These microorganisms may also be interesting models for early life on Earth, and possibly extra-terrestrial life forms. Of special note in this regard are their ability to grow autotrophically in mineral medium, the use of Fe(III) as a terminal electron acceptor, and a high Fe(III) reduction rate. Presuming that a reservoir of Fe(III) could be provided, JW/KA-2T would require little more than H2, CO2, trace minerals, and elevated temperatures from geothermal activity to thrive; i.e. as might be present in deep subsurface environments. Properties of Thermolithobacter ferrireducens, i.e. reducing Fe(III) in the range of pH60C 5.5–10.3 and temperature ranges of 50–90°C, indicate that this or closely related organisms could have contributed to geologically important iron reduction, such as forming the Precambrian Banded Iron Formations, especially since the resting cells showed an extended pH and temperature range and high Fe(III) reduction rates. Studies have indicated that, on Earth, ultra-fine grain magnetite is frequently the product of microbial Fe(III)-reduction (Gold 1992). Similar ultra-fine grains of magnetite have been observed in the Martian meteorite ALH84001 (McKay et al. 1996). It is of note to mention Thermoterrabacterium ferrireducens (Slobodkin et al. 1997) was isolated from a hot spring runoff at Calcite Spring a few meters away from the sample site from which strain JW/KA-2T was isolated. However, the sampling site from which strain JW/JH-Fiji-2 was isolated is far apart, which suggests that this bacterium could be widespread in various geothermal environments (Slobodkin and Wiegel 1997).
CO-oxidizing strain. The CO-oxidizing strain R1T was distinguished by 16S rRNA gene sequence analysis from any other known hydrogenogenic, CO-trophic bacteria. Strain R1T differed from Carboxydothermus hydrogenoformans, the type species of the genus to that strain R1T was originally assigned, by the inability of strain R1T to grow employing AQDS reduction and in the ability of strain R1T to grow fermentatively on several carbohydrates while C. hydrogenoformans is unable to grow fermentatively on carbohydrates.
Novel lineage Thermolithobacteria. In spite of their differences, strain R1T and strains JW/KA-1, JW/KA-2T, and JW-Fiji-2 possessed sufficient 16S rRNA gene sequence similarity to assign them to the same genus. Taking into account that strains JW/KA-1, JW/KA-2T, and JW-Fiji-2 only grow chemolithotrophically and strain R1T prefers chemolithoautotrophic growth (cell yield during the growth on CO is 10 times higher than during the growth on glucose, data not shown), we propose Thermolithobacter as a novel genus within the phylum ‘Firmicutes’.
The phylum ‘Firmicutes’ is divided into three classes: the ‘Clostridia’, Mollicutes, and ‘Bacilli’ (Garrity et al. 2002). Levels of 16S rRNA gene sequence similarity between the lineage containing the Thermolithobacter and well-established members of the three classes of the ‘Firmicutes’, i.e. represented by the type species of the type genus of the ‘Bacilli’ and ‘Clostridia’, Bacillus subtilis and Clostridium butyricum, respectively, are less than 85%, and the Thermolithobacter appear to form their own distinct lineage apart from the members of the three existing classes (Fig. 5). Even though Heliobacterium chlorum (a member of class 1) and Alicyclobacillus acidocaldarius (class 3) are the closest neighbors to the Thermolithobacter on the phylogenetic tree (Fig. 5), it should be noted that the classifications for these taxa are still tentative (Garrity et al. 2002). The 16S rRNA gene sequences of representative members of closely related (Schloss and Handelsman 2004) phylogenetic taxa outside the ‘Firmicutes’ were included in the tree (Fig. 5). The tree also shows the phylogenetic position of Thermolithobacter is indicative of a distinct lineage within the ‘Firmicutes’. It is therefore proposed that the Thermolithobacter represent a new class within phylum BXIII ‘Firmicutes’, Thermolithobacteria (Gibbons and Murray 1978; Garrity et al. 2002).
Phylogenetic tree of higher taxa. Phylogenetic tree constructed from the 16S rRNA gene with maximum likelihood correction for synonymous changes using the neighbor-joining algorithm. GenBank accession numbers are indicated in parentheses. The three classes of the phylum ‘Firmicutes’ are indicated. For the ‘Clostridia’ (class 1), the order 1 Clostridiales are indicated by (filled square) while the order 2 ‘Thermoanaerobacteriales’ and order 3 Halanaerobiales are indicated by (open circle) and (open square), respectively. The class ‘Bacilli’ is divided into the order 1 Bacillales (filled diamond) and order 2 ‘Lactobacillales’ (open diamond) while the members of the class Mollicutes are indicated by (filled circle) with the order number indicated; i.e. 1order 1 Mycoplasmatales, 2order 2 Entomoplasmatales, 3order 3 Acholeplasmatales, and 4order 4 Anaeroplasmatales (Garrity et al. 2002). To have a meaningful tree, each order is only represented by the type species of the type genus for the class and for the first family for each of the different orders. Numbers at nodes indicate bootstrap support values for 100 replicates with only values above 75 displayed on the tree. Scale bar denotes number of nucleotide substitutions per site. Escherichia coli was used as the outgroup
Description of Thermolithobacteria classis nov.
Thermolithobacteria (Ther.mo.li.tho.bac.te’ri.a. Gr. adj. thermos, hot; Gr. masc. n. lithos, stone; N. L. bacter masc. equivalent of Gr. neut. n. baktron, rod staff; suff. -ia, ending proposed by Gibbons and Murray (1978) to denote a class; N. L. neut. pl. n. Thermolithobacteria, thermophilic, lithotrophically growing rods).
Class of the ‘Firmicutes’. Segregation of these organisms is warranted by: (1) their distinct phylogenetic lineage within the ‘Firmicutes’, and (2) their obligate, or preferred, chemolithotrophic growth. Description is the same as that of the genus. Type order: Thermolithobacterales.
Description of Thermolithobacterales ord. nov.
Thermolithobacterales (Ther.mo.li.tho.bac.ter’ales. N.L. masc. n. Thermolithobacter, the type genus of the order; suff. -ales, ending to denote order; N.L. fem. pl. n. Thermolithobacterales, the order of the genus Thermolithobacter).
Description is the same as that for the genus. Type family: Thermolithobacteraceae
Description of Thermolithobacteraceae fam. nov.
Thermolithobacteraceae (Ther.mo.li.tho.bac.te.ra’ce.ae. N.L. masc. n. Thermolithobacter, the type genus of the family; N.L. suff. -aceae, ending denoting a family; N.L. fem. pl. n. Thermolithobacteraceae, the family of Thermolithobacter).
Description is the same as that for the genus. Type genus: Thermolithobacter.
Description of Thermolithobacter gen. nov.
Thermolithobacter (Ther.mo.li.tho.bac’ter. Gr. adj. thermos, hot; Gr. masc. n. lithos, stone; N. L. masc. n. bacter equivalent of Gr. neut. n. baktron, rod staff; N. L. masc. n. Thermolithobacter, thermophilic lithotrophically growing rods). Cells are short rods. Gram-type positive cell wall. Bacterium. Obligate anaerobe. Thermophile. Neutrophile. Chemolithotroph. DNA G + C content is 52–53 ± 1 mol%. Type species: Thermolithobacter ferrireducens.
Description of Thermolithobacter ferrireducens sp. nov.
Thermolithobacter ferrireducens (fer.ri.re.du’cens. L.n. ferrum, iron; N.L. part. adj. reducens, leading back, bringing back, and in the chemistry converting to a different state; N. L. part. adj. ferrireducens, reducing iron (III)). Has the characteristics of the genus. Rod-shaped cells, approximately 1.8 by 0.5 μm, occurring in singles, V-shaped pairs or chains. Exhibit tumbling motility via 2–3 peritrichously located flagella; spores not observed. Grows under anaerobic conditions in the presence of H2/CO2 in a temperature range of 50–75°C (opt. ∼73°C). The pH60C range for growth is 6.5–8.5 (opt. 7.1–7.3). Uses as electron acceptor, Fe(III), 9,10-anthraquinone-2,6-disulfonic acid, thiosulfate, and fumarate. No growth occurs with sulfur (precipitated or sublimated), nitrate, sulfate, ferric citrate, crystalline ferric hydroxide, Mn(IV), U(VI), Se(VI) or As(V). Besides growing with H2/CO2, the species is able to grow in the presence of H2 with casamino acids, yeast extract, fumarate, and crotonate, and also without the addition of H2 with formate. Resistant to tetracycline and ampicillin at 100 μg/ml (higher concentrations not tested), and resistant to 10 μg/ml rifampicin, erythromycin, streptomycin and chloramphenicol but not at 100 μg/ml (all tests performed at 60°C in mineral iron medium). The latter four were bactericidal at 100 g/ml. The phospholipid derived fatty acids consist mainly (78%) of terminal branched species i15:0, i17:0, and a17:0 which is consistent with the affiliation to the Gram-type positive bacteria. The most abundant respiratory quinone species is demethylmenaquinone-9 (84%) if grown with yeast extract and H2. The G + C content of the DNA of the type strain is 52.7 ± 0.3 mol%. Type strain is JW/KA-2T (= ATCC 700985T = DSM 13639T), isolated from a mixed sample of geothermally heated sediment and water collected from a hot spring outflow at Calcite Spring in the Yellowstone National Park (Wyoming, USA).
Description of Thermolithobacter carboxidivorans sp. nov.
Thermolithobacter carboxidivorans (car.bo.xy.di.vo’rans. N.L. neut. n. carboxydum carbon monoxide; L. part. adj. vorans devouring, digesting; N.L. part. adj. carboxidivorans, digesting carbon monoxide) has the characteristics of the genus. Cells are short, straight rods, about 0.5 by 1–2 μm, arranged singly or in short chains. Cells are motile due to peritrichous flagella. On solid medium produces round, white, semi-translucent colonies, 1 mm in diameter. Grows within the temperature range from 40 to 78°C (opt. 70°C). The pH for growth ranges from 6.6 to 7.6 (opt. 6.8–7.0). Grows on CO autotrophically, producing hydrogen as the sole reduced product. Growth and CO consumption are inhibited by penicillin, erythromycin, and chloramphenicol but not streptomycin, rifampicin, or tetracycline (all 100 μg/ml). Does not reduce SO 2−4 , S2O 2−3 , or fumarate during growth with CO. Does not reduce SO 2−4 , S2O 2−3 , or elemental sulfur with yeast extract, formate, acetate, pyruvate, citrate, succinate, lactate or H2:CO2. Does not grow on a H2:CO2 mixture in the presence or absence of either NO −3 , SO 2−3 , or fumarate. Does not grow on CO in the presence of Fe(III) citrate, Fe(III) oxide/hydroxide, or NO −3 . The G + C content of the DNA of the type strain is 52 ± 1 mol%.The type strain is strain R1T (= DSM 7242T, = VKM 2359T), isolated from a terrestrial hot spring at Raoul Island, Archipelago Kermadeck.
References
Amann G, Stetter KO, Llobet-Brossa E, Amann R, Anton J (2000) Direct proof for the presence and expression of two 5% different 16S rRNA genes in individual cells of Haloarcula marismortui. Extremophiles 4:373–376
Balashova VV, Zavarzin GA (1980) Anaerobic reduction of ferric iron by hydrogen bacteria. Microbiology 48:635–639
Balkwill DL, Kieft TL, Tsukuda T, Kostandarithes HM, Onstott TC, Macnaughton S, Bownas J, Fredrickson JK (2004) Identification of iron-reducing Thermus strains as Thermus scotoductus. Extremophiles 8:37–44
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Boone DR, Liu Y, Zhao Z, Balkwill D, Drake G, Stevens TO, Aldrich HC (1995) Bacillus infernus sp. nov., an Fe(III)- and Mn(IV)-reducing anaerobe from the deep terrestrial subsurface. Int J Syst Bacteriol 45:441–448
Byrer DE, Rainey FA, Wiegel J (2000) Novel strains of Moorella thermoacetica form unusually heat-resistant spores. Arch Microbiol 174:334–339
Cashion P, Hodler-Franklin MA, McCully J, Franklin M (1977) A rapid method for base ratio determination of bacterial DNA. Anal Biochem 81:461–466
Cox, M (1981) Preliminary geothermal investigation in the Lambasa area,Vanna Levu, Ministry of Lands and Mineral Resources Department, Geothermal Report Number 2, Supplementary sheet number 1, Fiji Government Printing Department, Fiji
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Garrity GM, Johnson KL, Bell J, Searles DB (2002) Taxonomic outline of the Procaryotes. In: Bergey’s manual of systematic bacteriology, 2nd edn, release 3.0 New York: Springer, Berlin Heidelberg New York, pp 1–366. DOI: 10.1007/bergeysoutline200210
Gaspard S, Vazquez F, Holliger C (1998) Localization and solubilization of the iron (III) reductase of Geobacter sulfurreducens. Appl Environ Microbiol 64:3188–3194
Geyer R, Peacock AD, White DC, Lytle C, Van Berkel GJ (2004) Atmospheric pressure chemical ionization and atmospheric pressure photoionization for simultaneous mass spectrometric analysis of microbial respiratory ubiquinones and menaquinones. J Mass Spectrom 39: 922–929
Geyer R, Peacock AD, Miltner A, Richnow HH, White DC, Kästner M (2005) In situ assessment of microbial activity using microcosms loaded with 13C-labelled benzene or toluene. Environ Sci Technol 39:4983–4989
Gibbons NE, Murray RGE (1978) Proposals concerning the higher taxa of the bacteria. Int J Syst Bacteriol 28:1–6
Götz D, Banta A, Beveridge TJ, Rushdi AI, Simoneit BRT, Reysenbach A-L (2002) Persephonella marina gen. nov., sp. nov. and Persephonella guaymasensis sp. nov., two novel thermophilic hydrogen-oxidizing microaerophiles from deep-sea hydrothermal vents. Int J Syst Evol Microbiol 52:1349–1359
Greene AC, Patel BKC, Sheehy A (1997) Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int J Syst Bacteriol 47:505–509
Gold T (1992) The deep, hot biosphere. Proc Natl Acad Sci USA 89:6045–6049
Guckert JB, Antworth CP, Nichols PD, White DC (1985) Phospholipid, ester-linked fatty acid profiles as reproducible assays for changes in prokaryotic community structure of estuarine sediments. FEMS Microbiol Ecol 31:147–158
Henstra AM, Stams AJM (2004) Novel physiological features of Carboxydothermus hydrogenoformans and Thermoterrabacterium ferrireducens. Appl Environ Microbiol 70:7236–7240
Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90°C. Arch Microbiol 144:324–333
Huss VAR, Festl H, Schleifer KH (1983) Studies on the spectrometric determination of DNA hybridization from renaturation rate. Syst Appl Microbiol 4:184–192
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (eds) Mammalian protein metabolism. Academic, New York, pp 21–132
Kashefi K, Lovley D (2000) Reduction of Fe(III), Mn(IV), and toxic metals at 100°C by Pyrobaculum islandicum. Appl Env Microbiol 66:1050–1056
Kashefi K, Tor JM, Holmes DE, Gaw Van Praagh CV, Reysenbach AL, Lovley DR (2002a) Geoglobus ahangari gen. nov., sp. nov., a novel hyperthermophilic archaeon capable of oxidizing organic acids and growing autotrophically on hydrogen with Fe(III) serving as the sole electron acceptor. Int J Syst Evol Microbiol 52:719–728
Kashefi K, Holmes DE, Reysenbach AL, Lovley DR (2002b) Use of Fe(III) as an electron acceptor to recover previously uncultured hyperthermophiles: isolation and characterization of Geothermobacterium ferrireducens gen. nov., sp. nov. Appl Environ Microbiol 68:1735–1742
Kashefi K, Holmes DE, Baross JA, Lovley DR (2003) Thermophily in the Geobacteraceae: Geothermobacter ehrlichii gen. nov., sp. nov., a novel thermophilic member of the Geobacteraceae from the “Bag City” hydrothermal vent. Appl Environ Microbiol 69:2985–2993
Kostka J, Nealson K (1998) Isolation, cultivation and characterization of iron- and manganese-reducing bacteria. In: Burlage RS, Atlas R, Stahl D, Geesey G, Sayler G (eds) Techniques in microbial ecology. Oxford, New York, pp 58–78
Lonergan DJ, Jentre HL, Coates JD, Phillips EJP, Schmidt TM, Lovley DR (1996) Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J Bacteriol 178:2402–2408
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287
Ljungdahl LG, Wiegel J (1986) Working with anaerobic bacteria. In: Demain AL, Solomon NA (eds) Manual of industrial microbiology and biotechnology. American Society for Microbiology, Washington, DC, pp 84–94
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Förster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, König A, Liss T, Lüßmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucl Acids Res 32:1363–1371
Marmur J (1961) A procedure for the isolation of desoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
McGeary D, Plummer C (eds) (1997) Physical geology: Earth revealed. WCW McGraw-Hill, New York
McKay DS, Gibson EK, Thomas-Keprta KL, Vali H, Romanek CS, Clemett SJ, Chillier XD, Maechling CR, Zare RN (1996) Search for past life on Mars: possible relic biogenic activity in martian meteorite ALH84001. Science 273:924–930
Menon S, Ragsdale SW (1996) Unleashing hydrogenase activity in carbon monoxide dehydrogenase/acetyl-CoA synthase and pyruvate:ferredoxin oxidoreductase. Biochemistry 35:15814–15821
Mesbah M, Premachandran U, Whitman W (1989) Precise measurement of the G + C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol 39:159–167
Olsen G, Matsuda H, Hagstrom R, Overbeek R (1994) FastDNAml: a tool for construction of phylogenetic trees of DNA sequences using maximum likelihood. CABIOS 10:41–48
Onyenwoke RU, Lee Y-J, Dabrowski S, Ahring B, Wiegel J (2006) Reclassification of Thermoanaerobium acetigenum X6BT as Caldicellulosiruptor acetigenus X6BT comb. nov. and emendation of the genus description. Int J Syst Evol Microbiol 56:1391–1395
Peteranderl R, Shotts EB, Wiegel J (1990) Stability of antibiotics under growth conditions for thermophilic anaerobes. Appl Environ Microbiol 56:1981–1983
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schloss PD, Handelsman J (2004) Status of the microbial census. Microbiol Mol Biol Rev 68:686–691
Slobodkin AI, Wiegel J (1997) Fe(III) as an electron acceptor for hydrogen oxidation in thermophilic anaerobic enrichment cultures from geothermal areas. Extremophiles 1:106–109
Slobodkin A, Reysenbach AL, Strutz N, Dreier M, Wiegel J (1997) Thermoterrabacterium ferrireducens gen. nov., sp. nov., a thermophilic anaerobic dissimilatory Fe(II)-reducing bacterium from a continental hot spring. Int J Syst Bacteriol 47:541–547
Slobodkin AI, Tourova TP, Kuznetsov BB, Kostrikina NA, Chernyth NA, Bonch-Osmolovskaya EA (1999) Thermoanaerobacter siderophilus sp. nov., a novel dissimilatory Fe(III)-reducing, anaerobic, thermophilic bacterium. Int J Syst Bacteriol 49:1471–1478
Sokolova TG, Gonzalez JM, Kostrikina NA, Chernyh NA, Tourova TP, Kato C, Bonch-Osmolovskaya EA, Robb FT (2001) Carboxydobrachium pacificum gen. nov., sp. nov., a new anaerobic, thermophilic, CO-utilizing marine bacterium from Okinawa Trough. Int J Syst Evol Microbiol 51:141–149
Sokolova TG, Kostrikina NA, Chernyh NA, Tourova TP, Kolganova TV, Bonch-Osmolovskaya E.A (2002) Carboxydocella thermautotrophica gen. nov., sp. nov., a novel anaerobic CO-utilizing thermophile from Kamchatkan hot spring. Int J Syst Evol Microbiol 52:1961–1967
Sokolova TG, Gonzalez JM, Kostrikina NA, Chernyh NA, Slepova TV, Bonch-Osmolovskaya EA, Robb FT (2004a) Thermosinus carboxydivorans gen. nov., sp. nov., a new anaerobic thermophilic, carbon-monoxide-oxidizing, hydrogenogenic bacterium from a hot pool of Yellowstone National Park. Int J Syst Evol Microbiol 54:2353–2359
Sokolova TG, Jeanthon C, Kostrikina NA, Chernyh NA, Lebedinsky AV, Stackebrandt E, Bonch-Osmolovskaya EA (2004b) The first evidence of anaerobic CO oxidation coupled with H2 production by a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Extremophiles 8:317–323
Sokolova TG, Kostrikina NA, Chernyh NA, Kolganova TV, Tourova TP, Bonch-Osmolovskaya EA (2005) Thermincola carboxydiphila gen. nov., sp. nov., a new anaerobic carboxydotrophic hydrogenogenic bacterium from a hot spring of Lake Baikal area. Int J Syst Evol Microbiol 55: 2069–2073
Svetlichnyi VA, Sokolova TG, Gerhardt M, Ringpfeil M, Kostrikina NA, Zavarzin GA (1991) Carboxydothermus hydrogenoformans gen. nov., sp. nov., a CO-utilizing thermophilic anaerobic bacterium from hydrothermal environments of Kunashir Island. Syst Appl Microbiol 14:254–260
Svetlichnyi VA, Sokolova TG, Kostrikina NA, Lysenko AM (1994) A new thermophilic anaerobic carboxydotrophic bacterium Carboxydothermus restrictus sp. nov. Microbiology (English translation of Mikrobiologiya) 63:294–297
Svetlitchnyi VA, Peschel C, Acker G, Meyer O (2001) Two membrane associated NiFeS-carbon monoxide dehydrogenases from the anaerobic carbon monoxide utilizing eubacterium Carboxydothermus hydrogenoformans. J Bacteriol 183:5134–5144
Valentine RC, Shapiro BM, Stadman ER (1968) Regulation of glutamine synthetase XII electron microscopy of the enzyme from E. coli. Biochemistry 7:2143–2152
Vargas M, Kashefi K, Blunt-Harris EL, Lovley DR (1998) Microbiological evidence for Fe(III) reduction on early Earth. Nature 395:65–67
Vetriani C, Jannasch HW, MacGregor BJ, Stahl DA, Reysenbach A-L (1999) Population structure and phylogenetic characterization of marine benthic Archaea in deep-sea sediments. Appl Environ Microbiol 65:4375–4384
White DC, Geyer R, Peacock AD, Hedrick DB, Koenigsberg SS, Sung Y, He J, Loffler FE (2005) Phospholipid furan fatty acids and ubiquinone-8: lipid biomarkers that may protect Dehalococcoides strains from free radicals. Appl Environ Microbiol 71:8426–8433
Wiegel J (1981) Distinction between the gram reaction and the gram type of bacteria. Int J Syst Bacteriol 31:88
Wiegel J (1998) Anaerobic alkalithermphiles, a novel group of extremophiles. Extremophiles 2:257–267
Wiegel J, Hanel J, Aygen K (2003) Chemolithoautotrophic thermophilic iron (III)-reducer. In: Ljungdahl LG, Adams MW, Barton LL, Ferry JG, Johnson MK(ed) Biochemistry and physiology of anaerobic bacteria. Springer, Berlin Heidelberg New York
Wolin EA, Wolin MJ, Wolfe RS (1963) Formation of methane by bacterial extracts. J Biol Chem 238:2882–2886
Acknowledgments
We would like to thank Kaya Aygen for his assistance during the purification of strain JW/KA-2T and Dr. Dorothy Byrer for the electron micrographs. We are grateful to Dr. Christopher Romanek and Robert Thomas for performing the X-ray diffraction analyses. We thank J.P. Euzeby for valuable assistance in using proper nomenclature. JMG acknowledges support from a Ramon y Cajal program and project REN2002–00041 both from the Spanish Ministry of Education and Science. This work was partly supported by Programms of Russian Academy of Sciences “Molecular and cell biology,” “Biosphere and evolution” and in its later stage by an NSF-MCB 0238407 grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Robb.
Rights and permissions
About this article
Cite this article
Sokolova, T., Hanel, J., Onyenwoke, R.U. et al. Novel chemolithotrophic, thermophilic, anaerobic bacteria Thermolithobacter ferrireducens gen. nov., sp. nov. and Thermolithobacter carboxydivorans sp. nov.. Extremophiles 11, 145–157 (2007). https://doi.org/10.1007/s00792-006-0022-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-006-0022-5