Abstract
Objectives
The aim of the present study was the qualitative and quantitative evaluation of osseous graft consolidation using allogeneic bone blocks for vertical bone augmentation in an animal model.
Material and methods
Standardised allogeneic and autologous bone blocks were fixed on the frontal skull of 20 adult female pigs and covered with a resorbable collagen membrane. Animals were sacrificed after 2 and 6 months. Specimens were histologically and histomorphometrically analysed focusing on the amount of vital bone, residual bone substitute material and connective tissue. Furthermore, the amount of expression of bone matrix proteins (collagen type I and osteocalcin) and de novo vessel formation (von Willebrand factor) were quantified by immunohistochemistry.
Results
Significantly more allogeneic bone blocks failed for both evaluation time points (p < 0.05). Allogeneic blocks showed significantly less vital bone with more connective tissue formation compared to autologous bone blocks. Increased vessel formation could be detected for both evaluation time points in the contact area of autologous bone with local bone. The expression of collagen type I and osteocalcin was significantly lower in the allogeneic bone graft.
Conclusions
Allogeneic cancellous bone blocks showed a significantly higher failure rate compared to autologous bone blocks. Allogeneic bone blocks seemed to negatively affect bone formation or negatively influence the host in the long term, and increased connective tissue formation and block loss should be anticipated.
Clinical relevance
In order to maintain patient safety and treatment success clinicians should be persuaded to make a conscious choice of the applied biomaterials with regard to their components and structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The alveolar ridge is characterised by an unavoidable and permanent resorption in the vertical and horizontal dimension after tooth loss. In order to enable aesthetic and functional dental implant rehabilitation without alveolar nerve irritation or perforation of the maxillary sinus, vertical bone augmentation can be essential for patients with extensive resorption. Distraction osteogenesis, guided bone regenerations (GBR), interposition and onlay block grafting techniques have been introduced for vertical augmentation in the mandible and the maxilla. In this context, block grafting, using an autologous bone graft, has been the procedure considered the reference [7]. The surgeon has to choose the appropriate donator region depending on the bone dimension/height needed for implant insertion. In the case of large osseous defects, extraoral donator regions (e.g. posterior/anterior iliac crest) should be preferred over intraoral donator regions (e.g. the retromolar region) [32]. Studies indicate that the average vertical bone gain is 4 to 5 mm. However, bone gain is associated with a significant resorption rate of up to 40% of the onlay graft, meaning over-contouring is essential [1, 31]. In this context, autologous bone is still considered to be the gold standard [9], but the limited availability; the tendency to undergo partial resorption; the need for additional surgery, associated surgical risks and the higher patient morbidity (limping, anaesthesia, paraesthesia, residual defects, pain); the higher costs and the longer treatment time represent significant limitations that make the establishment of alternative bone blocks, e.g. allogeneic bone substitutes, inevitable [14, 24, 25].
Allogeneic bone is available in different product lines, whereby fresh-frozen bone (FFBA), freeze-dried bone (FDBA), demineralised freeze-dried bone allograft (DFDBA) and mineralised processed bone (MPBA) from living or cadaver human donators can be differentiated. The application of allogeneic bone is a well-known technique in orthopaedics and dentistry. However, due to missing resounding success of tissue engineering technologies, allogeneic bone grafts are currently undergoing a renaissance, since recent strict processing guidelines have increased the safety of the material, practically eliminating the risk of patient cross-contamination (e.g. with hepatitis or HIV) [35]. The development of innovative and effective processing techniques leads to a reduction of the antigenic potential of allogeneic bone products, whereby the local and systemic activation of the immune system can be prevented and thus the healing process can be influenced positively. In this context, a FFBA application in an elective orthopaedic surgical intervention leads to a changing antibody profile to donor-specific HLA antigens and a subsequent listing as unacceptable for organ transplantation [26]. Nevertheless, current processing procedures are not able to offer absolute remnant-free allogeneic bone grafts, whereby the immunologic potential of cell remnants is unanswered so far [11, 12, 18]. Neither clinical nor preclinical studies exist, which compare allogeneic bone grafts of different processing techniques in their histological or immunohistochemical performance. The most widespread form of allograft for dental applications is a cellular cancellous bone material, which is mechanically and chemically processed and sterilised by irradiation. Products which dominate the market include chemical processing with perchlorate acetic acid or involving hydroxyl peroxide. The common products on the market in the USA and the European Union are DIZG/MTF allogeneic bone products (Argon Produktions-und Vertriebsgesellschaft GmbH & Co KG, Bingen, Germany), Maxgraft® allogeneic bone material (botiss medical AG, Berlin, Germany) and Tutoplast/Puros allogeneic material (Zimmer Dental GmbH, Freiburg, Germany).
Allogeneic bone grafts have a predominantly osteoconductive property, acting as a scaffold into which host cells migrate, proliferate, differentiate and produce new bone. Due to persisting collagen, allogeneic bone blocks are plastically stable for screw fixation, which makes the allogeneic material attractive for the practitioner.
Case reports already suggest that allogeneic bone blocks represent a promising treatment alternative to autologous bone grafting [23, 27]. However, it must be stated that evidence is limited, due to low patient numbers and a lack of defect standardisation. A clear statement concerning the reliable applicability of allogeneic bone blocks in comparison to autologous bone blocks for vertical bone augmentation is therefore not possible on the basis of current literature.
The goal of our preclinical study was to evaluate the consolidation of allogeneic and autologous bone blocks applied for vertical bone augmentation quantitatively and qualitatively. We hypothesised that there is no statistically significant difference concerning the clinical and the histological performance within the experimental (allogeneic) and the control (autologous) group. We also hypothesised that allogeneic bone blocks represent a suitable alternative grafting material in order to avoid autologous bone grafting for vertical bone augmentation.
Materials and methods
Study characteristics and outcome variables
The preclinical study was performed in 20 healthy female domestic pigs (86 ± 2 kg) to investigate the quantity and quality of osseous block consolidation simulating a vertical bone augmentation scenario by means of histomorphological and histological evaluation parameters. For this purpose, standardised prefabricated allogeneic (experimental group) and autologous (control group) bone blocks were fixed on the frontal skull of the pigs and covered with a resorbable membrane. Since there are no methodically comparable studies for power and sample size analysis, 20 animals (ten animals per evaluation time point) were selected for reliable data generation. Since a large variation in results of allogeneic bone blocks was anticipated, twice the number of allogeneic bone blocks (two per animal) were applied compared to autologous bone blocks (one per animal).
The primary outcome variable was the clinical, histological and histomorphometrical evaluation of osseous block consolidation (Toluidine Blue O) by quantifying the proportions of vital bone (VB), remaining bone substitute material (BSM) and connective tissue (CT) in % over the entire defect volume 2 and 6 months after defect preparation.
The secondary outcome variable was the histomorphometrical evaluation of bone matrix protein expression (collagen type I and osteocalcin) and vessel formation in the augmented sites.
The manuscript was prepared in accordance with the Animal Research: Reporting In Vivo Experiments (ARRIVE) Guidelines Checklist.
Animals
The domestic pig (Sus scrofa domesticus) represents a valuable model in biomedical research due to anatomical, physiological and metabolic similarities to humans. It is an established model for bone regeneration [21, 22, 29]. Twenty female domestic pigs were therefore used in the study following a protocol approved by the Pest county government department for food safety and animal health, Hungary (approval number: PEI/001/961-2/2013 including dogs, pigs and sheep for research addressing bone and hard tissue augmentative procedures and dental implant treatment). This includes keeping the animals under circadian day and night rhythm at an ambient room temperature of 18 ± 1 °C. After a period of 4 weeks for adaption, the experimental segment of the study started. Several animals were under veterinary monitoring for the entire study period.
Anaesthesia protocol
Before the surgical procedures, the pigs were fasted overnight and handled according to the following anaesthesia protocol. Intravenous administration of ketamine HCl (Ketavet®; Ratiopharm, Ulm, Germany) was performed after an intramuscular injection of medetomidine (Domitor®, Pfizer, Karlsruhe, Germany). Perioperative antibiosis was administered 1 h preoperatively and for 2 days post-operatively to reduce the risk of infection (streptomycin, 0.5 g/day, Grunenthal, Stolberg, Germany). A veterinarian performed the anaesthesia and the permanent peri-operative monitoring of vital signs. For post-operative pain control, analgesics (0.05 mg/kg every 12 h) (Temgesic®, Böhringer Mannheim GmbH, Mannheim, Germany) were administered for 3 days following surgery.
Surgical procedure
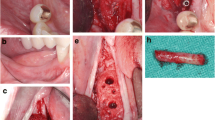
After application of a local anaesthetic in the area of the frontal skull (Ultracain DS forte®, Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany), a sagittal incision was performed and the soft tissue and the periosteum were mobilised. Per animal, two three-stepped allogeneic bone blocks simulating three different graft heights in one block (maxgraft® bonebuilder, botiss biomaterials GmbH, Zossen, Germany) with a step height of 6 mm, 9 mm and 12 mm, a length of 21 mm (7 mm per step) and a width of 10 mm were applied on the frontal skull and fixed with at least two osteosynthesis screws (KLS Martin, Gebrüder Martin GmbH & Co. KG; Tuttlingen, Germany) avoiding rotation. Autologous blocks were harvested adjacent to the recipient site. In order to prepare identical autologous bone blocks, a prefabricated template in the mentioned three-step shape was adhered to on the blocks and the corresponding block configuration was prepared with the help of a drill. In order to guarantee a gap-less and stable block position, the graft bed was prepared with an average depth of 1 mm. Following the grafting procedures, a non-cross-linked native bioresorbable collagen membrane of porcine origin (Jason® membrane; botiss biomaterials GmbH, Zossen, Germany) was adapted over the entire defect area. The periosteum and skin over the defects were sutured in two layers (Vicryl® 3.0; Vicryl 1.0®; Ethicon Co., Norderstedt, Germany).
Animal sacrifice and harvesting of specimens
After the designated healing period of 2 and 6 months, randomly selected animals were sacrificed. The pigs were sedated by an intramuscular injection of azaperone (1 mg/kg) and midazolam (1 mg/kg). Euthanasia was performed by an intravascular injection of 20% pentobarbital solution into an ear vein until cardiac arrest. The skulls were immediately dissected and stored at − 80 °C.
Specimen fixation
To identify bone blocks, cone beam computed tomography (CBCT) analyses (SCS Med Series® H23; SCS Sophisticated Computertomographic Solutions GmbH; Aschaffenburg, Germany) (Department of Radiology, University of Erlangen-Nuremberg; Director: Prof. Dr. M. Uder) were performed for all specimens in the frontal and transverse planes. In order to render the organic matrix insoluble, the specimens were dissected and subsequently fixed by immersion in 1.4% paraformaldehyde at room temperature. Performing an ascending alcohol series at room temperature (Shandon Citadel 1000, Shandon GmbH, Frankfurt, Germany) specimens were dehydrated. Xylol was used as an intermediate fixative and Technovit 9100 (Heraeus Kulzer, Kulzer Division, Wertheim, Germany) for embedding. To avoid any negative influence of polymerisation heat, the polymerisation was performed in a cold atmosphere (4 °C). After 20 h, the specimens were completely polymerised and cut in the middle of the stepped bone blocks through their longitudinal axis using a precision saw (EXAKT Advanced Technologies GmbH, Norderstedt, Germany) in order to process one half for light-microscopic and the other half for the immunohistochemical evaluation.
Histological preparation
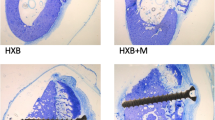
Specimens were ground into thin sections (30 μm) using a precision saw (EXAKT Advanced Technologies GmbH, Norderstedt, Germany) and a special grinding machine (EXAKT Advanced Technologies GmbH, Norderstedt, Germany). The slides were transferred in 10% H2O2 solution for 15 min. Followed by rinsing under cold running water, the specimens were stained for 15 min with Toluidine Blue O (Sigma-Aldrich Chemie GmbH, Munich, Germany). Excess stain was removed by rinsing the specimens under running water. This specific staining causes mineralised laminated tissue to stain as uncoloured to pale blue cells; cell cores, osteoid fringes, cement lines, collagen fibres and soft tissue colours itself differently blue; cartilage matrix and early wound healing areas metachromatic red-violet and calcified matrix dark-blue. The Toluidine Blue O-stained specimens were then digitised using an optical microscope (Axioskop, Carl-Zeiss AG, Jena, Germany, magnification 10) with integrated video camera and stored in TIFF format (Figs. 1 and 2).
Histological evaluation parameters
The evaluation of the Toluidine Blue O-stained specimens will distinctly show allogeneic block margins. Measuring in percentage of total graft volume, the following parameters were assessed to describe the quality and quantity of osseous graft consolidation:
-
Proportion of vital bone (VB = mineralised bone + osteoid) in the graft volume (GV) [VB/GV]
-
Proportion of residual bone substitute material (RBSM) in the GV [RBSM/GV]
-
Proportion of connective tissue (CT) in the GV [CT/GV]
Immunohistochemistry
The other halves of the specimens were used for immunohistochemistry. For this purpose, specimens were cut into 3-μm sections using a microtome (Leica microsystems, Heidelberg, Germany). Specimen preparation included rehydration with ethanol, deacrylation in 2-methoxyethylacetate, decalcification in ethylenediaminetetraacetate (EDTA), and unmasking of the antigens in citrate at 99 °C. To determine vessel formation, tissue sections were immunostained with primary antibodies directed against the von Willebrand factor (vWF) (DAKO, Hamburg, Germany; concentration 1:10,000). In order to describe the early phase of osseous graft consolidation primary antibodies against collagen type I (Abcam, Cambridge, UK; concentration 1:20,000), and for the late phase, primary antibodies against osteocalcin (Takara, Bio Inc. Seta 3-4-1, Otsu, Shiga 520-2193, Japan; concentration 1:30,000) were applied. The staining procedures were performed using the labelled streptavidin-biotin method and an autostainer (Cytomation Autostainers plus, Dako TM, Glostrup, Denmark). A secondary antibody (DAKO Diagnostics GmbH, Germany) was added to complex the primary antibody. Finally, the addition of StreptAB/HRP (DAKO Diagnostics GmbH, Germany) enabled the binding of the actual dye, AEC+ (DAKO Diagnostics GmbH, Germany), leading to a red stain in the regions localising the perspective antigen. The procedure was completed using nuclear haematoxylin counterstaining. Each specimen was accompanied by a negative control. The stained sections were digitised using an optical microscope (Axioskop, Carl-Zeiss AG, Jena, Germany, magnification 20) with integrated video camera and stored in TIFF format (Figs. 3, 4 and 5).
Anti-collagen type I stained autologous bone block 6 months after augmentation procedures. Collagen type I positive bone proportions are stained deeply red (*). Collagen Type I negative osseous tissue proportions (background) are pale red coloured (**). Intraosseous soft tissue proportions are coloured white and cell bodies blue (***)
Anti-osteocalcin stained autologous bone block 6 months after augmentation procedures. Osteocalcin positive bone proportions are stained deeply red (*). Osteocalcin negative osseous tissue proportions (background) are pale red coloured (**). Intraosseous soft tissue proportions are coloured pink (***)
Immunohistochemical evaluation parameters
In order to quantify the expression of vessels (anti-vWF) as well as the protein expression of early (collagen type I) and late (osteocalcin) osteogenesis three regions of interest (ROIs) with direct contact to local bone were selected. The expression of bone matrix proteins (collagen type I and osteocalcin) was defined as the area stained positive in proportion to the complete bony area in the region of interest and was denoted in percent.
Image analyses
The evaluation of the light microscopy and the immunohistochemistry specimens were performed by using the image processing software and evaluated with the image processing software Bioquant Osteo (Bioquant Osteo Software 2013 v13.2.6, BIOQUANT Image Analysis Corporation, Nashville, TN, USA). With the help of this program, it is possible to distinguish different tissue fractions by their individual colour spectra. The program allows tissue fractions to be assigned different colours and to use them as metric variables for calculations of various bone indices.
Statistical analysis
The statistical analysis was performed using a commercially available software program (IBM SPSS Software, Ehningen, Germany). Median values as well as standard deviations among animals were calculated for each group. To determine distribution, the data rows were examined using the Kolmogorov-Smirnov test. The non- parametric Mann-Whitney U test was used for intergroup-group comparison. A p value of less than 0.05 was considered to be significant.
Results
Clinical observations
All animals survived the operation as well as the post-operative period without problems. The initial post-operative healing phase was considered as uneventful in all pigs. No wound dehiscence or wound infections could be observed. However, during the further study course, in eight of the 20 animals, signs of chronic inflammatory progress could be observed in the area of allogeneic grafting, in the sense of reddening, swelling and fistula. No animal lost weight during any stage of the study. The general behaviour and feeding habits of all animals were considered as uneventful. Overall, 31 bone blocks failed (24 allogeneic and seven autologous bone blocks). Therefore, 15 allogeneic and 18 autologous bone blocks were included in the histological, histomorphometrical and immunohistochemical analyses.
Histological evaluation
For both evaluation time points, differences within the allogeneic and the autologous groups could be detected in descriptive histological analysis. Several allogeneic bone blocks were surrounded by formation of connective tissue (Fig. 1). No allogeneic bone block showed osseous consolidation in the area of the graft-block interface; however, a few blocks showed de novo bone formation in the central aspect. Some allogeneic grafts showed alongside chronic inflammatory reaction with connective tissue formation signs of acute inflammatory reaction in the sense of a granulomatous inflammation with adjacent osteolysis. In high magnifications, allogeneic bone blocks showed organic material consisting of cells that varied in type and number and cell debris. In contrast, the autologous bone blocks showed less connective tissue formation and fewer signs of acute and chronic inflammation. However, four autologous bone blocks were also considered as failures, due to an overarching inflammation of an adjacent allogeneic bone block. In an exceptional case, an autologous bone block showed a thin connective layer between the graft and the local bone. Autologous blocks showed osseous consolidation with living and metabolically active osteocytes (osteoid formation) for both evaluation time points. Concerning de novo vessel formation in the contact area of the applied bone blocks and the local bone, significantly more intraosseous vessels could be detected for the autologous bone graft compared to the allogeneic grafts.
Histomorphometrical evaluation
Toluidine Blue O
Using Toluidine Blue O staining, the proportions of vital bone (= transplanted bone + newly formed bone), residual bone substitute material and connective tissue within the augmented area were quantified. Focusing on vital bone after 2 months as well as after 6 months, statistically significantly more vital bone could be evaluated for the autologous bone blocks. On average, for autologous bone grafts, 67.36 ± 7.89% (median) after 2 months and 60.11 ± 7.56% after 6 months of vital bone could be quantified, whereas in the allogeneic grafts, on average, 0 ± 0.85% after 2 months and 0 ± 0.89% could be detected (Fig. 6). Concerning connective tissue proportion in autologous bone grafts, 32.64 ± 7.89% after 2 months and 39.90 ± 7.56% after 6 months were found, with significantly higher connective tissue formation in allogeneic grafts with 62.12 ± 3.98% after 2 months and 54.08 ± 8.69% after 6 months (Fig. 7). For the allogeneic block group, after 2 months, the proportion of calcified residual bone substitute material was 36.92 ± 3.44% and after 6 months, 45.60 ± 8.59% (Fig. 8). Several medians and standard deviations are illustrated in Table 1.
Immunohistochemistry
Within the autologous and the allogeneic bone blocks, significant differences concerning the expression of the bone matrix protein expression of collagen type I and osteocalcin could be detected. Whereas after 2 months, in autologous bone grafts, the collagen type I expression was 12.15 ± 7.49% (median) and after 6 months, an expression of 5.55 ± 3.33% could be quantified, which is characteristic for collagen type I expression as a protein of the early bone formation (Fig. 9). The corresponding expression within the newly formed bone in the allogeneic bone grafts was statistically significantly lower for both evaluation time points (2 months: 4.2 ± 4.03%; 6 months 1.72 ± 4.04%). The expression of osteocalcin, as a matrix protein of late bone formation, was predominantly consistent in the autologous bone grafts for both evaluation time points with 10.16 ± 3.55% after 2 months and 9.15 ± 5.02% after 6 months. Focusing on allogeneic bone blocks, osteocalcin expression was statistically significantly less compared to autologous grafts (2 months: 1.46 ± 1.12% and after 6 months 2.96 ± 2.62%) (Fig. 10). Several medians and standard deviations are illustrated in Table 2.
Discussion
The preclinical randomised controlled study aimed to evaluate the quality of osseous consolidation of allogeneic bone blocks applied for vertical bone augmentation procedures by histological and immunohistochemical evaluation in order to make a clear statement concerning its applicability in this challenging situation.
In order to generate results with a high level of transferability to humans, we chose the domestic pig due to similar bone anatomy, morphology, healing capacity, remodelling, bone density and lamellar bone structure [10]. For multiple block application, we selected the porcine calvaria as recipient site, since preparation of the forehead is simple to perform, and due to the flat surface topography of the frontal skull, which allows sufficient block fixation [34]. Due to the mentioned advantages, the forehead of the pig represents a well-cited method for investigations focusing on bone regeneration and testing of bone substitute materials and therefore an appropriate model to prove allogeneic bone block applicability.
The study was undertaken because clinical results concerning allogeneic bone application are discussed controversially in the literature, which is limited in number, quality and long-term results. A number of authors state that allogeneic bone grafts represent a reliable alternative to autologous bone, whereas quite a few clinicians are cautious about this statement [2, 3, 6, 8, 19]. A number of advantages are obvious, since no donor site preparation is necessary, while patient morbidity is lower and the bone quantity is not limited. Furthermore, it is possible to order customised CAD/CAM fabricated allogeneic bone blocks, which can represent a successful treatment option for complex defect situations in the maxillary aesthetic zone or the posterior mandible for implant insertion [2, 3, 6]. Further clinical studies underline the applicability of allogeneic bone grafting techniques for horizontal bone augmentation accompanied by reduced patient morbidity and decreased surgery time using allograft alone [33] or in combination with autologous bone/platelet-rich fibrin concentrates [30]. Other studies were able to show that allogeneic bone grafts may represent a potential resorbable scaffold for growth factors in order to improve allogeneic block graft incorporation [20], coming to the conclusion that allogeneic bone blocks represent an alternative for autologous bone grafting [16], for soft tissue/aesthetic results [28] undergoing similar resorption dynamics compared to autologous bone. However, the results of several studies should be interpreted with care, since conclusions were stated with a limited number of patients [2, 3, 16] on the basis of radiographic results [17, 33] with no standardised defect configuration or treatment protocol.
In this context, the generated histomorphometrical, histological and immunohistochemical results cannot confirm the mentioned data, since most of the blocks showed no comparable results in comparison to the autologous control block group. We observed acute as well as chronic inflammatory processes surrounding the allografts, leading to graft loss and significant connective tissue formation, which resembles a massive foreign body reaction. Thus, we reject the hypothesis that allogeneic bone blocks represent a reliable alternative for autologous bone blocks for vertical bone augmentation procedures.
Regarding possible causes of graft failure, a number of circumstances need to be considered. One essential point represents immobility of the graft, which is a prerequisite for sufficient blood clot and callus formations leading to osseous graft consolidation. In our study, several bone blocks were fixed with two or three osteosynthesis screws, which led to absolutely stable fixation of the blocks. Where the contact between graft and local bone was not ideal, the local bone surface was flattened by means of a chisel in order to guarantee gapless graft-to-local-bone contact. In addition to graft immobility and graft-to-local-bone contact, osseous graft consolidation depends on the metabolic activity of the recipient site. In our study, the recipient site and its “defect configuration” is challenging, since vertical augmentation procedures were performed in a non-space providing defect configuration (simulating the atrophic jaw), which means that osseous graft consolidation solely occurs from the basal aspect. Furthermore, allogeneic/autologous bone blocks were fixed on cortical bone, which represents a bradytrophic base, characterised by significantly less vascularisation compared to cancellous bone. However, it must be stated that these circumstances were identical for the allogeneic experimental and the autologous control groups, with the difference that successful graft consolidation could be observed for the autologous bone block group in contrast to allogeneic bone blocks. Independent of the applied biomaterial, our results underline the fact that the more demanding the environment/defect configuration is, the greater are the requirements for the selected graft. In this context, autologous bone graft, which is characterised by osteoinductive, osteogenic and osteoconductive properties, showed a better consolidation rate compared to the allogeneic block graft. Thus, our study results confirm that autologous bone block remains the “gold standard” for vertical bone augmentation procedures. However, the clear intergroup difference was unexpected. Due to massive connective tissue formation and acute inflammation adjacent to allogeneic blocks, soft/hard tissues could be observed. We therefore hypothesise that the allogeneic material per se represents the reason for grafting failure. Problems and complications after allogeneic bone material application are well known in orthopaedics; however, literature concerning allogeneic grafting failure in implant dentistry is rare. The popularity of the product can be seen in the frequency of its use. In the USA, per year 800,000 allogeneic bone grafts were used in orthopaedics [5], which mirrors the situation in Europe. However, due to poor revascularisation, complications in allograft applications in the sense of non-union or graft fracture occur in 15 to 20% of cases. In this context, grafting failure in orthopaedics must be assessed differentially, since normally, a sufficient soft tissue covering with a limited risk of graft exposure exists [5]. This is quite in contrast to grafting failure in implant dentistry, where soft tissue capacity is limited, with the consequence of persisting graft exposure to oral cavity possibly leading to local or descending life-threatening abscess formations. Reasons for the higher incidence of allogeneic grafting failure could be the fact that inflammatory reactions to human leukocyte antigen class I and II molecules especially in frozen allogeneic bone products were noted [13].
Furthermore, hypersensitivity of the recipient should be considered if bone grafts cryopreserved with dimethyl sulphoxide are used. The “NOTIFY” project, conducted by the World Health Organisation, reported problems such as adverse events of immunologic inflammation indicating the high possibility of a host-versus-graft reaction, where grafting failure using allografts is plausible [15].
In order to find reasons for chronic inflammatory reactions, in vitro characterisations of allogeneic grafts of different industrial manufacturers were conducted. Fretwurst et al. and Lorenz et al. showed in their structural histological analysis of available allografts significant multiple debris and immunogenic residues [11, 12, 18]. More precisely, the histological evaluations performed by Lorenz et al. [18] detected cellular remnants within the osteocyte lacunae and at the outer trabecular surfaces, i.e. former osteoblasts and osteoclasts, together with remnants of the former inter-trabecular adipose and connective tissue, i.e. collagenous structures and connective tissue cells or cell remnants. However, it must also be stated that histological descriptions only show the presence of cellular remnants. A statement concerning vitality is not possible so far. The questions concerning insufficient purification and the impact of cell remnants on clinical performance are not finally resolved. In a clinical prospective study Lorenz et al. demonstrated that cell remnants seemed not to affect bone formation or influence the host in the long term. However, Lorenz et al. observed in harvested graft biopsies > 50% connective tissue formation with a residual bone substitute material proportion of > 25% after 6 months of healing [19]. It could be hypothesised that the inflammatory reaction due to cell remnants led to higher connective tissue formation-induced M1 macrophage polarisation as already described by Brown et al. [4]. Summarising Lorenz’s results, major allogeneic blocks proportions were not osseous consolidated at the time point of dental implant insertion. We anticipate a limited success rate of the implant in the long term, according to the results of Draenert et al. [8]. Draenert et al. inserted in a prospective clinical study dental implants in allogeneic bone blocks immediately (one-time approach) after vertical augmentation procedures using allogeneic bone blocks or after a healing period of 3 or 4 months (two-time approach). The study aimed to evaluate the survival of the implanted bone material, soft tissue healing and the incidence of peri-implantitis by the inclusion of 20 cases. However, the study was terminated after six cases due to the unexpectedly high number of severe complications including an implant failure rate of 83% (ten of 12 implants; five out of six patients), early complete loss of the augmentation with soft tissue defects (two cases), early soft tissue maceration without loss of coverage and complete early bone healing with later peri-implantitis and bone loss after prosthetic loading (two cases) and complication-free bone healing with subsequent peri-implantitis after prosthetic loading (one case). According to the results of Draenert et al. our results suggest that in challenging vertical and/or horizontal defect situations with a significant bone volume demand, the application of allogeneic bone blocks could lead to limited treatment success leading to chronic and acute inflammatory reactions with consequent graft failure. Our results indicate that for successful allogeneic bone grafting procedures, a defect configuration of high regenerative potential (three-wall defect configurations: extraction socket, sandwich osteotomy) and a high metabolic rate (cancellous) with sufficient soft tissue volume are essential. Further preclinical and clinical studies are necessary to find the defect configuration which allows the selection of allogeneic grafting materials. Along with overreaction to remaining xenogenic human proteins, massive allogeneic bone block failure could result from possible hypersensitivity of the porcine immune system against chemicals used during graft processing, e.g. diethyl ether and ethanol.
Due to this fact, one possible limitation of the study is that graft applicability is not characterised by special stainings for acute and/or chronic inflammatory processes. Upcoming studies should focus on the quantification of vital bone, connective tissue, residual bone graft proportion, vessel formation and the expression of bone matrix proteins on the inflammatory potential of bone substitute material and their effect on osteoimmunological reactions.
Conclusion
The histological and histomorphometrical results of the present study demonstrate that the application of allogeneic cancellous bone blocks showed a significantly less bone ingrowth rate compared to autologous bone blocks. Allogeneic bone blocks seemed to affect the bone formation or influence the host in the long term, and higher connective tissue formation and block loss should be anticipated. Further studies focusing on the influence of bone substitute material on osteoimmunological reactions are essential in order to optimise bone graft selection for grafting procedures.
Change history
24 July 2020
In the article by M��st et al., entitled ���Osseous ingrowth in allogeneic bone blocks applied for vertical bone augmentation: a preclinical randomized controlled study.
References
Bernstein S, Cooke J, Fotek P, Wang HL (2006) Vertical bone augmentation: where are we now? Implant Dent 15:219–228
Blume O, Hoffmann L, Donkiewicz P, Wenisch S, Back M, Franke J, Schnettler R, Barbeck M (2017) Treatment of severely resorbed maxilla due to peri-implantitis by guided bone regeneration using a customized allogenic bone block: a case report. Materials (Basel) 10:1213
Blume O, Back M, Born T, Smeets R, Jung O, Barbeck M (2018) Treatment of a bilaterally severely resorbed posterior mandible due to early tooth loss by Guided Bone Regeneration using customized allogeneic bone blocks: A case report with 24 months follow-up data. J Esthet Restor Dent 30:474–479
Brown BN, Valentin JE, Stewart-Akers AM, Mccabe GP, Badylak SF (2009) Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30:1482–1491
Delloye C, Cornu O, Druez V, Barbier O (2007) Bone allografts: what they can offer and what they cannot. J Bone Joint Surg (Br) 89:574–579
Deluiz D, Santos Oliveira L, Ramoa Pires F, Reiner T, Armada L, Nunes MA (2017) Muniz Barretto Tinoco E: Incorporation and remodeling of bone block allografts in the maxillary reconstruction: a randomized clinical trial. Clin Implant Dent Relat Res 19:180–194
Draenert FG, Huetzen D, Neff A, Mueller WE (2014) Vertical bone augmentation procedures: basics and techniques in dental implantology. J Biomed Mater Res A 102:1605–1613
Draenert FG, Kammerer PW, Berthold M, Neff A (2016) Complications with allogeneic, cancellous bone blocks in vertical alveolar ridge augmentation: prospective clinical case study and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 122:e31–e43
Dragoo MR, Sullivan HC (1973) A clinical and histological evaluation of autogenous iliac bone grafts in humans. II. External root resorption. J Periodontol 44:614–625
Eitel F, Seiler H, Schweiberer L (1981) Morphologic examination of animal-experiment results: comparison with regeneration of the human bone-structure. I. Research methods (author's transl). Unfallheilkunde 84:250–254
Fretwurst T, Spanou A, Nelson K, Wein M, Steinberg T, Stricker A (2014) Comparison of four different allogeneic bone grafts for alveolar ridge reconstruction: a preliminary histologic and biochemical analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 118:424–431
Fretwurst T, Gad LM, Steinberg T, Schmal H, Zeiser R, Amler AK, Nelson K, Altmann B (2018) Detection of major histocompatibility complex molecules in processed allogeneic bone blocks for use in alveolar ridge reconstruction. Oral Surg Oral Med Oral Pathol Oral Radiol S2212-4403:30054–3
Friedlaender GE, Strong DM, Tomford WW, Mankin HJ (1999) Long-term follow-up of patients with osteochondral allografts. A correlation between immunologic responses and clinical outcome. Orthop Clin North Am 30:583–588
Herford AS, Dean JS (2011) Complications in bone grafting. Oral Maxillofac Surg Clin North Am 23:433–442
Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noel L, Strong DM (2012) Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation Project NOTIFY. Int Orthop 36:633–641
Jun CM, Yun JH (2016) Three-dimensional bone regeneration of alveolar ridge defects using corticocancellous allogeneic block grafts: histologic and immunohistochemical analysis. Int J Periodontics Restorative Dent 36:75–81
Kloss FR, Offermanns V, Kloss-Brandstatter A (2018) CEComparison of allogeneic and autogenous bone grafts for augmentation of alveolar ridge defects-A 12-month retrospective radiographic evaluation. Clin Oral Implants Res
Lorenz J, Schlee M, Al-Maawi S, Chia P, Sader RA, Ghanaati S (2017) Variant purification of an allogeneic bone block. Acta Stomatol Croat 51:141–147
Lorenz J, Kubesch A, Al-Maawi S, Schwarz F, Sader RA, Schlee M, Ghanaati S (2018) Allogeneic bone block for challenging augmentation-a clinical, histological, and histomorphometrical investigation of tissue reaction and new bone formation. Clin Oral Investig 22:3159–3169
Misch CM (2017) Bone augmentation using allogeneic bone blocks with recombinant bone morphogenetic protein-2. Implant Dent 26:826–831
Moest T, Koehler F, Prechtl C, Schmitt C, Watzek G, Schlegel KA (2014) Bone formation in peri-implant defects grafted with microparticles: a pilot animal experimental study. J Clin Periodontol 41:990–998
Moest T, Wehrhan F, Lutz R, Schmitt CM, Neukam FW, Schlegel KA (2015) Extra-oral defect augmentation using autologous, bovine and equine bone blocks: a preclinical histomorphometrical comparative study. J Craniomaxillofac Surg 43:559–566
Monje A, Pikos MA, Chan HL, Suarez F, Gargallo-Albiol J, Hernandez-Alfaro F, Galindo-Moreno P, Wang HL (2014) On the feasibility of utilizing allogeneic bone blocks for atrophic maxillary augmentation. Biomed Res Int 2014:814578
Nkenke E, Schultze-Mosgau S, Radespiel-Troger M, Kloss F, Neukam FW (2001) Morbidity of harvesting of chin grafts: a prospective study. Clin Oral Implants Res 12:495–502
Nkenke E, Weisbach V, Winckler E, Kessler P, Schultze-Mosgau S, Wiltfang J, Neukam FW (2004) Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: a prospective study. Int J Oral Maxillofac Surg 33:157–163
O'Sullivan ED, Battle RK, Zahra S, Keating JF, Marson LP, Turner DM (2017) Allosensitization following bone graft. Am J Transplant 17:2207–2211
Schlee M, Rothamel D (2013) Ridge augmentation using customized allogenic bone blocks: proof of concept and histological findings. Implant Dent 22:212–218
Schlee M, Dehner JF, Baukloh K, Happe A, Seitz O, Sader R (2014) Esthetic outcome of implant-based reconstructions in augmented bone: comparison of autologous and allogeneic bone block grafting with the pink esthetic score (PES). Head Face Med 10:21
Schlegel KA, Lang FJ, Donath K, Kulow JT, Wiltfang J (2006) The monocortical critical size bone defect as an alternative experimental model in testing bone substitute materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:7–13
Stopa Z, Siewert-Gutowska M, Abed K, Szubinska-Lelonkiewicz D, Kaminski A, Fiedor P (2018) Evaluation of the safety and clinical efficacy of allogeneic bone grafts in the reconstruction of the maxilla and mandible. Transplant Proc 50:2199–2201
Tonetti MS, Hammerle CH, European Workshop on Periodontology Group C (2008) Advances in bone augmentation to enable dental implant placement: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 35:168–172
Troeltzsch M, Troeltzsch M, Kauffmann P, Gruber R, Brockmeyer P, Moser N, Rau A, Schliephake H (2016) Clinical efficacy of grafting materials in alveolar ridge augmentation: a systematic review. J Craniomaxillofac Surg 44:1618–1629
Venet L, Perriat M, Mangano FG, Fortin T (2017) Horizontal ridge reconstruction of the anterior maxilla using customized allogeneic bone blocks with a minimally invasive technique - a case series. BMC Oral Health 17:146
Von Wilmowsky C, Moest T, Nkenke E, Stelzle F, Schlegel KA (2014) Implants in bone: part II. Research on implant osseointegration: material testing, mechanical testing, imaging and histoanalytical methods. Oral Maxillofac Surg 18:355–372
Waasdorp J, Reynolds MA (2010) Allogeneic bone onlay grafts for alveolar ridge augmentation: a systematic review. Int J Oral Maxillofac Implants 25:525–531
Acknowledgements
The study was performed in cooperation with the Semmelweis-University, Budapest, Hungary. Animal care keeping and surgical procedures were performed in the European Animal Research Centre (“EARC”; 2053 Herceghalom, Hungary, Gesztenyes ut 1; Certified for “Biological evaluation of medical devices” (EN ISO 10993-2:2006). Specimen processing was undertaken in the laboratories of the Department of Oral and Maxillofacial Surgery, University of Erlangen-Nürnberg, Erlangen, Germany. Botiss biomaterials GmbH, Zossen, Germany, supported this study by providing the applied allogeneic bone blocks and membranes. The authors have no conflicts of interest. The work of Dr. Endre Felszeghy, Elke Diebel and Andrea Schönherr is highly appreciated.
Funding
This project was supported by a grant from the ITI Foundation, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. The study was approved by the Pest county government department for food safety and animal health, Hungary (approval number: PEI/001/961-2/2013).
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moest, T., Frabschka, J., Kesting, M.R. et al. Osseous ingrowth in allogeneic bone blocks applied for vertical bone augmentation: a preclinical randomised controlled study. Clin Oral Invest 24, 2867–2879 (2020). https://doi.org/10.1007/s00784-019-03151-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03151-0