Abstract
Gingival melanin hyperpigmentation is an esthetic concern for many individuals. In this study, we compared the standard surgical removal method with two different Er,Cr:YSGG laser settings in order to find the best treatment method. In 33 dental arches, the following three treatment groups were comparatively evaluated: (1) surgical stripping, (2) removal with laser setting 1 (4.5 W, 50 Hz, 100% water, 80% air, 60 μs, 800 μm Tip; MZ8), and (3) laser setting 2 (2.5 W, 50 Hz, 20% water, 40% air, 700 μs, 800 μm Tip; MZ8). We comparatively evaluated pain, patient satisfaction and wound healing, treatment time, and the amount of bleeding. Re-pigmentation was evaluated after 1 and 12 months by Hedin and Dummet pigmentation scores. Laser setting 1 had the best results regarding pain and patient satisfaction, although not statistically significant (P > 0.05). Wound healing results were better using lasers compared to surgical stripping (P < 0.05). Laser setting 1 was a faster procedure with mild amounts of bleeding. The least amount of bleeding was seen with laser setting 2. After 1 month, only two cases of the laser setting 2-treated areas showed an isolated pigmented area in the papilla; at 12 months, the mean Hedin indexes were still less than 2 and mean Dummett index less than 1 in all treatment techniques, with the lowest scores seen in the laser setting 1 sites. Based on our results, Er,Cr:YSGG laser can be more convenient for gingival depigmentation compared to surgical blade. Although not statistically significant, laser setting 1 with shorter pulse duration and higher water spray showed better overall results. However, laser setting 2, with longer pulse duration and less water spray, resulted in better coagulative effects and can be used to control bleeding wherever necessary in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Health and appearance of gingival tissues influence the attractiveness of a smile. Gingival color is one of these factors that is determined by the number and size of underlying blood vessels, epithelial thickness, degree of keratinization, and residing pigments within the gingiva. Melanin, carotene, reduced hemoglobin, and oxyhemoglobin are the main pigments found in the oral mucosa [1, 2]. Melanin is a brown pigment synthesized in the cytoplasm of melanocytes. After activation of melanocytes by factors like stress hormones, sunlight, etc., chemical messengers like melanocyte stimulating hormone are produced. Melanocytes are then induced to create melanin-containing granules called melanosomes. This process occurs when the enzyme tyrosinaseconverts tyrosine into a molecule named dehydroxyphenylalanine (DOPA). Tyrosinase also changes DOPA into a secondary chemical dopaquinone. After a series of reactions, dopaquinone is converted into either dark melanin (eumelanin) or light melanin (pheo-melanin). Melanin is then transferred to keratinocyte cells of skin and oral epithelium [3]. Excessive melanin deposition in the basal and suprabasal cell layers of the gingival epithelium creates pigmented areas in gums [4]. We normally consider this kind of hyperpigmentation of the gingiva a physiological variation mainly reported in dark skinned individuals. These people have more completely melanized granules, also forming larger complexes with sizes of about 1–3 mm. The size and amount of melanin in these granules seem to be directly proportional to the degree of pigmentation [5, 6]. Smoking is an environmental factor that also effects gingival pigmentation. Hedin et al. first reported greater gingival pigmentation in smokers compared to non-smokers [7]. They also reported that quitting smoking could result in a decrease in gingival melanin [8]. Melanin in the oral mucosa attaches to free radicals produced as the result of exposure to cigarette smoke and polycyclic compounds like nicotine and benzoperylene found in cigarette smoke. It acts as a protective barrier against oxidative stress. This can explain its increased production by melanocytes in smokers [9,10,11].
Although not considered a medical concern, complaint of black gums and the demand for its esthetic correction is quite common, especially if gums are visible during speech and smiling. Gingival depigmentation is a periodontal plastic surgery procedure, and several different methods have been employed for depigmentation of gums to create a more pleasant smile. Removal by a surgical scalpel, bur abrasion, cryosurgery, and electro surgery, and chemical removal by 90% phenol and 95% alcohol are successful previously reported methods [12,13,14,15,16,17]. Gingivectomy with free gingival auto grafts and acellular dermal matrix allograft (ADMA) are other reported techniques [18, 19]. Recently, lasers have been recognized as a reliable method for this kind of treatment with many reported advantages. Many laser systems such as CO2 laser, Nd:YAG, argon, and different diode lasers as well as the erbium family of lasers (Er:YAG, Er,Cr:YSGG) have been used for this purpose [2, 20,21,22,23,24,25,26,27]. The erbium family of lasers, when used with correctly employed parameters, have an excellent tissue ablation mechanism which results in minimal thermal damage to the underlying tissues and high levels of disinfection [28].
Laser interaction is primarily determined by the laser irradiation affinity for specific chromophores inside the tissue. A chromophore is a molecule or substance capable of absorbing specific laser wavelengths. Er,Cr:YSGG with a 2780 nm and Er:YAG with a 2890 nm wavelength have high absorption in water. The erbium lasers’ energy is predominantly absorbed in tissue water and rapidly heats it to ablative temperatures creating micro explosions and ablation. Tissue ablation occurs before the absorbed energy is spread out into the surrounding tissue by the process of thermal diffusion [29,30,31]. This makes erbium lasers suitable devices for periodontal treatments with minimal side effects. However, inappropriate application and inaccurate settings for different tissues may lead to gingival recession, damage to the periosteum and bone, causing pain discomfort and delayed wound healing [26]. It is therefore important to use the correct laser settings to achieve favorable results. In this study, we comparatively evaluated the standard blade technique with two different settings of the Er,Cr:YSGG (2780 nm) laser in a randomized clinical trial study in order to find the most suitable technique for clinical applications.
Materials and methods
From patients referring to our university’s Periodontology Department seeking esthetic treatment of their pigmented gingival tissue, 36 arches of patients with physiologic melanin pigmentation in the anterior part of maxilla or mandible were selected. Each patient signed an informed consent form. The study was also independently reviewed and approved by the university research ethics board (IR.Zaums.REC.1394.181) and registered in the Iranian Registry of Clinical Trials (IRCT ID IRCT2016113018493N3).
The inclusion criteria were over 18 years of age, nonsmoking, systemically healthy individuals with a healthy periodontal status, without periodontal pockets and a Loe’s gingival index of 1 or less and a plaque index of less than 10%. Melanin pigmentation indexes were also considered in the inclusion criteria as Dummett index of 2 or 3 and a Hedin index of 3 or 4 [32] (Table 1). The exclusion criteria were pregnant or breastfeeding patients, being under orthodontic treatment, having bleeding on probing, and plaque index of more than 20%.
The arches were randomly assigned to 3 groups of 12 with block randomization. Three treatment techniques were compared in a randomized double blind split mouth design. In each group, two different treatments were randomly allocated to each side. The same surgeon performed all procedures. A periodontist not aware of the procedures evaluated and scored the healing of treated sites and helped patients fill out the satisfaction and pain questioners. The patients were not informed about the type of procedure used in each site. Preoperative Dummett index for intensity of pigmentation and Hedin index for extent of pigmentation were recorded for each site [7, 33].
Each patient was treated in one session, and to separate the two treatments, a 5 min rest was given between the treatments. Before depigmentation in the blade-treated site, the inactive laser was held from a distance with water spray for a few seconds to create a placebo condition. In patients, wanting treatment in both arches treatment in the other arch was done at least 14 days after the first treated arch.
Before starting treatments in each arch, we applied a 1 mm thickness of topical anesthetic gel 20% benzocaine (G-Benzotop, Nova DFL) for 2 min. The blade-treated sides received local infiltration with lidocaine (2%, 1:80,000 epinephrine). Treatments extended from midline to the distal line angle of the first premolars. Surgical stripping was performed by a #15 scalpel. An approximately 1.5-mm thickness of gingiva was evenly removed.
Laser treatments were performed by a 2780 nm Er,Cr:YSGG laser (Waterlase, Biolase, USA). The tested laser settings were as follows (Table 2): laser setting 1 4.5 W, 50 Hz, 60 μs pulse duration (H mode), 80% air, 100% water (water flow rate of 17 ml/min); and laser setting 2 2.5 W, 50 Hz, 700 μs pulse duration (S mode), 20% water, 40% air (water flow rate of 9 ml/min). A Mz8 laser tip with a diameter of 800 μm was used with contact mode with overlapping brush strokes in both settings. The papillary point and free gingival margins were usually free of pigment and were left untouched as far as possible to prevent gingival recession. The operator, assistant, and patient wore laser safety glasses during the procedures. Photographs were taken at baseline, 1st, 7th, and 30th day from all patients.
Pain, patient satisfaction, and gingival wound healing and repimentation were evaluated post-operatively.
We evaluated pain using a Visual Analogue Scale (VAS) of 1–10, immediately after treatment and on the first and seventh postoperative days.
Patient satisfaction was evaluated on day 7 using a self-designed questioner. The validity and reliability of the questionnaire were previously evaluated.
Gingival wound healing was evaluated and scored as follows: 0 = tissue necrosis, 1 = ulcer formation, 2 = incomplete epithelialization, and 3 = complete epithelialization. Severity of bleeding during procedures (1: none, 2: slight, 3: moderate, 4: severe) and time taken to complete treatment in each procedure were recorded [34].
We also evaluated treatment relapse in each site using the Dummett and Hedin indices, after 1 and 12 months.
After completion of each procedure, the treated site was gently compressed and cleaned with a sterilized gauze soaked in normal saline. No periodontal dressing was applied. We instructed to avoid flossing and brushing the treated area for a week and rinse with warm normal saline two times a day from the night after surgery and avoid spicy, hard, or hot foods, and drinks. No analgesics were prescribed; however, for ethical considerations in case of severe pain, patients could use 325 mg of acetaminophen after informing the practitioner, but this excluded them from the study.
Three arches from two patients were excluded during treatment; therefore, 33 arches were comparatively evaluated at the end. Post hoc Tukey’s test was used with a SPSS 21 software for data analysis, and a P value of < 0.05 was considered statistically significant.
Results
From the 22 patients recruited, 14 needed gingival depigmentation in both arches and eight only in the maxilla. Two patient dropped out during the study, one because of use of analgesics after treatment and the other for not coming for follow-up visits.
Finally, 33 arches (66 treated sides, 22 in each treatment group) from 20 patients with a mean age of 24 ± 5.88 were comparatively evaluated (Table 3).
Pain
According to our results, though, the mean amount of pain did not have a statically meaningful difference between the treatments at different evaluation times (P > 0.05). However, the lowest amount of pain during procedures was reported in the laser setting 1-treated sites (Table 4). Blade-treated sites had higher reported pain on the first and seventh day after treatment compared to laser groups (Fig. 1). When comparing the pain score distributions in the laser setting 2-treated sites, there were three (13.6%) cases who evaluated their intraoperative pain as severe and one as unbearable. However, laser setting 1-treated sites reported pain as mild in most cases (59.1%) (Fig. 2).
Patient satisfaction
Satisfaction scores just after treatment showed that the laser setting 1 and blade-treated sites had the highest satisfaction scores (24/65 ± 3.16 and 24/68 ± 3.99) and laser setting 2 had the lowest satisfaction. After 1 week, the laser setting 1 group still had the highest satisfaction score, but the blade group showed the least amount of satisfaction score. However, these results were also not statistically significant (Table 4).
Gingival wound healing
We evaluated healing on days 1, 3, 7, and 30 after treatment. Overall healing was uneventful, and we could observe complete epithelialization after 30 days in all treated sites. Overall, laser-treated sites showed better healing. The blade-treated sites had lower healing scores on 1, 3, and 7 days after treatment which was statistically significant on day 7 (P = 0,001) (Table 4). Better healing scores could be found in the laser setting 1 group on day 1 and 3. Complete epithelialization percentage was higher in laser-treated groups compared to the blade on day 7. All groups had complete epithelialization after 30 days (Fig. 3).
Bleeding during the procedure and treatment duration
The amount of bleeding was significantly less in laser-treated sites compared to the blade (P = 0.001). The laser setting 2 setting had the least amount of bleeding which was statistically significant. Treatment time was longer in the laser setting 2-treated sites (P = 0.010) (Table 5 and Fig. 4).
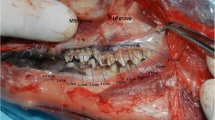
a Intra-oral preoperative view. b Surgical stripping treatment. c Laser setting 1 treatment (using the gold hand piece, 4,5 W 50 Hz Mz8 tip 100% water 80% air). d Immediate postoperative view (blade on right side, laser setting 1 on left side). e One-day postoperative view. f Three-day postoperative view. g Seven-day postoperative view. h One-month postoperative view
Re-pigmentation
After 1 and 12 months, we evaluate the treated patients with regard to re-pigmentation. All three techniques showed a marked reduction in Hedin and Dummett (DOPI) indices compared to baseline (Table 6).
After 1 month, only two laser setting 2-treated areas showed an isolated pigmented area in the papilla, and the mean Hedin and DOPI indices were very low (0.09 ± 0.29). After 12 months, in all sites, re-pigmentation was staring to appear. However, they were mostly light brown small solitary pigmented areas, and patients were still satisfied with the overall results. There was only one case in the laser setting 2-treated group with a strip of regimented area (Hedin score 3). The mean Hedin indices were less than 2 and mean Dummett index of less than 1 in all treatment techniques and not statistically significant between the groups. The difference between pigmentation scores of the groups was statistically significant at 1 month, but they were not statistically significant at 12 month (Table 6).
Discussion
Different methods of esthetic treatment for gingival melanin hyperpigmentation have been practiced and studied previously. Since its approval for soft tissue treatments by the US Food and Drug Administration (FDA) in 1998, Er,Cr:YSGG laser has had promising results in periodontics and oral surgery [35, 36].
In this study, we have for the first time comparatively evaluated the clinical application of two different settings of Er,Cr:YSGG and the gold standard method of removal with a surgical blade in a randomized split mouth design to find the most suitable setting for clinical applications.
According to our results when evaluating the amount of pain experienced during the procedure, the highest VAS scores were seen in laser setting 2 sites. The blade-treated sites showed lower pain scores which was predictable since we used local infiltration, and the pain scores reported just after treatment were probably only due to the pain from injections. The laser setting 1 had the least reported pain score at all-time intervals. When statistically compared, none of these amounts were statistically significant.
The reason for this lower pain experience with the laser setting 1 seems to be due to the higher ablative effect seen in this setting. When using H mode, we have very short pulse durations (60 μs) compared to S mode (700 μs) resulting in higher peak powers and a greater ablative effect. The higher water spray in this setting may also lead to less thermal and coagulative effects on the tissue. This could explain the less pain experienced by patients during and just after the procedure. The pain sensation tended to increase when removing deeper pigments and pigmentations near the mucogingival junction, which were also areas where in some cases, we had minor amounts of bleeding with this setting. No bleeding was observed with laser setting 2 due to the thermal effects it provides.
Pain was also evaluated on days 1 and 7 after the procedure in which the blade group had the highest pain experienced during these periods compared to the laser groups (Table 4). Laser setting 1 sites had the least mean levels of pain experience at both evaluation times.
Hegde et al. also reported higher pain results using VAS evaluation during days 1 and 7 after treatment with a blade compared to CO2 and Er:YAG lasers [25]. In a case series reported by Butchibabu et al., the blade technique was compared with diode laser and pain was evaluated by VAS on days 1, 3, and 7 after the procedure. They also reported higher pain in the blade-treated sites compared to laser treatment. Although similar to our report, their results were also not statistically significant [25, 27]. Studies by Azzeh and Kishore have also reported less pain when using Er:YAG lasers [34, 37]. Case reports have used Er,Cr;YSGG for gingival melanin pigmentation removal previously. They also reported this laser to be covenant, with almost no bleeding and pain. This is probably due to creation of a fibrin layer and coagulated proteins on the wound surface as a biologic barrier and also coagulation of nerve endings resulting in a painless treatment [38].
In our study, a questioner was used to evaluate patients’ satisfaction of the treatment 1 week later. This has not been evaluated in previous studies. The highest satisfaction score was found in the laser setting 1 group but the blade group showed the lowest scores. This result was logical since the blade group had higher pain and slower healing results during this period. Laser setting 1-treated areas on the other hand showed better healing. This laser setting also resulted in quite a fast treatment with negligible pain, bleeding, and satisfying change in color visible from immediately after treatment.
In this study, the blade group had lower healing scores and slower healing results compared to laser-treated groups. This was statistically significant on day 7. Erbium laser setting 1 showed better healing results after 1 and 3 days, but after a week, the laser 2 group had better healing scores, although these findings were not statistically significant. An epithelial layer had formed in most laser-treated groups after 1 week. After 1 month, all treatment groups showed complete healing, epithelialization, and tissue thickness recovery.
In a case series by Murphy [24], three methods of bur abrasion, surgical blade, and diode laser were compared on three patients. In their report, the fastest healing was in the blade group, which could probably be due to the thermal damage that might occur with the diode laser’s coagulation effect. This was different from our results since we do not have this effects when using the Er,Cr:YSGG laser. The erbium laser also has less penetration into gingival tissue and an ablative mechanism of action that only affects the surface layer of tissue. This results in minimum damage to deeper layers and better wound healing compared to the blade. This was similar to the results obtained in a study by Rosa et al. on Er:YAG. They also observed fast epithelialization of gingiva in 1 week with a healthy appearance in all five cases. This is due to the fact that erbium laser wave lengths are highly absorbed by the water in the tissue and rapidly heat the tissue to ablative temperatures causing tissue removal through ablation. The water sprayed on the operation field and the air stream could also help avoid thermal damage to the surrounding tissues [31, 39].
The width of the thermally changed layer in dog gingival connective tissue has been reported to be only 5–25 μ when using an Er:YAG in contact mode with water spray [40]. Photobiostimulation and bactericidal effects of the these lasers could also be favorable for wound healing [28, 41]. The scattered low levels of laser energy during treatment might act as low-level laser therapy leading to a cascade of photobiostimulative events that might affect cellular metabolic processes and promotes beneficial biological effects resulting in better repair of the gingival tissue. Ogita et al. have reported increased proliferation and a significant change in protein expression after low-level laser treatment of human gingival fibroblast cells with Er:YAG laser [41, 42].
A report by Hedge et al. also compared three methods of blade, Er:YAG, and CO2 lasers with a 6-month follow-up. They found the slowest healing with the CO2 laser compared to Er:YAG. This laser also has greater coagulative effects on the tissue. On the other hand, the erbium laser had better results [25]. A review article by Bakhshi et al. has reported faster healing results for the erbium laser family especially Er,Cr:YSGG compared to other lasers. As mentioned, these lasers tend to ablate the tissue through their water-mediated ablation mechanism with minimal damage to the underlying tissues. A fibrin layer forms on the gingiva in the first 24 h after treatment; within a week, healing is almost complete with a normal tissue color, and no scarring similar to the results obtained in our study [43]. However, although the blade technique is simple, effective, and fast, it results in bleeding. Therefore, small areas of hematoma may form which take longer to turn into a fibrin layer. The thicker fibrin layer causes a small delay in healing compared to laser treatment. This was seen in a few of our cases with deeper pigmentations (Fig. 5c). Similar to our results, many other studies have also reported superiorities of lasers especially the erbium lasers over conventional blade treatment [16, 24, 27, 37, 38]. However, up to our knowledge, this is the first randomized controlled trial comparing removal of physiologic melanin pigmentation by Er,Cr:YSGG laser and its different settings with the standard blade removal technique.
Our results of evaluation of re-pigmentation after 1 month showed only two patients from the laser setting 2 groups having small light areas of pigmentations reappearing in the papilla (Hedin index = 1) which seemed to be pigmentation left due to incomplete removal of melanin. This was probably due to the coagulative effect of this setting on gingival tissue, producing brown-black discolorations which make it harder to distinguish deeper melanin pigmentation during operation. On the other hand, with the L1 setting and its higher ablative effects and water spray as a coolant, greater ablation and minimal coagulation could be observed; this together with the rinsing effect of water provided better visualization of the operative field. Removal of the pigmented tissue could therefore be performed more accurately and completely [30].
After 12 months, all three treatment techniques showed similar trends in re-pigmentation. In all cases, isolated areas of light brown pigmentation (Mean Dummett index of less than 1, mean Hedin index of less than 2) had appeared in areas with deeper pigmentations at baseline, mostly the interdental papilla and in some cases small areas in the attached gingiva or mucogingival junction. In only one treated site, the re-pigmentation area had formed a short melanin strip (Hedin index = 3). However, the esthetic results were still satisfactory for all patients. Similar to our results, physiologic recurrence has been reported after different time periods, but usually, it takes more than 6 months [2, 34]. It seems that the migration theory described by Perlmutter and Tal could be an acceptable explanation [5]. According to this theory, active melanocytes remaining adjacent to depigmented areas migrate to these areas causing re-pigmentations. Depigmentation results would be more stable with treatment techniques that can accurately eliminate melanocytes. In this study with laser setting 1, there was no carbonization, and with the water spray, cleansing of the surface the remaining melanin pigmentations seemed to be more easily visible during treatment and therefore resulted in better removal in sites treated with this setting. Therefore, the reevaluation scores of melanin indices were lower in this group after 1 and 12 months (Table 6).
Conclusion
Based on our results, Er,Cr:YSGG laser can be considered a more convenient method of gingival depigmentation compared to surgical blade. Although not statistically significant, laser setting 1 with short pulse duration and higher water spray showed better overall results and can remove pigmentations safely and effectively. However, laser setting 2, with longer pulse duration and less water spray resulted in better coagulative effects and less bleeding and could be used to control bleeding wherever necessary in clinical practice.
References
Dummett CO (1960) Oral pigmentation. J Periodontol 31(5):356–360
Tal H, Oegiesser D, Tal M (2003) Gingival depigmentation by erbium:YAG laser: clinical observations and patient responses. J Periodontol 74(11):1660–1667. https://doi.org/10.1902/jop.2003.74.11.1660
Lerner AB, Fitzpatrick TB (1950) Biochemistry of melanin formation. Physiol Rev 30(1):91–126
Dummett CO (1945) Clinical observations on pigment variations in healthy oral tissues of the Negro. J Dent Res 24(1):7–13
Perlmutter S, Tal H (1986) Repigmentation of the gingiva following surgical injury. J Periodontol 57(1):48–50. https://doi.org/10.1902/jop.1986.57.1.48
Barrett AW, Scully C (1994) Human oral mucosal melanocytes: a review. J Oral Pathol Med 23(3):97–103
Hedin CA (1977) Smokers’ melanosis. Occurrence and localization in the attached gingiva. Arch Dermatol 113(11):1533–1538
Hedin CA, Pindborg JJ, Axell T (1993) Disappearance of smoker’s melanosis after reducing smoking. J Oral Pathol Med 22(5):228–230
Vellappally S, Fiala Z, Smejkalova J, Jacob V, Somanathan R (2007) Smoking related systemic and oral diseases. Acta Med (Hradec Kralove) 50(3):161–166
Moravej-Salehi E, Moravej-Salehi E, Hajifattahi F (2015) Relationship of gingival pigmentation with passive smoking in women. Tanaffos 14(2):107–114
Hedin CA, Axell T (1991) Oral melanin pigmentation in 467 Thai and Malaysian people with special emphasis on smoker’s melanosis. J Oral Pathol Med 20(1):8–12
Roshna T, Nandakumar K (2005) Anterior esthetic gingival depigmentation and crown lengthening: report of a case. J Contemp Dent Pract 6(3):139–147
Almas K, Sadig W (2002) Surgical treatment of melanin-pigmented gingiva; an esthetic approach. Indian J Dent Res 13(2):70–73
Tal H, Landsberg J, Kozlovsky A (1987) Cryosurgical depigmentation of the gingiva. A case report. J Clin Periodontol 14(10):614–617
Gnanasekhar JD, al-Duwairi YS (1998) Electrosurgery in dentistry. Quintessence Int (Berlin, Germany: 1985) 29(10):649–654
Kathariya R, Pradeep AR (2011) Split mouth de-epithelization techniques for gingival depigmentation: a case series and review of literature. J Indian Soc Periodontol 15(2):161–168. https://doi.org/10.4103/0972-124x.84387
Kumar S, Bhat GS, Bhat KM (2013) Comparative evaluation of gingival depigmentation using tetrafluoroethane cryosurgery and gingival abrasion technique: two years follow up. J Clin Diagn Res 7(2):389–394
Tamizi M, Taheri M (1996) Treatment of severe physiologic gingival pigmentation with free gingival autograft. Quintessence Int (Berlin, Germany : 1985) 27(8):555–558
Pontes CC, Novaes AB, Taba M (2006) Evaluation of the efficacy of the acellular dermal matrix allograft with partial thickness flap in the elimination of gingival melanin pigmentation. A comparative clinical study with 12 months of follow-up. J Esthet Restor Dent 18(3):135–143
Atsawasuwan P, Greethong K, Nimmanon V (2000) Treatment of gingival hyperpigmentation for esthetic purposes by Nd: YAG laser: report of 4 cases. J Periodontol 71(2):315–321
Ozbayrak S, Dumlu A, Ercalik-Yalcinkaya S (2000) Treatment of melanin-pigmented gingiva and oral mucosa by CO 2 laser. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 90(1):14–15
Trelles MA, Verkruysse W, Segui JM, Udaeta A (1993) Treatment of melanotic spots in the gingiva by argon laser. Br J Oral Maxillofac Surg 51(7):759–761
Yousuf A, Hossain M, Nakamura Y, Yamada Y, Kinoshita J, Matsumoto K (2000) Removal of gingival melanin pigmentation with the semiconductor diode laser: a case report. J Clin laser Med Surg 18(5):263–266. https://doi.org/10.1089/clm.2000.18.263
Murthy MB, Kaur J, Das R (2012) Treatment of gingival hyperpigmentation with rotary abrasive, scalpel, and laser techniques: a case series. J Indian Soc Periodontol 16(4):614–619. https://doi.org/10.4103/0972-124x.106933
Hegde R, Padhye A, Sumanth S, Jain AS, Thukral N (2013) Comparison of surgical stripping; erbium-doped: yttrium, aluminum, and garnet laser; and carbon dioxide laser techniques for gingival depigmentation: a clinical and histologic study. J Periodontol 84(6):738–748. https://doi.org/10.1902/jop.2012.120094
Bergamaschi O, Kon S, Doine A, Ruben M (1993) Melanin repigmentation after gingivectomy: a 5-year clinical and transmission electron microscopic study in humans. Int J Periodontics Restorative Dent 13(1):85–92
Butchibabu K, Koppolu P, Tupili MK, Hussain W, Bolla VL, Patakota KR (2014) Comparative evaluation of gingival depigmentation using a surgical blade and a diode laser. J Dent Lasers 8(1):20
Gutknecht N (2015) Laser supported reduction of specific microorganisms in the periodontal pocket with the aid of an Er,Cr:YSGG laser: a pilot study. ScientificWorldJournal 2015:450258. https://doi.org/10.1155/2015/450258
Verma SK, Maheshwari S, Singh RK, Chaudhari PK (2012) Laser in dentistry: an innovative tool in modern dental practice. Nat J Maxillofac Surg 3(2):124–132. https://doi.org/10.4103/0975-5950.111342
Pang P, Sebastiano Andreana D, Aoki A, Coluzzi D, Obeidi A, Olivi G, Parker S, Rechmann P, Sulewski J, Caroline Sweeney M (2010) Laser energy in oral soft tissue applications. J Laser Dent 18(3):123–131
Diaci J, Gaspirc B (2012) Comparison of Er: YAG and Er, Cr: YSGG lasers used in dentistry. J Laser Health Acad 2012(6):1855–9913
Loe H (1967) The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol 38(Suppl 6):610–616. https://doi.org/10.1902/jop.1967.38.6.610
Dummett CO, Gupta OP (1964) Estimating the epidemiology of oral pigmentation. J Natl Med Assoc 56:419–420
Kishore A, Kathariya R, Deshmukh V, Vaze S, Khalia N, Dandgaval R (2014) Effectiveness of Er:YAG and CO2 lasers in the management of gingival melanin hyperpigmentation. Oral Health Dent Manag 13(2):486–491
Jacboson B, Berger J, Kravitz R, Patel P (2003) Laser pediatric crowns performed without anesthesia: a contemporary technique. J Clin Pediatr Dent 28(1):11–12
Food and Drug Administration (2009) Summary of safety and effectiveness information: Waterlase Millennium, surgical laser instrument. http://www.accessdata.fda.gov/cdrh_docs/pdf8/K083927.pdf.2001
Azzeh MM (2007) Treatment of gingival hyperpigmentation by erbium-doped:yttrium, aluminum, and garnet laser for esthetic purposes. J Periodontol 78(1):177–184. https://doi.org/10.1902/jop.2007.060167
Fekrazad R, Chiniforush N (2014) One visit providing desirable smile by laser application. J Laser Med Sci 5(1):47–50
Kang HW, Rizoiu I, Welch AJ (2007) Hard tissue ablation with a spray-assisted mid-IR laser. Phys Med Biol 52(24):7243–7259. https://doi.org/10.1088/0031-9155/52/24/004
Rosa DS, Aranha AC, Eduardo Cde P, Aoki A (2007) Esthetic treatment of gingival melanin hyperpigmentation with Er:YAG laser: short-term clinical observations and patient follow-up. J Periodontol 78(10):2018–2025. https://doi.org/10.1902/jop.2007.070041
Talebi-Ardakani MR, Torshabi M, Karami E, Arbabi E, Esfahrood ZR (2016) In vitro study of Er: YAG and Er, Cr: YSGG laser irradiation on human gingival fibroblast cell line. Acta Med Iran 54(4):251–255
Ogita M, Tsuchida S, Aoki A, Satoh M, Kado S, Sawabe M, Nanbara H, Kobayashi H, Takeuchi Y, Mizutani K, Sasaki Y, Nomura F, Izumi Y (2015) Increased cell proliferation and differential protein expression induced by low-level Er:YAG laser irradiation in human gingival fibroblasts: proteomic analysis. Lasers Med Sci 30(7):1855–1866. https://doi.org/10.1007/s10103-014-1691-4
Bakhshi M, Rahmani S, Rahmani A (2015) Lasers in esthetic treatment of gingival melanin hyperpigmentation: a review article. Lasers Med Sci 30(8):2195–2203. https://doi.org/10.1007/s10103-015-1797-3
Acknowledgements
The authors would like to thank the staff at the Oral and Dental Research Center of Zahedan University of Medical Sciences for their valuable contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was supported by a research grant from Zahedan University of Medical Sciences, Oral and Dental Research Center, and no external funding, apart from the support of this institution, was available for this study (Project Grant no. 7346). The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in this study on human participants were in accordance with the ethical standards of our institutional research committee (IR.Zaums.REC.1394.181), the 1964 Helsinki Declaration, and its later amendments. The study is registered in the Iranian Registry of Clinical Trials (IRCT ID IRCT2016113018493N3).
Informed consent
An informed consent was obtained from all participants.
Rights and permissions
About this article
Cite this article
Gholami, L., Moghaddam, S.A., Rigi Ladiz, M.A. et al. Comparison of gingival depigmentation with Er,Cr:YSGG laser and surgical stripping, a 12-month follow-up. Lasers Med Sci 33, 1647–1656 (2018). https://doi.org/10.1007/s10103-018-2501-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2501-1