Abstract
Objective
To evaluate the efficiency of depigmentation, patient perceptions, and the recurrence rates of physiological gingival pigments during a 2-year follow-up after ablative depigmentation using two laser wavelengths: diode 940 nm and Er,Cr:YSGG 2780 nm.
Materials and methods
Sixty patients exhibiting physiological melanin hyperpigmentation in the buccal maxillary gingiva were randomly divided into two equal groups treated with an Er,Cr:YSGG laser at 2780 nm, and a 940 nm diode laser, respectively. The depigmentation procedure essentially involves the ablation of epithelial tissue exhibiting melanin pigmentation. The intensity and extensity indices of gingival pigments were evaluated at baseline, 1 month, 1 year, and 2 years.
Results
At all time points following depigmentation treatment, oral pigmentation index (OPI) and melanin pigmentation index (MPI) scores were less significantly (p <0.05) compared to the baseline in both groups. Treatment was significantly faster with Er,Cr:YSGG laser and required no anesthesia, with faster healing and less postoperative discomfort after 1-week of treatment, compared to the diode laser treatment (p <0.001). The re-pigmentation intensity and extensity were higher significantly in the Er,Cr:YSGG group than in the diode group at 1 year and 2 years (p <0.05).
Conclusion
Both lasers efficiently removed gingival pigments with comparable clinical outcomes and overall positive patient experience. Diode laser treatment exhibited better long-term stability of gingival color, with a lower incidence of re-pigmentation.
Clinical relevance
The color of the gingiva plays an important role in the esthetics of oral soft tissues and the overall ideal smile. Laser-assisted gingival depigmentation is an effective, comfortable, and reliable technique with good esthetical outcomes. The rate of re-pigmentation was affected by the laser wavelength and the technique used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The color of the oral mucosa varies between individuals and is determined by several factors, including the number and melanogenic activity of the melanocytes, the thickness of the keratinized epithelium, and the degree of vascularization [1, 2]. The color of the gums is one of the gingival factors that play an important role in soft tissue esthetics and the overall ideal smile [3]. Although physiological hyperpigmentation is not considered a medical disorder, the appearance of a pigmented gingiva is considered by many patients to be unaesthetic, especially for those who have a gummy smile. Laypersons are highly observant of changes in the color of the gingiva due to pigmentation and rate it as highly unaesthetic [4].
Physiological hyperpigmentation of the oral mucosa is clinically manifested as multifocal or diffuse melanin pigmentation in variable amounts in different ethnic groups [5]. Hyperpigmentation of the gum is due to excessive melanin accumulation by the melanocytes, which are mostly found in the basal and supra-basal layers of the epithelium. On average, the epithelium of the vestibular gingiva is approximately 0.30 ± 0.07 mm thick [6].
Several treatment modalities have been suggested in the literature for gingival depigmentation, such as scalpel surgery[7], abrasion[8], electrocautery[9], cryosurgery[10], radiosurgery[11], chemical cauterization[12], and laser [13, 14].
Laser surgery is superior to conventional mechanical surgery in the tissue ablation, decontamination, hemostasis, and there is potentially less operative and postoperative pain [15]. Recently, laser ablation has been recognized as one of the most effective, comfortable, and reliable techniques for gingival depigmentation [16]. Different lasers have been used for gingival depigmentation, including diodes [17], Nd:YAG [18], Er:YAG [19], Er:Cr:YSSG [20], and CO2 [21] lasers with varying results.
Two successful strategies have been suggested for the use of laser in gingival depigmentation, which depend on the wavelength absorption specification. In the surgical/ablative approach, the gingival epithelium is vaporized with melanin. All surgical wavelengths could be utilized in this approach. The second approach is non-surgical or non-ablative, where a specific wavelength, such as visible diode laser (445 nm) and near-infrared diode laser (810 nm), degranulates the melanosomes or denatures the melanin without de-epithelization of the gingival epithelium [22].
However, re-pigmentation has been documented to occur after all techniques to different degrees. The definite mechanism of re-pigmentation has not yet been clarified [23]. Active melanocytes from the adjacent pigmented tissues might migrate to treated areas causing relapse. The huge dissimilarity in the re-pigmentation duration can be linked to the method used, smoking behavior, and the patient’s ethnicity [24, 25].
This randomized clinical trial evaluated the recurrence rates of physiological gingival pigments during a 24-month follow-up and patient perceptions after ablative depigmentation using two laser wavelengths; diode 940 nm and Er,Cr:YSGG 2780 nm.
Materials and methods
Study sample
Sixty healthy patients (22 males and 38 females, aged 21 to 43 years) who presented with a chief complaint of dark-brown to black gingival hyperpigmentation between April 2015 and September 2018 were included in the present clinical prospective study. The participants were randomly divided into two groups (30 patients each). Randomization was performed using a randomization table using a computer-generated randomization list (SPSS v23.0; IBM corp., Armonk, NY, USA) with an allocation ratio of 1:1.
Inclusion and exclusion criteria
All of the patients were selected according to the following study inclusion and exclusion criteria.
The inclusion criterion was as follows: all patients exhibiting physiological melanin hyperpigmentation of score 1 or more according to the oral pigmentation index (OPI) in the buccal maxillary gingiva [26]. The exclusion criteria were pathologic hyperpigmentation, participants with a systemic condition that could affect tissue healing (e.g., autoimmune diseases); pregnancy and lactation, a history of smoking, previous mucogingival surgery at the region to be treated, and any contraindication for laser treatment.
Patients were informed of the nature and potential risks of the proposed surgical procedures and they reviewed and signed an informed consent form. The Declaration of Helsinki guidelines were followed throughout the study. The study was approved by Scientific Research and Graduate Studies Council, The Faculty of Dental Medicine, Damascus University, Damascus, Syria.
Clinical indices
The participants underwent a comprehensive periodontal examination, including the oral hygiene index (PI) and gingival index (GI), at all time points. Clinical gingival parameters, such as swelling, redness, and ulceration, were evaluated at week 1 and month 1, postoperatively. Gingival pigments were detected at baseline, 1 month, 1 year, and 2 years in the maxilla.
Intraoral photographs were taken using a DSLR camera with EF 100 mm f/2.8 Macro lens and Macro Twin Lite (ISO 100, aperture f/25, shutter speed 1/160, flash power 1/2). To achieve blinding, the photos were sent to three calibrated external experts, who were blinded to the treatment. External examiners classified the intensity and extensity of gingival pigments according to the following two indices:
-
Score 0: no clinical pigmentation (pink-colored gingiva)
-
Score 1: mild clinical pigmentation (mild light brown color)
-
Score 2: moderate clinical pigmentation (medium brown or mixed pink and brown)
-
Score 3: heavy clinical pigmentation (deep brown or bluish-black color)
-
Score 0: no pigmentation
-
Score 1: solitary unit(s) of pigmentation in the papillary gingiva without extension between neighboring solitary units.
-
Score 2: formation of continuous ribbon extending from neighboring solitary units
The gingival appearance, including the homogeneity of the color of the gingiva after 1 month of treatment, was evaluated according to the following suggested index (Fig. 3):
-
Score 1: homogeneous pink vital appearance of the gingiva
-
Score 2: non-homogeneous pale pink appearance of the gingiva
a Preoperative case of gingival hyperpigmentation. b The clinical appearance of the gingiva of the case exhibited a homogeneous vital pink color after 1 month of depigmentation. c Preoperative view for another case of gingival hyperpigmentation. d Non-homogeneous pale pink (non-vita) appearance for gingiva after 1 month of depigmentation
Treatment
Surgical procedure and laser parameters
The depigmentation procedure essentially involves ablation of the epithelial layer of the buccal gingiva exhibiting melanin pigmentation and was performed by the same operator (Fig. 4).
Participants were randomly allocated to the two research groups:
Group A was treated with an Er,Cr:YSGG laser 2,780 nm (iPlus Waterlase, Biolase, USA)Footnote 1 with an MZ6 cylindrical tip (tip 600 μm, 45 mJ/pulse, average power 2.25 W, frequency 50 Hz, pulse duration 60 μs, energy density 43 J/cm2 at the fiber’s tip, water 50%, and air 40%). The procedure was performed completely without infiltration anesthesia for all the patients of this group with the laser tip at an angulation of ~30° at a distance of ~1 mm from the gingival tissue. The laser tip was advanced in a scanning movement from the cervical-apical direction in all pigmented areas. The following settings were used to achieve hemostasis in case bleeding was present (tip 600 μm, 30 mJ/pulse, average power 1.5 W, frequency 50 Hz, pulse duration 700 μs, energy density 28.7 J/cm2 at the fiber’s tip, water 10%, and air 20%).
Group B was treated with diode 940 laser (Epic™, Biolase, USA).Footnote 2 The procedure was performed with a pencil-sized handpiece containing a 400 μm lasing fiber (400 μm initiated tip, average power 0.8 watts, Pulsed mode, Duty cycle 20%, Pulse duration 10 μs, energy density 127.4 J/cm2 per second at the fiber’s tip, no water or air). The procedure was started without anesthesia and infiltration anesthesia was injected in group B according to the patient subjective response of intraoperative pain. (Ubistesin™ 1/200,000, 3 M ESPE AG, Germany).Footnote 3 The laser tip was placed at an angle of approximately 30° to the gingival surface. Short light paint brush strokes were used in the cervical-apical direction in all pigmented areas. The actual lasing time for ablation of the gingival epithelium of the six maxillary teeth was recorded in minutes for both laser wavelengths.
Post-operative instructions
No periodontal pack or additional material was applied to support the healing process. Verbal and written postoperative instructions were given to the patients. The patient was cautioned to avoid traumatic foods, alcohol, and acidic beverages during the first week.
Patient perceptions
A self-administered modified version of Melzack’s McGill Pain Questionnaire was used by each patient to evaluate the treatment provided. The questionnaire was obtained at 7 days post-surgery (pain/discomfort, swelling, bleeding) using a five-point Likert scale with numerical values 0 = “not at al,” 1 = “mild,” 2 = “moderate,” 3 = “severe,” and 4 = “very severe.” Patient perceptions regarding esthetic and total satisfaction were evaluated using another questionnaire at 1-month post-surgery using a five-point Likert scale with numerical values 0 = “not at al,” 1 = “a little,” 2 = “somewhat,” 3 = “strongly,” and 4 = “very strongly.”
Statistical analyses
Data analysis was performed using computer software (SPSS v.17.0, IBM, Chicago, IL). The level of significance was set at 0.05, for all analyses. The Mann-Whitney U test was used to evaluate the depigmentation effectiveness, clinical appearance, gingival pigmentation indices, and patient perception. Differences between groups and time points were compared using the Wilcoxon test. The Kruskal-Wallis test was used to compare the gingival pigmentation indices between the two lasers according to the time intervals.
Results
Statistical analysis was performed for 60 patients (30 in each group). At the baseline examination, no statistically significant differences were observed in the OPI and MPI between both treatment groups (p >0.05). Throughout the study, all patients maintained an acceptable level of plaque control (PI < 18%) without statistical significant differences between groups in PI and GI indices at all time points (p >0.05).
Depigmentation effectiveness and clinical appearance
The effectiveness of the depigmentation treatment was examined 1 month after the treatment by comparing the OPI scores at baseline and 1 month. Both laser wavelengths exhibited similar effectiveness in removing gingival pigments without significant difference (p=0.49). At all follow-up time points after the depigmentation treatment, OPI and MPI scores were less significant compared to the recorded scores at baseline in both groups (p <0.05). The ablation of the gingival epithelium of the six maxillary incisors with Er,Cr:YSGG laser needed an average time of 13.4 ± 2.1 min, which was significantly faster than the diode laser 18.2 ± 2.7 min (p <0.001). The Er,Cr:YSGG group showed a homogeneous pink vital appearance of the gingiva in 56.8% of cases compared to 43.2% in the diode group. Swelling, redness, and ulceration of the gingiva during 1-week follow-up was less frequent in Er,Cr:YSGG group. However, this difference was not statistically significant (p =0.082) (Fig. 5).
Re-pigmentation
The results regarding the gingival pigmentation indices, OPI, and MPI are presented in Tables 1 and 2, respectively. Re-pigmentation was observed in 50% of cases in the Er,Cr:YSGG group after 1 year, with significant differences, compared to 1-month scores for both OPI (p =0. 001) and MPI (p <0 .001). The frequency of re-pigmentation increased significantly to 83.3% after 2 years of OPI (p <0.001) and MPI (p =0.004). On the other hand, re-pigmentation appeared in 23.3% and 36.7% of cases treated with diodes at 1 and 2 years, respectively. In the diode group, no significant differences were noted when comparing 1-month to 1-year OPI scores (p =0.056) and MPI scores (p =0.082). At the 2-year follow-up, a significant difference in pigmentation indices was recorded only in OPI (p = 0.034), while the difference in MPI was not significant (p = 0.157) (Figs. 6 and 7).
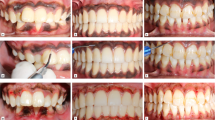
a Preoperative intraoral buccal view of the patient with gingival pigmentation in diode group. b Immediate postoperative after the ablative depigmentation procedure with diode 940 nm laser. c The clinical appearance 1 month postoperative. d No incidence of repigmentation after 1 year of the treatment. e 2 years postoperative follow-up
a Preoperative intraoral buccal view of the patient with gingival pigmentation in Er,Cr:YSGG laser group. b Immediate postoperative after the ablative depigmentation procedure with Er,Cr:YSGG 2780 nm laser. c The clinical appearance 1 month postoperative. d Mild repigmentation at 1 year postoperative follow-up. e Moderate repigmentation at 2 years postoperative follow-up
The Kruskal-Wallis test was used to compare the OPI and MPI indices between the two laser wavelengths according to the time interval. No statistically significant difference in indices was detected between the diode group and the Er,Cr:YSGG group after 1 month of treatment (p < 0.05). OPI scores were higher in the Er,Cr:YSGG group than in the diode group, with significant differences at 1 year and 2 years follow-up. Similarly, MPI scores were significantly higher in the Er,Cr:YSGG group at 1 and 2 years of follow-up.
Patient perceptions
The results of patients’ perceptions of morbidity and esthetic appearance are presented in Table 3. A significant difference was noticed between the two lasers in terms of pain during the ablative depigmentation with higher pain scores among the diode group (p<0.001). In the Er,Cr:YSGG group, the patients reported higher satisfaction scores with regard to the esthetic appearance after 1 week of surgery compared to the diode group. No significant difference was observed between the two laser groups in terms of pain during the first week, eating discomfort, speaking discomfort, bleeding, esthetic appearance after 1 month, and depigmentation effectiveness after 1 month.
Discussion
Despite the considerable interest in using the laser in gingival depigmentation, our knowledge regarding their use is largely limited, based on available data. Mostly, case reports or case series with small samples have been reported.
This study aimed to compare the clinical outcomes, the rate of re-pigmentation, and patient perceptions after laser-assisted ablative gingival depigmentation with diode 940 nm laser and Er,Cr:YSGG 2780 nm laser. The ablative technique includes surgical excision of the gingival epithelium, which differs from the non-ablative laser treatment of gingival pigmentation, which is based on the principle of selective photo thermolysis with specific near-infrared wavelengths that are highly absorbed by melanin-like diodes 445 nm and 810 nm [28].
In the present study, both wavelengths were efficient in removing the gingival pigments. Wound healing was uneventful in all patients. Clinical results were comparable at 1-month postoperative follow-up for both groups, whereas the pink color of the gingiva, epithelialization, and tissue thickness recovery were the same as the neighboring untreated gingiva.
We observed faster and complete re-epithelialization during the first week in the Er,Cr:YSGG laser group. This might be explained by the lower thermal damage of gingival tissue in the erbium family of lasers compared with diode lasers. The width of the thermally affected layer in gingival connective tissue has been reported to be approximately 5–25 μm [29]. In contrast to our results, and other results reported in the literature,[14, 29] Giannelli et al. recorded prolonged healing following erbium laser when compared to the diode [30]. The use of different laser parameters may explain this inconsistency in the results between the studies. Although the wavelength of each laser determines the absorption rate, characteristics of every tissue, and the thermal effect, the emission parameters of each laser system may influence the thermal damage inflicted on the soft tissue [31]. Although the 940 nm diode is absorbed mainly by hemoglobin and melanin, the photothermal ablation of soft tissues with a diode of 940 nm is difficult to achieve clinically without causing serious collateral thermal damage due to the high energy density level required. Therefore, the initiation of the diode laser tip was required to use low power settings (0.8 W) to raise the temperature of the tip. This gives the chance to achieve tissue excision using the thermal conducting effect when the laser tip is in contact with the targeted tissue.
Er,Cr:YSGG laser achieved depigmentation treatment without anesthesia, whereas infiltration anesthesia was used in the 940 nm diode group. This finding is consistent with the results from previous reports [14]. The free-running pulsed mode of Er,Cr:YSGG wavelength with short pulse duration (60 μs) provides a chance to deliver the ablation threshold energy density in the shortest duty cycle, whereas the duration between laser pulses was much greater than the thermal relaxation time of gingival tissues. Water irrigation and air are additional features when using Er,Cr:YSGG laser, which helps to cool down the tissue and eliminate pain during the ablation procedure.
Unlike other research carried out in this area [14, 32], the total time of laser irradiation was shorter in the Er,Cr:YSGG laser group than in the 940 nm laser group. Kaya et al. reported that the diode laser required a shorter treatment time than the Er:YAG laser. In the present study, the depigmentation procedure was completed in one session, while Kaya et al. needed multiple sessions of laser treatment on a weekly basis until the excess pigmentation was removed [32]. Agha et al. found that the duration of the procedure was faster using the diode than the Er,Cr:YSGG laser [14]. The speed of laser tip was not recorded; in addition, the means of the total time of treatment or total time of laser irradiation were not clear in both previous studies.
The visualization of melanin pigments was excellent with Er,Cr:YSGG laser compared to diode laser, which may produce carbonization at the treated surface, which confuses the clinician with the residual pigments. Both Er,Cr:YSGG and diode laser tips have been in a systematic manner with 30 angulations between the tip and the gum surface to assist in the control of the excision depth and ensure that the complete epithelium is removed.
Complete hemostasis was achieved by the diode, while mild bleeding was recorded in some Er,Cr:YSGG clinical cases. Diode lasers penetrate much deeper into the soft tissue, causing sustained heat to provide rapid vessel shrinkage, unlike the Er:YAG laser. In addition, diode laser wavelengths are highly absorbed by hemoglobin [32].
Almost all patients perceived mild to moderate post-surgical pain and discomfort. This finding is in good agreement with many studies that have shown that the overall incidence of post-surgical pain is low following laser surgery [15], [21,22,23], [33]. The Er,Cr:YSGG laser group recorded the least intraoperative and postoperative pain and the highest satisfaction scores at 1 week postoperatively. These results support our explanation that the healing process was faster in the Er,Cr:YSGG laser group with less collateral thermal damage than the diode laser group.
In general, the patients reported high levels of satisfaction with good esthetic results for both treatment modalities after 1 month but with no statistical differences. The patient did not distinguish between the homogeneous pink and pale pink appearance of the gum 1 month after the procedure, while the examiners found that the Er,Cr:YSGG laser showed a more homogeneous pink vital appearance for the gum in comparison to the diode laser group. Laypersons are usually less discriminating than dentists when they judge the effects of changes in gingival display on the perception of smile esthetics [34].
Based on our results, re-pigmentation was lower in the diode group at 23.3% and 36.7% at 1 and 2 years of follow-up, respectively. At 2 years, 83.3% of patients showed re-pigmentation in the Er,Cr:YSGG laser group. More re-pigmentation was observed in patients with a high baseline OPI score. This finding is consistent with Agha et al.’s findings [14]; follow-up after 2 years revealed that re-pigmentation mainly occurs in the Er,Cr:YSGG patients, whereas most patients treated with diode had no recurrence.
Multiple studies using different methods have reported different rates of recurrence after gingival melanin depigmentation. Re-pigmentation during the first year after treatment with erbium lasers has been reported in past studies [18, 19]. Hegde et al. showed more re-pigmentation in the Er:YAG laser-treated sites than in the surgically or CO2 laser-treated sites. The authors explained the results based on the fact that the absorption of Er:YAG laser is superficial thus, allowing ablation without any thermal damage to adjacent tissues [21]. Gholami et al. showed isolated areas with light brown pigmentation in all cases treated with Er,Cr:YSGG laser after 12 months [20]. Nammour et al. in a recent study compared the relapse rate after depigmentation after 5 years between three different laser wavelengths (Er:YAG, CO2, and diode 980 nm) in a randomized clinical trial. Diode lasers provide long-term stability in treatment. The Er:YAG laser group showed the earliest return of pigmentation after an average of 9 months [35].
In contrast to recent studies, Kaya et al. reported that there was no re-pigmentation in any patient treated with an Er:YAG laser. Similarly, the study reported the absence of pigments in patients treated with diode laser for 14 months. The multi-session protocol of depigmentation used in the study may explain this finding [32]. Chandra et al. recorded a slight recurrence of solitary units of pigments with mild intensity in 50% of cases treated with diode laser after 6 months [33], while another study using a diode laser showed that one in ten patients exhibited re-pigmentation at 18 months [36].
The migration of the active melanocytes from the adjacent pigmented tissues to treated areas was suggested as a possible mechanism of re-pigmentation [37]. The large variation in time of re-pigmentation in different laser studies may be related to the technique used and the patient’s race. For example, inadequate depth of penetration of the laser beam and incomplete removal of melanin may lead to faster recurrence. The Er,Cr:YSGG laser has a superficial penetration depth (15 μm) in the gingival tissues because it is highly absorbed by water, which is the main component of the gingival epithelium. Also Er,Cr:YSGG laser does not coincide with the absorption spectrum of melanin.
On the other hand, diode lasers have deeper penetration and are present in the absorption spectrum range of melanin. The unintended irradiation caused by this deep thermal effect of diode laser results in the sealing of blood vessels in the surrounding tissue and may lead to delayed migration of melanocytes. In addition, the diode laser might be absorbed by the pigment-containing cells that may have become arrested in the lamina propria, such as the melanophages or melanophores, and also has some specific effects on the cells that reduced their activity [38]. The functional activity of melanocytes in the basal cell layer of the epithelium is influenced by signals from the neighboring fibroblasts in connective tissues [39]. These factors may explain the lower rate of re-pigmentation in diode laser-treated sites.
Conclusions
Er,Cr:YSGG 2790 nm and 940 nm diode were efficient in removing the gingival pigments, and the clinical outcomes were comparable at one-month post-surgery. The Er,Cr:YSGG laser treatment as a procedure was faster, less traumatic with shortened healing time, and an overall positive experience for the patients in comparison with the 940 nm diode. The long-term stability of gingival color was better in diode laser treatment, with a lower incidence of re-pigmentation compared to the final outcome with the Er,Cr:YSGG laser.
Notes
Iplus Waterlase, Biolase, Irvine, CA, USA.
Epic X, Biolase, Irvine, CA, USA.
Ubistesin™ 1/200,000, 3M ESPE AG, Germany.
References
Feller L, Masilana A, Khammissa RAG, Altini M, Jadwat Y, Lemmer J (2014) Melanin: the biophysiology of oral melanocytes and physiological oral pigmentation. Head Face Med 10:1–7. https://doi.org/10.1186/1746-160X-10-8
Squier CA, Kremer MJ (2001) Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr 2001:7–15. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003443
Jones J, McFall WTJ (1977) A photometric study of the color of health gingiva. J Periodontol 48:21–26. https://doi.org/10.1902/jop.1977.48.1.21
Batra P, Daing A, Azam I et al (2018) Impact of altered gingival characteristics on smile esthetics: Laypersons’ perspectives by Q sort methodology. Am J Orthod Dentofac Orthop 154:82–90.e2. https://doi.org/10.1016/j.ajodo.2017.12.010
Müller S (2010) Melanin-associated pigmented lesions of the oral mucosa: presentation, differential diagnosis, and treatment. Dermatol Ther 23:220–229. https://doi.org/10.1111/j.1529-8019.2010.01319.x
Breathnach AS (1981) Differentiation of Human Oral Stratified Epithelia. J Anat 133:460
Kathariya R, Pradeep AR (2011) Split mouth de-epithelization techniques for gingival depigmentation: a case series and review of literature. J Indian Soc Periodontol 15:161–168
Murthy MB, Kaur J, Das R (2012) Treatment of gingival hyperpigmentation with rotary abrasive, scalpel, and laser techniques: a case series. J Indian Soc Periodontol 16:614–619. https://doi.org/10.4103/0972-124X.106933
Gufran K (2016) A comparative evaluation of two different techniques for esthetic management of gingival melanin hyperpigmentation: a clinical study. J Dent Res Rev 3:13. https://doi.org/10.4103/2348-2915.180109
Narayankar SD, Deshpande NC, Dave DH, Thakkar DJ (2017) Comparative evaluation of gingival depigmentation by tetrafluroethane cryosurgery and surgical scalpel technique. a randomized clinical study. Contemp Clin Dent 8:90–95. https://doi.org/10.4103/ccd.ccd_1017_16
Mahesh H, Harish M, Shashikumar B, Ramya K (2012) Gingival pigmentation reduction: a novel therapeutic modality. J Cutan Aesthet Surg 5:137–140. https://doi.org/10.4103/0974-2077.99458
Shimada Y, Tai H, Tanaka A, Ikezawa-Suzuki I, Takagi K, Yoshida Y, Yoshie H (2009) Effects of ascorbic acid on gingival melanin pigmentation in vitro and in vivo. J Periodontol 80:317–323. https://doi.org/10.1902/jop.2009.080409
Luk K, Anagnostaki E (2017) Impact of laser dentistry in management of color in aesthetic zone. In: lasers in dentistry—current concepts. Springer International Publishing, Cham, pp 337–358
Taher Agha M, Polenik P (2020) Laser Treatment for melanin gingival pigmentations: a comparison study for 3 laser wavelengths 2780, 940, and 445 nm. Int J Dent 2020:1–11. https://doi.org/10.1155/2020/3896386
Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y (2009) Application of lasers in periodontics: true innovation or myth? Periodontol 2000(50):90–126. https://doi.org/10.1111/j.1600-0757.2008.00283.x
Bakhshi M, Rahmani S, Rahmani A (2015) Lasers in esthetic treatment of gingival melanin hyperpigmentation: a review article. Lasers Med Sci 30:2195–2203. https://doi.org/10.1007/s10103-015-1797-3
Suragimath G, Lohana MH, Varma S (2016) A split mouth randomized clinical comparative study to evaluate the efficacy of gingival depigmentation procedure using conventional scalpel technique or diode laser. J Lasers Med Sci 7:227–232. https://doi.org/10.15171/jlms.2016.40
Ribeiro FV, Cavaller CP, Casarin RCV, Casati MZ, Cirano FR, Dutra-Corrêa M, Pimentel SP (2014) Esthetic treatment of gingival hyperpigmentation with Nd:YAG laser or scalpel technique: a 6-month RCT of patient and professional assessment. Lasers Med Sci 29:537–544. https://doi.org/10.1007/s10103-012-1254-5
Kishore A, Kathariya R, Deshmukh V, Vaze S, Khalia N, Dandgaval R (2014) Effectiveness of Er:YAG and CO2 lasers in the management of gingival melanin hyperpigmentation. Oral Health Dent Manag 13:486–491
Gholami L, Moghaddam SA, Rigi Ladiz MA, Molai Manesh Z, Hashemzehi H, Fallah A, Gutknecht N (2018) Comparison of gingival depigmentation with Er,Cr:YSGG laser and surgical stripping, a 12-month follow-up. Lasers Med Sci 33:1647–1656. https://doi.org/10.1007/s10103-018-2501-1
Hegde R, Padhye A, Sumanth S, Jain AS, Thukral N (2013) Comparison of surgical stripping; erbium-doped:yttrium, aluminum, and garnet laser; and carbon dioxide laser techniques for gingival depigmentation: a clinical and histologic study. J Periodontol 84:738–748. https://doi.org/10.1902/jop.2012.120094
Muruppel AM, Jagadish Pai BS, Bhat S et al (2020) Laser-assisted depigmentation-an introspection of the science, techniques, and perceptions. Dent J 8. https://doi.org/10.3390/DJ8030088
Lin YH, Tu YK, Lu CT, Chung WC, Huang CF, Huang MS, Lu HK (2014) Systematic review of treatment modalities for gingival depigmentation: a random-effects poisson regression analysis. J Esthet Restor Dent 26:162–178. https://doi.org/10.1111/jerd.12087
Kaur H, Jain S, Sharma RL (2010) Duration of reappearance of gingival melanin pigmentation after surgical removal - a clinical study. J Indian Soc Periodontol 14:101–105. https://doi.org/10.4103/0972-124X.70828
Araki S, Murata K, Ushio K, Sakai R (1983) Dose-response relationship between tobacco consumption and melanin pigmentation in the attached gingiva. Arch Environ Health 38:375–378. https://doi.org/10.1080/00039896.1983.10545823
DUMMETT CO, GUPTA OP (1964) Estimating the epidemiology of oral pigmentation. J Natl Med Assoc 56:419–420
Hanioka T, Tanaka K, Ojima M, Yuuki K (2005) Association of melanin pigmentation in the gingiva of children with parents who smoke. Pediatrics 116:e186–e190. https://doi.org/10.1542/peds.2004-2628
Mun JY, Jeong SY, Kim JH, Han SS, Kim IH (2011) A low fluence Q-switched Nd:YAG laser modifies the 3D structure of melanocyte and ultrastructure of melanosome by subcellular-selective photothermolysis. J Electron Microsc 60:11–18. https://doi.org/10.1093/jmicro/dfq068
Rosa DSA, Aranha ACC, de Eduardo CP, Aoki A (2007) Esthetic treatment of gingival melanin hyperpigmentation with Er:YAG laser: short-term clinical observations and patient follow-up. J Periodontol 78:2018–2025. https://doi.org/10.1902/jop.2007.070041
Giannelli M, Formigli L, Bani D (2014) Comparative evaluation of photoablative efficacy of erbium: yttrium-aluminium-garnet and diode laser for the treatment of gingival hyperpigmentation. a randomized split-mouth clinical trial. J Periodontol 85:554–561. https://doi.org/10.1902/jop.2013.130219
Cercadillo-Ibarguren I, España-Tost A, Arnabat-Domínguez J et al (2010) Histologic evaluation of thermal damage produced on soft tissues by CO2, Er,Cr:YSGG and diode lasers. Med Oral Patol Oral Cir Bucal 15:e912–e918. https://doi.org/10.4317/medoral.15.e912
Kaya GŞ, Yavuz GY, Sümbüllü MA, Day E (2012) A comparison of diode laser and Er:YAG lasers in the treatment of gingival melanin pigmentation. Oral Surg Oral Med Oral Pathol Oral Radiol 113:293–299. https://doi.org/10.1016/j.tripleo.2011.03.005
Chandra GB, VinayKumar MB, Walavalkar NN et al (2020) Evaluation of surgical scalpel versus semiconductor diode laser techniques in the management of gingival melanin hyperpigmentation: a split-mouth randomized clinical comparative study. J Indian Soc Periodontol 24:47–53. https://doi.org/10.4103/jisp.jisp_186_19
Sohail K, Nawaz E, Durrani O et al (2016) Comparison of perceptions of laypersons, dentists and orthodontists to altered smile aesthetics. Pak Orthod J 7:76–82
Nammour S, El Mobadder M, Namour M et al (2020) A randomized comparative clinical study to evaluate the longevity of esthetic results of gingival melanin depigmentation treatment using different laser wavelengths (diode, CO2, and Er:YAG). Photobiomodulation Photomed Laser Surg 38:167–173. https://doi.org/10.1089/photob.2019.4672
Singh V, Giliyar SB, Kumar S, Bhat M (2012) Comparative evaluation of gingival depigmentation by diode laser and cryosurgery using tetrafluoroethane: 18-month follow-up. Clin Adv Periodontics 2:129–134. https://doi.org/10.1902/cap.2012.110008
Perlmutter S, Tal H (1986) Repigmentation of the gingiva following surgical injury. J Periodontol 57:48–50. https://doi.org/10.1902/jop.1986.57.1.48
Murphy GF, Shepard RS, Paul BS, Menkes A, Anderson RR, Parrish JA (1983) Organelle-specific injury to melanin-containing cells in human skin by pulsed laser irradiation. Lab Investig 49:680–685
Yamaguchi Y, Passeron T, Watabe H, Yasumoto KI, Rouzaud F, Hoashi T, Hearing VJ (2007) The effects of dickkopf 1 on gene expression and Wnt signaling by melanocytes: mechanisms underlying its suppression of melanocyte function and proliferation. J Invest Dermatol 127:1217–1225. https://doi.org/10.1038/sj.jid.5700629
Acknowledgements
The authors wish to thank Josep Arnabat-Domínguez, Kenneth Luk, and Sana Farista for their expertise and contribution as external examiners throughout all aspects of the study.
Author information
Authors and Affiliations
Contributions
Walid Altayeb and Georgios E. Romanos substantially contributed to the conception and design of the study. Walid Altayeb was involved in clinical procedures and data collection. Ahmed Abdullah was involved in the statistical data analysis. Omar Hamadah, MHD Bahaa Aldin Alhaffar, and Walid Altayeb were involved in drafting the manuscript. All authors have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Georgios E. Romanos and Omar Hamadah critically revised the article for important intellectual content and gave final approval on the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies involving animals performed by any of the authors. Informed consent was obtained from all individual participants involved in the study.
Competing interests
The authors declare no competing interests.
Additional information
Summary
A randomized controlled trial to evaluate the effectiveness of two types of intraoral lasers in treating gingival hyperpigmentation and the rate of re-pigmentation.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altayeb, W., Hamadah, O., Alhaffar, B.A. et al. Gingival depigmentation with diode and Er,Cr:YSGG laser: evaluating re-pigmentation rate and patient perceptions. Clin Oral Invest 25, 5351–5361 (2021). https://doi.org/10.1007/s00784-021-03843-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-03843-6