Abstract
Objectives
The aim of the present study was the histological investigation of an allogeneic spongious bone block for horizontal and vertical ridge augmentation in humans. The amount of new bone, soft tissue, and residual bone substitute were histomorphometrically assessed after a mean healing period of 6 months.
Materials and methods
Fourteen patients received augmentation with an allogeneic spongious bone block (Tutobone®, Tutogen Medical, Neunkirchen, Germany). After 6 months of healing, 28 implants were placed with simultaneous harvesting of bone biopsies for histological and histomorphometrical analysis. Moreover, samples from the bone blocks were collected as blanks and analyzed histologically. The formation of new bone, connective tissue, and remaining bone substitute material as well as vascularization and formation of multinucleated giant cells (MNCGs) within the augmentation bed were analyzed.
Results
New bone formation could be observed primarily in close proximity to the bone block. Histomorphometrical analyses showed 18.65 ± 12.20% newly formed bone, 25.93 ± 12.36% allogeneic spongious bone block, and 53.45 ± 10.34% connective tissue. MNCGs were observed on the biomaterial surface. Furthermore, organic residues were evident, as donor-related cellular remnants within the osteocyte lacunae were found in the blank bone blocks and in the analyzed biopsies.
Conclusion
Despite the presence of donor-related organic remnants, the bone block shows the ability to serve as a scaffold for new bone formation. Within the limits of the present study, the detect organic remnants seemed not to affect the bone formation or influence the host in the long term.
Clinical relevance
Clinicians have to make a conscious choice of the applied biomaterials with regard to their components and structure to support tissue regeneration and maintain patient safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past decades, dental implants have become a reliable and common approach for replacing missing teeth and retaining dentures since their introduction by Branemark [1]. Because of bone loss by atrophy, resective surgery, or traumatic influences, the bone amount in the prospective implant site is often reduced and requires augmentation of the alveolar crest. The achievement and maintenance of a sufficient amount and quality of hard tissue are important for a long-lasting and stable placement of dental implants. Several surgical techniques such as sinus floor augmentation and horizontal or vertical augmentation have proved to form a sufficient implant bed with more or less burden to patients and challenge to surgeons [2]. Autologous bone, e.g., harvested from the iliac crest or different enoral sites, is postulated to be the gold standard in augmentation procedures because of its osteoinductive, osteoconductive, and osteogenic potential [2,3,4,5]. However, harvesting autologous bone has numerous associated disadvantages such as donor site morbidity, the requirement for general anesthesia, and the risk of additional complications in the donor site [6,7,8,9,10].
Thus, in the past years, numerous approaches have been made to avoid autologous bone transfer and therefore avoid additional burdens and risks to patients. Beside xenogeneic bone substitute materials and synthetic bone substitute materials, allogeneic bone substitute materials from living or deceased donors may provide an almost equal alternative to autologous bone [10,11,12,13,14,15,16]. A clinical study evaluating the efficacy of allogeneic bone block grafts used for ridge augmentation before implant placement showed good integration into the recipient site and stable augmented bone. The author concluded that the use of allogeneic bone block grafts is a viable alternative to autogenous grafts in selected patients with alveolar ridge deficiencies [10].
Especially in vertical and horizontal augmentation procedures, which are esthetically important, such as the upper anterior region, or challenging, such as the lower molar region, vascularization is necessary for sufficient integration of the graft material. Block augmentations with allogeneic bone blocks combine a three-dimensional matrix for ingrowth of bone from the graft-host interface with dimensional stability and tailored fit to the recipient area [10, 17]. However, the small surface contact to vital bone in relation to the volume of the applied graft questions the complete bony fusion of the grafted bone block.
Moreover, the purification of naturally derived bone substitute materials including allogeneic and xenogeneic materials by removal of cellular remnants, thus reducing their antigenicity, is an important issue, not only for patient safety and ethical considerations but also for biomaterial scientists and practitioners. Some allogeneic bone blocks aim to include organic extracellular matrix in the form of collagen as an additional component to enhance new bone formation, because collagen is known to support bone regeneration [18]. However, the inclusion of collagen is often accompanied by the preservation of further organic structures and cellular remnants. In a recent ex vivo investigation of our group of different blank bone blocks from xenogeneic and allogeneic origin that were analyzed histologically and histochemically to detect inorganic matrix, cellular, or organic matrix components, it was shown that the investigated allogeneic bone blocks exhibited the required structure of trabecular, lamellar arranged bone, with additional signs of organic components such as collagen, fatty-like structures, and cellular remnants [19]. The aim of the present clinical study was to evaluate the clinical application of a commercially available allogeneic spongious bone block (Tutobone®, Tutogen Medical, Neunkirchen, Germany) for horizontal and vertical augmentation of the alveolar crest in the upper anterior and lower molar region before the placement of dental implants. The present investigation was specifically focused on cellular tissue response within the augmentation bed to the allogeneic bone block. By conducting histological and histomorphometrical analyses, the fraction of newly formed bone, connective tissue, and remaining bone substitute within the implantation bed was determined.
Materials and methods
Study design
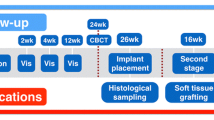
In the present study, 14 patients (9 women, 5 men) who received horizontal augmentation, vertical augmentation, or a combination of both in the upper (9 patients) or the lower (5 patients) jaw with a commercially available for clinical application allogeneic spongious bone block (Tutobone®, Tutogen Medical, Neunkirchen, Germany) were enrolled. The study was approved by the ethics commission of the University Clinic, Frankfurt, Germany (377/16) and conducted according to the World Medical Association Declaration of Helsinki (version 2008) and the STROBE guidelines for observational studies. The enrolled patients had a mean age of 60 years (range, 26–78 years) and had to meet the following criteria: reduced alveolar crest in the vertical and/or horizontal dimension for placement of dental implants that does not allow placement of implants simultaneous to a GBR procedure, minimum age of 18 years (or older), no untreated periodontal disease, smoking more than 15 cigarettes per day, no general disease that influences surgical treatment, no oncologic anamnesis or oncologic treatment in the head and face region, compliance with the study. Treatment was conducted in a private practice by one of the authors (M.S.). All patients were informed about indication of surgery, treatment alternatives, disadvantages, and drawbacks of implanted biomaterial and gave informed consent before the surgical procedure. At an average of 6 months after augmentation (range, 5–9 months) with the spongious allogeneic bone block, a total of 28 dental implants (Astra Osseo Speed, Astra Tech Implant system, Dentsply Sirona Implants, York, USA) were placed in the augmented regions. Simultaneously with implantation, 14 bone biopsies, one per patient, were taken for histological and histomorphometrical analysis of the augmented region. In patients who received more than one implant within the augmentation region, the sample for histological analysis was randomly assigned. Inclusion criteria for participating in the present study were, besides a reduced dentition in combination with a reduced alveolar crest, an adequate oral hygiene, no persistent infection of the augmentation site, and no general or medical contraindications such as bisphosphate treatment or radiation. Table 1 provides a detailed overview of included patients and the performed augmentation procedures. In addition to the analysis of human bone biopsies, five randomly allocated blanc allogeneic bone blocks were analyzed histologically to determine the structure and components of the bone block.
Surgical procedure
In all 14 patients, the augmentation procedure was conducted under local anesthesia (Ultracain 1:200.000). Through a crestal incision and mobilization of a full-thickness mucoperiostal flap, the alveolar crest was exposed, and according to the morphology of the alveolar crest, the allogeneic spongious bone block was individually tailored chairside to the prospective augmentation site. After placement, the block was fixed with at least two osteosythesis retaining screws with a diameter of 1.2 mm and a length between 10 and 14 mm (MONDE pre-implant Osteosynthesis, Mondeal Medical Systems, Mühlheim, Germany). In all augmentation procedures, a collagen membrane (Bio-Gide, Geistlich Biomaterials, Wolhusen, Switzerland) was used for covering the augmentation site. The flap was enlarged by a horizontal periostal incision, and primary wound closure was achieved with absorbable tension-free single sutures. Postoperative medication consisted of penicillin for 5 days, a 0.2% chlorhexidine mouth rinse, and 400 mg ibuprofen. At an average of 6 months (range, 5–9 months) after augmentation, a total of 28 implants were inserted in the augmented regions through a crestal approach. In this context, it has to be mentioned that the healing period varies between 5 and 9 months and was chosen individually and is therefore kind of heterogeneous. In the study protocol, a minimal healing period of 5 months was determined.
Simultaneous to implant drilling, biopsies from the augmented regions, where the implants were inserted, were harvested with trephine burrs of 3 to 5 mm according to the inserted implant’s diameter for histological and histomorphometrical analysis.
Figure 1 shows clinical images of horizontal and vertical augmentation with the allogeneic bone block in the lower jaw of the right side in patient 6.
Clinical images of patient 6 with horizontal and vertical augmentation in the lower jaw of the right side. a and b show the atrophic augmentation site previous to augmentation procedure. c shows the allogeneic bone block adapted to the morphology of the jaw and fixed with osteosynthesis screws. d shows the augmentation site at re-entry after a healing period of 7 months. The allogeneic bone block shows well integration. e shows implant drilling in regions 45, 46, and 47. f and g show insertion of dental implants in the augmentation site. h shows the bone biopsy extracted simultaneous with implant placement for histological and histomorphometrical analysis
Investigated biomaterial
For augmentation of the alveolar crest, an allogeneic spongious bone block (Tutobone®, Tutogen Medical, Neunkirchen, Germany) was used. The biomaterial is manufactured from human tibia or femur head spongiosa, harvested after joint replacement from living donators, and processed by the Tutoplast® procedure consisting of, among others, gamma sterilization. This processing results in a natural trabecular system of interconnecting pores with a preserved collagen matrix. At the same time, this procedure aims to remove unwanted materials such as cells, fat, antigens, and viruses, and to inhibit pathogens.
Tissue preparation and histology for blanc and human bone biopsies
In accordance with previously described methods, the harvested biopsies were fixed in 4% formalin for 24 h. The further processing consisted of decalcification in 10% tris-buffered ethylenediamine tetraacetic acid (EDTA) (Carl Roth, Karslruhe, Germany) at 37 °C for 4 days and dehydration in alcohol in a series of increasing concentrations and, finally, in xylol. Subsequently, the processed biopsies were embedded in paraffin longitudinally. Sections of 3 to 4 μm were cut from the biopsies’ central region with a microtome (Leica, Wetzlar, Germany). The slices were stained with hematoxylin and eosin, Masson-Goldner’s trichrome, Azan, and tartrate-resistant acid phosphatase (TRAP) according to previously described methods [12, 20,21,22,23].
Qualitative histological and quantitative histomorphometrical analysis
To evaluate the tissue reaction and the potential inflammatory reaction to the allogeneic bone block, the processed sections were analyzed histologically with a conventional light microscope (Nikon, Tokyo, Japan) in combination with a digital camera with a sight control unit (Nikon, Tokyo, Japan). Each biopsy was assessed by two independent investigators (J.L and A.K).
In addition to the histological analysis, a histomorphometric evaluation was performed to determine the tissue composition within the augmentation bed (ratio of newly formed bone, connective tissue, and remaining bone substitute material) as well as number of multinucleated giant cells (MNCGs), vessel density (i.e., number of vessels per mm2), and the percent vascularization (i.e., area of vessels within the implantation bed). According to previously published methods, images were recorded with a DS-Fi1 digital camera and analyzed with the software NIS-Elements (Nikon, Tokyo, Japan) [12, 20,21,22,23]. One hundred to 120 images of the region of interest were matched to a total scan of the implantation bed and the peri-implant tissue with an Eclipse 80i histological microscope (Nikon, Tokyo, Japan) and an automatic scanning table (Prior Scientific, Rockland, MA).
Statistical analysis
Data from the quantitative histomorphometric analysis are shown as mean ± standard deviation (SD) and a one-way univariate analysis of variance accompanied by least significant difference (LSD). Post hoc assessment was used to compare groups using SPSS 16.0.1 software (SPSS Inc., Chicago, IL, USA). Differences were considered significant (*) at P < 0.05 and highly significant (**) at P < 0.01 as well as (***) at P < 0.001. Graphs were prepared with SigmaPlot 11.0 software (SigmaPlot, Systat Software Inc., Erkrath, Germany).
Results
Clinical results
In all 14 patients, an uneventful healing could be observed. All bone blocks could be placed stable leading to no mobility or dislocation. One further patient (female, 78 years of age) initially included in the present study with augmentation of the lower jaw of the right side presented a sterile soft tissue dehiscence without signs of an acute infection (pus, secretion) after 3 months. The bone block showed at that time point an advanced integration and therefore implant placement was considered at an earlier time point. However, as 3 months of integration time was significantly shorter than the integration time of the other 14 patients, this patient was excluded from further investigation. In the other 14 patients, no signs of early or late wound dehiscence or wound infections could be observed. At the re-entry for implant placement, the bone block showed well integration in the host tissue and no signs of rejection or necrosis could be found. All implants placed in the augmentation could be placed in a satisfying primary stability and showed well osseointegration and could therefore be used for prosthetic rehabilitation, consisting of single crowns or bridge constructions.
Qualitative histology of the analyzed bone block blank samples
The blanc bone block samples showed a trabecular structure with preserved lamellar substructure. The intertrabecular area exhibited organic remnants. In addition to cellular residues within some osteocyte lacunae, the haversian canals showed remaining extracellular material (Fig. 2a–d).
Ex vivo histological analysis of the bone block. a, b Cellular residues within the osteocyte lacunae (black arrows) as well as within the intertrabecular area (red arrows), (Giemsa staining, a = × 400 magnification; b = × 100 magnification). c organic residues within the intertrabecular region including multinucleated giant cell (red arrow), (Azan staining, × 400 magnification). d cellular residues within the osteocytes lacunae (black arrows) as well as within the haversian canal (yellow arrow), (Giemsa staining, × 200 magnification)
Qualitative histology of the analyzed biopsies
Histological analysis of the bone cores extracted from the allogeneic spongious bone block augmentation beds showed the bone substitute material adjacent to the residual bone in all evaluated biopsies (Fig. 3a, b). The differentiation between the biomaterial, i.e., the allogeneic bone block, and the newly formed bone was possible according to the brighter appearance of the biomaterial compared to the newly formed bone. Moreover, the newly formed bone matrix was detectable within the bone substitute material and exhibited a more woven structure (Fig. 4a). New bone formation seemed to start from the bone-block interface and grow toward the peripheral regions of the block. Osteoblasts on the surface of the bone substitute material seemed to generate new bone formation. Regarding the cellular colonization within the augmentation bed, mononuclear cells were observed within each investigated biopsy. Furthermore, the tissue reaction constituted of multinucleated giant cells (MNGCs) distributed all over the biopsy located in close proximity the biomaterial surface (Fig. 4b). The TRAP enzyme staining showed that most of the MNGCs were TRAP-positive (Fig. 4c). Each biopsy showed a large amount of connective tissue. Within the allogeneic bone block, primarily empty lacunae from former osteocytes were observed; however, some organic material was observed in some osteocyte lacunae (Fig. 4d).
a Total scan of the augmentation region in longitudinal section shows the interface (dashed line) between the residual bone (RB) and the newly generated bone (red arrows) adherent to the allograft bone substitute material (BSM). The (BSM) appears surrounded by the connective tissue, Azan staining, × 100 magnification, 100-μm scale bar. b A horizontal augmentation using the allograft bone block at the time of biopsy harvesting (dashed line = biopsy; RB = residual bone; CT = connective tissue; BSM = bone substitute material)
a The allograft bone substitute material (BSM) adjacent to the newly generated bone (asterisks), Azan staining, × 40 magnification, 500-μm scale bar. b The interface between the allograft bone substitute material (BSM) and the newly formed bone (asterisks) as well as the presence of multinucleated giant cells in close proximity to the bone substitute material (black arrow heads), Azan staining, × 400 magnification, 20-μm scale bar. c TRAP-positive multinucleated giant cells (black arrow heads) in close proximity to the allograft surface TRAP staining, × 100 magnification, 100-μm scale bar. C1 TRAP-positive multinucleated giant cells within the surrounded connective tissue at higher magnification, TRAP staining, × 200 magnification, 50-μm scale bar. d The allograft bone substitute material (BSM) including cellular remnants within the osteocyte lacunae (black arrows), hematoxylin and eosin staining, × 200 magnification, 100-μm scale bar
Histomorphometric analysis of the tissue composition, vascularization, and MNCG formation within the extracted bone cores
Tissue composition
In addition to histologic analysis of the bone biopsies, the tissue composition within the implantation bed as well as vascularization and MNCG formation were determined. Quantitative analysis showed a ratio of newly formed bone of 18.65 ± 12.20%, allogeneic spongious bone block of 25.93 ± 12.36%, and connective tissue of 53.45 ± 10.34% (Fig. 5).
Subanalysis of the tissue composition in patients who received horizontal augmentation and patients who received a combination of horizontal and vertical augmentation revealed non-statistically significant differences (newly formed bone 19.48 ± 9.23% in HA and 15.61 ± 12.02% in HA + VA, allogeneic spongious bone block 24.55 ± 13.98% in HA and 28.14 ± 12.06% in HA + VA, connective tissue 55.12 ± 12.12% in HA and 55.72 ± 11.85% in HA + VA).
Vascularization
Analysis of the vascularization showed newly formed, homogeneously distributed vessels within the biopsies. The histomorphometric analyses of the vascularization within the implantation bed showed a vessel density of 4.73 ± 3.59 vessels/mm2 and a percent vascularization of 0.51 ± 0.52% (Fig. 6).
Multinucleated giant cell formation
The histomorphometric analysis of the total number of biomaterial-associated TRAP-positive MNCGs showed 10.13 ± 3.32 MNCG/mm2 in the augmentation site, whereas few MNCGs were sparsely detectable within the residual bone in some biopsies, 0.82 ± 2.97 MNCG/mm2 (Fig. 7).
Discussion
In the present study, a commercially available allogeneic spongious bone block was used for horizontal and vertical augmentation in case of alveolar crest atrophy to form a sufficient implantation bed before implant placement. Histological analysis of the blank bone block and the harvested biopsies was performed to evaluate the structure and components of this bone block as well as its regenerative potential. Moreover, histomorphometrical analysis was performed to determine the tissue composition within the implantation bed, i.e., the ratio of newly formed bone, connective tissue, and bone substitute material. Furthermore, vascularization and presence of TRAP-positive MNCGs were assessed.
Qualitative histologic results showed preserved donor-related organic remnants within the blank bone blocks before implantation, reflecting the lack of purification of this biomaterial. Knowing that this biomaterial is commercially available for clinical application, it should be highlighted that allogeneic bone blocks are derived from humans. Thus, purification is of utmost importance to avoid disease transmission, genetic transmission, and adverse immune response such as host-versus-graft reactions. Generally, purification methods aim to eliminate immunogenic components and inactivate pathogens. However, lack of standardized purification processes, especially in bone blocks, is reported in the literature [19, 24]. Recently, our group compared the components as stated by the manufacturer of five commercially available allogeneic and xenogeneic bone blocks with the histological analysis of their structure and constitutions. The results showed discrepancies between the components stated by the manufacturer and the histologic results in three of five blocks with regard to the presence of organic remnants such as fatty and connective tissue and cellular residues [19]. Thereby, the present study evaluated the commercially available allogeneic bone block histologically after clinical application. The histological analysis of the biopsies showed that the bone block’s augmentation region included newly formed bone and connective tissue with primarily mononuclear cells in the implantation bed. In addition, MNGCs were shown on the biomaterial surface. Initial new bone formation was observable in direct proximity to the bone block. However, the formation of connective tissue was predominant, as shown by histomorphometrical analysis. This finding is in accordance with the primary difficulties in vertical and horizontal augmentation procedures, especially in cases of advanced alveolar atrophy. This study highlighted that the allogeneic bone block rather serves as a scaffold and space holder for the ingrowth of bone by osteoconduction, instead of undergoing a full remodeling. In this context, it has to be mentioned that in general healing period needs to be prolonged in case of vertical augmentation or horizontal augmentation of larger volumes to allow sufficient vessel ingrowth within the augmentation bed [5, 13].
The present study also demonstrated that the majority of MNGCs showed the expression of TRAP and were accordingly classified as TRAP-positive MNGCs. MNGCs are cells formed by the union of several distinct cells (usually macrophages, which can appear in case of infection or in case of a foreign body within the human organism (foreign body giant cell). By staining MNGCs with the enzyme tartrate-resistant acid phosphatase (TRAP), the cells can be divided in TRAP-positive and TRAP-negative subforms. TRAP is highly expressed by osteoclasts and activated macrophages, which allows a statement about the activity of the MNGCs in the tissue reaction to biomaterials [25].
The presence of MNGCs, especially TRAP-positive within the biomaterial augmentation region, was previously described as foreign body giant cells [21, 22]. Moreover, different studies described the biomaterial-related MNGCs as foreign body giant cells [26, 27]. Thereby, the presence of these cells within the implantation material reflects that, although human-derived, this bone block induced the aforementioned MNGCs. This kind of cell has also been previously observed within the implantation bed of synthetic and xenogeneic bone substitute materials, which were frequently shown to exhibit only mineralized extracellular bone matrix without further organic components [19, 20, 28]. In former clinical studies of our group, the ability of bone substitute materials of different origin (synthetic, xenogeneic) to enhance new bone formation in the implantation bed has been investigated. It was shown that the tissue reaction, especially the formation of MNGCs, is dependent on the biomaterial’s physico-chemical properties [12, 20,21,22,23]. In a split-mouth sinus augmentation trial, the tissue reaction to a xenogeneic, bovine-based and a synthetic, hydroxy-apatite-based bone substitute material were compared. It could be detected that the synthetic bone substitute material was populated with significantly more MNGCs compared to the xenogeneic bone substitute and induced a MNGC-triggered tissue reaction, which was accompanied by a comparable higher vascularization. In contrast, the tissue reaction to the bovine-based bone substitute material consisted of mainly mononuclear cells, only a few MNGCs, and a comparably lower vascularization. However, regarding the tissue formation, no significant difference in new bone formation or bone graft resorption could be observed [21]. The present histological and histomorphometrical investigation of the allogeneic bone substitute material with preserved donor-related organic structures completes the investigations of the aforementioned xenogeneic and synthetic bone substitute materials on the understanding of the cellular mechanisms and its relation to the physico-chemical properties of the investigated biomaterials. Furthermore, investigation of a human biopsy extracted 3 years after sinus augmentation with a synthetic bone substitute material also showed the presence of MNGCs after a relatively long biomaterial-host period [22]. The foreign body response to the synthetic bone substitute material seems to be encapsulated without further contribution to degradation-related regeneration [22]. On the basis of the previous clinical observations and the present outcomes, it appears as if one reason for the induced foreign body reaction is the physico-chemical composition of the applied bone substitute material and the quality of its surface, rather than the origin from which it was derived. Thus, with the present outcomes as the completion of our clinical investigation series, it becomes obvious that the formation of foreign body MNGCs is independent of the bone substitute origin, i.e., synthetic, xenogeneic, or allogeneic. A further aspect that has to be discussed when investigating or applying allogeneic biomaterials is impurity. The histological evaluation of the biopsies allowed a qualitative differentiation between the donor bone block and the newly built bone. The bone block lacunae were filled with osteocytes residues, which are most likely to be donor related. The applied histological and histomorphometrical methodology, however, does not allow a statement if the cellular remnants show biological activity. Furthermore, within the limits of this study, it is still questionable to what extent the impurity of bone substitute materials has an effect on the long-term integration or encapsulation of the biomaterials as well as the clinical performance of the dental implants placed afterward. Therefore, long-term studies are highly needed to evaluate the function and in situ survival period of implants inserted in an allogeneic augmentation bed. Nonetheless, it might be that because of the necessary processing (e.g., sterilization), the biological capacity of the biomaterial is reduced and transforms the donor tissue in a biologically inactive scaffold. The histomorphometric results showing predominantly the formation of connective tissue (17.69 ± 11.33% newly formed bone, 26.81 ± 13.56% allogeneic spongious bone block, and 55.50 ± 10.85% connective tissue) questioning the capacity of this biomaterial to support new bone formation. The high amount of connective tissue can be explained with the type of augmentation performed in the present study (horizontal and vertical augmentation). As seen in the histological results, the amount of newly formed bone decreased from the bone-block interface toward the peripheral region, where mostly connective tissue was visible; therefore, the high amount of connective tissue can most likely be explained by the large augmentation volume. However, clinically, it was possible to insert implants within the augmentation region with sufficient primary stability. This could be explained by the stable lamellar structure of the present biomaterial that could serve as an adequate implantation bed. Moreover, the histomorphometrical analysis is in accordance with the results of previous studies, which evaluated an allograft fresh-frozen bone in three-dimensional atrophied posterior mandible showing similar histomorphometrical results (18.9 ± 8.1% newly formed bone and 32.5 ± 14.8% allograft residual bone) [29, 30]. Independently of the histological and histomorphometrical results of the present study, a clinical study by one of the authors (M.S.) could shown that bone grafting with an allogeneic bone block achieved equivalent results to autologous bone grafts regarding the esthetic outcome of the implant-retained prosthetics after a mean follow-up period of 49.5 ± 25.8 months [31].
In conclusion, the three-dimensional allogeneic bone blocks are able to form a stable framework and scaffold for larger three-dimensional defects. In contrast to particulate bone substitute materials, tension or pressure from the covering soft tissue or loading forces do not impair the stability of the graft. The aim of biomaterial research should therefore be to develop a bone substitute material that combines the advantages of allogeneic bone blocks, such as the scaffold function for bone ingrowth and the framework stability, with a high purity and safety. Therefore, there still seems to be the need for autologous bone transfer, which is accompanied by several disadvantages to the patient, as mentioned previously. A further promising approach for increasing the regenerative capacity of biomaterials is an autologous fibrin matrix (platelet-rich fibrin, PRF) [32, 33]. This concentrate of peripheral-venous blood cells is a type of natural drug delivery system and has proved to carry numerous cells and growth factors that are involved in the tissue regeneration process [32, 33]. The combination with a bone substitute material of high biocompatibility and safety that serves as three-dimensional framework could make autologous bone transfers less necessary.
However, the development of bone substitute materials that can remove the need for autologous bone transfer, especially in cases of severe atrophy and defects of challenging anatomy, is in an early phase. Furthermore, the results of the present study demonstrated the critical aspects in applying allogeneic bone grafts, which include organic and cellular remnants to attract clinicians’ attention to the choice of the biomaterials and their component to support tissue regeneration with regard to patient safety.
Conclusion
The present study reports the use of an allogeneic spongious bone block for horizontal and vertical crest augmentation before implant placement. Histologic and histomorphometric analysis showed a foreign body reaction to the bone block in the peri-implant tissue without signs of rejection or adverse effects. Within the implantation bed 18.65 ± 12.20% newly formed bone, 25.93 ± 12.36% allogeneic spongious bone block, and 53.45 ± 10.34% of connective tissue could be detected histomorphometrically. A cellular reaction consisting of primarily mononuclear cells, but also TRAP-positive MNCGs was observed. Furthermore, blank bone block samples and biopsies showed that osteocyte lacunae are filled with donor-related cellular residues. Thus, the present study outlined the critical aspects about allogeneic block purification to draw clinicians’ attention to the choice of the used biomaterials and its constituents. However, it is still unclear to what extent their presence in bone substitute materials have an effect on the regeneration capacity of the biomaterials as well as the clinical performance of the dental implants placed afterward.
References
Branemark P, Adell R, Albrektsson T, Lekholm U, Lundkvist S, Rockler B (1983) Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 4(1):25–28
Jensen S, Terheyden H (2009) Bone augmentation procedures in localized defects in the alveolar ridge: clinical results with different bone grafts and bone-substitute materials. Int J Oral Maxillofac Implants 24(Suppl):218–236
Damien C, Parsons J (1991) Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater 2:187–208
Esposito M, Grusovin M, Felice P, Karatzopoulos G, Worthington H, Coulthard P (2009) Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev 4:CD003607
Chiapasco M, Casentini P, Zaniboni M (2009) Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants 24(Suppl):237–259
Younger E, Chapman M (1989) Morbidity at bone graft donor sites. J Orthop Trauma 3:192–195
Cordaro L, Torsello F, Miuccio M, di Torresanto V, Eliopoulos D (2011) Mandibular bone harvesting for alveolar reconstruction and implant placement: subjective and objective cross-sectional evaluation of donor and recipient site up to 4 years. Clinical Oral Impl Res 22:1320–1326
Sauerbier S, Rickert D, Gutwald R, Nagursky H, Oshima T, Xavier SP, Christmann J, Kurz P, Menne D, Vissink A, Raghoebar G, Schmelzeisen R, Wagner W, Koch FP (2011) Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: a controlled, randomized, single-blinded clinical and histological trial-per-protocol analysis. Tissue Eng A 17(17–18):2187–2197
Weibull L, Widmark G, Ivanoff C, Borg E, Rasmusson L (2009) Morbidity after chin bone harvesting–a retrospective long-term follow-up study. Clin Implant Dent Relat Res 11:149–157
Peleg M, Sawatari Y, Marx R, Santoro J, Cohen J, Bejarano P, Malinin T (2010) Use of corticocancellous allogeneic bone blocks for augmentation of alveolar bone defects. Int J Oral Maxillofac Implants 25:153–162
Norton M, Odell E, Thompson I, Cook R (2003) Efficacy of bovine bone mineral for alveolar augmentation: a human histologic study. Clin Oral Implants Res 14:775–783
Stübinger S, Ghanaati S, Orth C, Hilbig U, Saldamli B, Biesterfeld S, Kirkpatrick C, Sader R (2009) Maxillary sinus grafting with a nano-structured biomaterial: preliminary clinical and histological results. Eur Surg Res 42:143–149
Aghaloo T, Moy P (2007) Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants 22(Suppl):49–70
Avila G, Neiva R, Misch C, Galindo-Moreno P, Benavides E, Rudek I, Wang H (2010) Clinical and histologic outcomes after the use of a novel allograft for maxillary sinus augmentation: a case series. Implant Dent 19:330–341
Lyford R, Mills M, Knapp C, Scheyer E, Mellonig J (2003) Clinical evaluation of freeze-dried block allografts for alveolar ridge augmentation: a case series. Int J Periodontics Restorative Dent 23:417–425
Waasdorp J, Reynolds M (2010) Allogeneic bone onlay grafts for alveolar ridge augmentation: a systematic review. Int J Oral Maxillofac Implants 25:525–531
McAllister B, Haghighat K (2007) Bone augmentation techniques. J Periodontol 78(3):377–396
Regazzoni C, Winterhalter K, Rohrer L (2001) Type I collagen induces expression of bone morphogenetic protein receptor type II. Biochem Biophys Res Commun 4;283(2):316–11
Ghanaati S, Barbeck M, Booms P, Lorenz J, Kirkpatrick C, Sader R (2014) Potential lack of “standardized” processing techniques for production of allogeneic and xenogeneic bone blocks for application in humans. Acta Biomater 10(8):3557–3562
Ghanaati S, Barbeck M, Lorenz J, Stuebinger S, Seitz O, Landes C, Kovács A Kirkpatrick C, Sader R (2013) Synthetic bone substitute material comparable with xenogeneic material for bone tissue regeneration in oral cancer patients: first and preliminary histological, histomorphometrical and clinical results. Ann Maxillofac Surg 3:126–138
Lorenz J, Kubesch A, Korzinskas T, Barbeck M, Landes C, Sader R, Kirkpatrick C, Ghanaati S (2015) TRAP-positive multinucleated giant cells are foreign body giant cells rather than osteoclasts: results from a split-mouth study in humans. J Oral Implantol 41(6):e257–e266
Lorenz J, Barbeck M, Sader R, Russe P, Choukroun J, Kirkpatrick C, Ghanaati S (2016) Foreign body giant cell related encapsulation of a synthetic material three years after augmentation. J Oral Implantol 42(3):273–277
Ghanaati S, Barbeck M, Willershausen I, Thimm B, Stübinger S, Korzinskas T, Obreja K, Landes C, Kirkpatrick C, Sader R (2013) Nanocrystal-line hydroxyapatite bone substitute leads to sufficient bone tissue formation already after 3 months: histological and histomorpho-metrical analysis 3 and 6 months following human sinus cavity augmentation. Clin Implant Dent Relat Res 15:883–892
Fretwurst T, Spanou A, Nelson K, Wein M, Steinberg T, Stricker A (2014) Comparison of four different allogeneic bone grafts for alveolar ridge reconstruction: a preliminary histologic and biochemical analysis. Oral Surg, Oral Med, Oral Pathol oral Radiol 118(4):424–431
Minkin C (1982) Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int 34(3):285–290
Anderson J, Rodriguez A, Chang D (2008) Foreign body reaction to biomaterials. Semin Immunol 20(2):86–100
Barbeck M, Booms P, Unger R, Hoffmann V, Sader R, Kirkpatrick C, Ghanaati S (2017) Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells—new insights into the material-mediated healing process. J Biomed Mater Res A 105(4):1105–1111
Barbeck M, Udeabor SE, Lorenz J, Kubesch A, Choukroun J, Sader R, Kirkpatrick C, Ghanaati S (2014) Induction of multinucleated giant cells in response to small sized bovine bone substitute (Bio-Oss™) results in an enhanced early implantation bed vascularization. Ann Maxillofac Surg 4(2):150–157
Dias R, Sehn F, de Santana Santos T, Silva E, Chaushu G, Xavier S (2016) Corticocancellous fresh-frozen allograft bone blocks for augmenting atrophied posterior mandibles in humans. Clin Oral Implants Res 27(1):39–46
Spin-Neto R, Stavropoulos A, Coletti F, Pereira L, Marcantonio E, Wenzel A (2015) Remodeling of cortical and corticocancellous fresh-frozen allogeneic block bone grafts—a radiographic and histomorphometric comparison to autologous bone grafts. Clin Oral Implants Res 26(7):747–752
Schlee M, Dehner J, Baukloh K, Happe A, Seitz O, Sader R (2014) Esthetic outcome of implant-based reconstructions in augmented bone: comparison of autologous and allogeneic bone block grafting with the pink esthetic score (PES). Head Face Med 28;10:21
Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C, Choukroun J (2014) Advanced platelet-rich fibrin (A-PRF)—a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol 40(6):679–689
Choukroun J, Ghanaati S (2016) Reduction of g-force within PRF-(platelet-rich-fibrin) concentrates advances patients’ own inflammatory cells and platelets: first introduction of the low speed centrifugation concept. European Journal of Trauma and Emergency Surgery (accepted)
Acknowledgments
The authors would like to thank Mrs. Verena Hoffmann and Mrs. Poju Chia for their excellent technical assistance.
Funding
The work was supported by the Clinic for Maxillofacial and Plastic Surgery, Johann Wolfgang Goethe University in Frankfurt Am Main, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the present study involving human participants were in accordance with the ethical standards of the ethics commission of the University of Frankfurt (377/16) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Markus Schlee and Shahram Ghanaati have equal authorship.
Rights and permissions
About this article
Cite this article
Lorenz, J., Kubesch, A., Al-Maawi, S. et al. Allogeneic bone block for challenging augmentation—a clinical, histological, and histomorphometrical investigation of tissue reaction and new bone formation. Clin Oral Invest 22, 3159–3169 (2018). https://doi.org/10.1007/s00784-018-2407-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2407-0