Abstract
Objectives
The objective of the study was to conduct a systematic review of literature assessing botulinum toxin type A (BoNT-A) safety and adverse effects in the treatment of myofascial pain (MFP) and trigeminal neuralgia (TN).
Materials and methods
The search for articles by two specific researchers involved the PubMed, EMBASE, Web of Science, and Scopus databases. Specific terms were used, and no publication time and language restrictions were applied. Clinical trials that investigated the effects of BoNT-A among participants with myofascial pain in masticatory muscles or trigeminal neuralgia were considered eligible for this systematic review. Data for each study were extracted and analyzed according to a PICO-like structured reading.
Results
The search strategy provided 436 citations. After analysis, 16 citations were included, seven for MFP and nine for TN. In all studies, BoNT-A was well tolerated and improved pain. The most common adverse effects were temporary regional weakness, tenderness over the injection sites, and minor discomfort during chewing. Most studies reported a spontaneous resolution of adverse effect.
Conclusions
It can be concluded that BoNT-A treatment is well tolerated, since minor adverse effects were the most frequently reported; however, it is recommended that future studies aim to assess the safety and possible adverse effects of multiples applications or high doses of this treatment.
Clinical relevance
BoNT-A has been increasingly diffused in dentistry, being used for the management of masticatory myofascial pain and trigeminal neuralgia. Nonetheless, there is no consensus about its efficacy and adverse effects that could occur when this treatment is applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Botulinum toxin type A (BoNT-A) has been widely used to control several motor disorders due to local, long-lasting, and also reversible paralytic effects [1, 2]. In the beginning, when BoNT-A was used to control muscle hyperactivity, an analgesic effect preceding muscle paralysis was observed and attributed to its neuromuscular effect [3, 4]. Currently, different studies have shown a direct analgesic effect [5,6,7] independent from muscle relaxation; for this reason, the drug gained new indications in the pain-control field, including the orofacial and neck area [8,9,10].
Consequently, BoNT-A has been progressively introduced as a treatment option to control pain associated to many conditions [2] such as spasticity, temporomandibular disorders (TMD), movement disorders, and bruxism [4, 11]. Several studies [8, 12,13,14,15] also reported positive effects of BoNT-A injections for trigeminal neuralgia (TN) [16], which can already be considered as a level-A treatment according to the Therapeutics and Assessment Subcommittee of the American Academy of Neurology [17, 18]; in addition, a recent systematic review showed moderate evidence for BoNT-A therapeutic effects for myofascial pain which encourage clinicians to use it [19].
BoNT-A treatment is considered generally safe, since doses used for the mentioned conditions are distant from the lethal doses [20]. However, some minor (e.g., edema and infection due to the puncture at BoNT-A injection time) [21, 22] and mild adverse effects (e.g., not desired muscle paralysis, muscle weakness, and swallowing and chewing difficulties) have been reported [21, 23]. Experimental studies have also described severe side effects after BoNT-A injections such as changes in muscle fiber size and composition, replacement of contractile tissue for fat, and even loss of bone volume and density [22, 24]. In addition, bone changes were reported in patients receiving repeated injections of high doses of BoNT-A [25, 26].
Despite all existing evidence about BoNT-A use [19, 27], there are few studies summarizing its adverse effects on the orofacial area. Thus, based on these premises and considering the rapid increase in the use of the neurotoxin, the present manuscript aims to systematically review the findings from all studies assessing BoNT-A safety and the report of the possible adverse effects.
Materials and methods
The present systematic review methodology was approved and registered (protocol- CRD42017079250) in the International Prospective Register of Systematic Reviews (PROSPERO).
Search strategy
A systematic search was conducted to identify articles assessing the adverse effects produced by BoNT-A applications, as well as the efficacy of this treatment for myofascial pain in the orofacial and for trigeminal neuralgia. PubMed, EMBASE, Web of Science, and Scopus databases were explored using the Medical Subject Headings (MeSH) and related terms which were divided into two groups as follows: for masticatory myofascial pain (myofascial pain) OR (temporomandibular joint disorders) OR (TMD) AND (“botulinum toxin”) OR (botox) OR (dysport) OR (myobloc) OR (onabotulinumtoxina) AND (adverse effects) OR (safety) OR (tolerability). For trigeminal neuralgia (trigeminal neuralgia) OR (neuropathic pain) AND (“botulinum toxin”) OR (botox) OR (dysport) OR (myobloc) OR (onabotulinumtoxina) AND (adverse effects) OR (safety) OR (tolerability).
On phase 1, citations were first screened by titles and abstracts (TiAb screening) by two independent researchers (G.D.C and R.L.P). On phase 2, potential articles were then obtained in full text and carefully read to screen for those whose purposes were not in accordance with the aim of the present review. Any disagreement between the reviewers was solved by a third researcher (V.R.M.L).The eligibility of the studies was based on the following criteria:
-
1.
Participants must be over 18 years old;
-
2.
Clinical trials that investigated the effects of BoNT-A among participants with myofascial pain in masticatory muscles or TN were considered eligible for this systematic review independently if they present a control or a comparison group;
-
3.
For masticatory myofascial pain, diagnostic criteria should be based on the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) or on the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD);
-
4.
For TN, diagnostic criteria should be based on the “International Classification of Headache Disorders (ICHD), 3rd edition (beta version).”
No publication time and language restrictions were applied.
Data collection and assessment of papers
Data for each study were extracted and analyzed according to a PICO-like structured reading, which comprises the population/problem (P), intervention (I), comparison group (C), and outcomes (O) of each study (Table 1). The following question was adopted to conduct data collection: “Are botulinum toxin injections (I) tolerable and safe for the treatment (O) of patients with masticatory myofascial pain and/or with trigeminal neuralgia (P), when compared to other treatments (C)?”
Quality assessment for the included randomized clinical trials was based on the “Cochrane Handbook of Systematic Reviews of Interventions” and for the included cohort studies, it was based on the Critical Appraisal Skills Programme (CASP).
Results
Literature search outflow
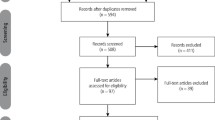
The search strategy provided 436 citations, of which 158 were overlaps. Thus, 278 citations were evaluated for eligibility (Fig. 1). Based on the reported criteria, 59 papers were read in full text and, after consensus, 16 citations were included in this systematic review (eight randomized controlled clinical studies (RCTs) and eight cohort studies) (Table 1).
Included studies
Among the 16 included studies, seven treated patients diagnosed with TMD (five RCTs and two Cohorts) and nine treated patients diagnosed with TN (three RCTs and six cohorts). Quality assessment for the RCTs by Cochrane Collaboration’s risk of bias tool and the quality assessment for the cohort studies by CASP are shown in Tables 2 and 3, respectively.
TMD studies
Regarding the seven TMD studies (Table 1), 246 patients (age range from 20 to 71 years) with masticatory myofascial pain were assessed. The total number of participants in each study varied from 10 [28] to 116 [29] with a prevalence of females. Follow-ups fluctuated from 28 days [30] to 6 months [31], and dosage of BoNT-A injection in the masseter and temporalis muscles ranged from 10 to 150 U. Only Abboud et al. [32] used 30 to 180 U of BoNT-A into one to six painful muscles (masseter, anterior temporalis, sternocleidomastoid, and posterior digastric muscles). In four studies [28, 30, 31, 33], the control group received 0.9% NaCl injections. One study [34] compared BoNT-A injection with fascial manipulation and only one study [29] reported no comparison group.
In all studies, the treatment was well tolerated, and BoNT-A injections improved pain and maximum mouth opening. The most common adverse effects were temporary regional weakness, tenderness over the injection sites, and minor discomfort during chewing [30, 32,33,34]. In addition, three studies reported asymmetric smile [28, 30, 32], and just one study [29] reported mild to severe adverse effects such as reduction in the size of the masticatory muscle (especially, masseter muscles), paraesthesia, eye drooping or muscle weakness, difficulty swallowing, speech changes, perioral swelling, and bruising. Regarding quality assessment, most of the RCTs were classified as “poor quality,” while only one study was classified as “good quality". Cohort studies varied in quality scores from 2 to 4 (Tables 2 and 3).
TN studies
As for TN (Table 1), 359 patients (age range from 28 to 85 years) were assessed with prevalence of females. The total number of participants in each study varied from 13 [35] to 100 [36]. Follow-ups fluctuated from 60 days [35] to 6 months [14, 37], and dosage of BoNT-A injection ranged from 15 U [38] to 200 U [39]. The site of the injection was intradermal, submucosal, and varied according to the patient trigger zone, except for one study in which BoNT-A injections were applied into the maxillary and mandibular nerves [37]. In three studies, the control group received 0.9% NaCl [40,41,42], four studies presented no comparison group [14, 35, 37, 38], one study compared different doses of BoNT-A [36], and another one compared the effect of BoNT-A among patients of different ages [39].
In all studies, BoNT-A injections significantly reduced pain intensity, pain attack frequency, and the number of acute medications. Two studies reported patients without side effects after treatment [35, 37]. BoNT-A was generally well tolerated, and no serious adverse effects were reported [38]. The most common adverse effects were a short-term facial weakness on the injection side [37], short-term facial asymmetry, transient edema, itching, and pain at the injection area [35, 36, 40, 41] (2014). One study reported transient paralysis of the buccal branch of the facial nerve (Bohluli et al., 2011), and one study reported transient whole-body discomfort, mild left eye ptosis, and slight oral deviation (Liu et al., 2018). Most studies showed a spontaneous resolution of adverse effects. Regarding the quality assessment, the RCTs were classified as “poor quality” to “fair quality,” and the cohort studies varied in quality scores from 1 to 9.5 (Tables 2 and 3).
Discussion
Results of the present systematic review showed that among the 16 included studies, only minor adverse effects are the most frequent after BoNT-A injections (edema, itching, and pain at the injection site). Mild adverse effects such as regional muscle weakness, short-term facial asymmetry, difficulty swallowing, speech changes, asymmetric smile, and slight oral deviation were found in less proportion, and only two studies reported severe side effects such as transient paraesthesia of the buccal branch of the facial nerve [14] and reduction in the size of the masticatory muscles [29]. Most studies reported a spontaneous resolution of minor and mild adverse effects and concluded that BoNT-A treatment is well tolerated. Notwithstanding, it is important to highlight that the majority of the selected studies did not aim to assess BoNT-A adverse effects, limiting data to self-reported adverse effects that should be carefully interpreted.
Botulinum toxin injection is an off-label therapy in dentistry. The lack of well-designed studies due to the absence of validated clinical protocols and the standardization of dosage and dilution between commercial brands contribute to increase the controversy around this treatment [43]. For these reasons, a diversity of treatment protocols was found in the included studies, with doses varying between 10 and 150 U for masticatory myofascial pain and 15 and 200 U for TN, a fact that certainly could influence the development of adverse effects. The neuromuscular effect of BoNT-A is dose- and muscle-size dependent [4], which means that bulkier muscles require higher doses to achieve a satisfactory therapeutic effect, a fact that turns even more difficult to establish protocols since there is a variety of muscles size. This pattern was also found in the included studies between the masseter and temporalis muscle, inasmuch as masseter muscles are bulkier than temporalis and require more units of BoNT-A to achieve an adequate clinical effect. Notwithstanding, none of the included studies assessed muscles size in order to propose a doses protocol.
Since BoNT-A is used in different doses, it is logical to hypothesize that higher doses and even repeated injections of this treatment could be determinant factors to develop adverse effects on muscular tissue. Muscle weakness, which is an undesired effect when BoNT-A is injected for analgesic reasons, was the most reported adverse effect. Even though this issue is spontaneously resolved in the first 3 months of treatment, none of the studies evaluated this variable objectively. In addition, muscle weakness can lead to a series of other effects such as the reduction of occlusal and bite force for a period of 12 to 18 weeks [26, 42, 44] and the decline in masticatory performance [45]. These findings can be explained by the duration of BoNT-A into muscles. The toxin reaches its maximum muscle effect between day 14 and 21 after injection, and it is sustained for about 90 days before it gradually diminishes [4]. This means that the effects of a single application of BoNT-A in muscle function seems to be transient. On the other hand, a reduction in the size of the masticatory muscles after two applications of BoNT-A was reported in Khawaja et al. [29] and Lee et al. [26], who also reported a significant decrease in masseter muscle thickness and cross-sectional areas after 6 months of BoNT-A treatment with high doses. For both studies, no data about muscle recovery was reported. Taken together, this data could confirm that higher doses or repeated applications of this treatment could lead to possible structural changes in muscle fibers. From a physiological point of view, the inhibition of the exocytose of acetylcholine toward motor endplate by BoNT-A causes a direct paralytic effect on muscles, a fact that could provoke atrophy and a reduction in muscle size. Experimental studies have demonstrated that muscle atrophy after BoNT-A injections is due to the decrease of fibers size [46], replacement of contractile tissue for fat [47], changes in muscle-fiber composition (i.e. from IIa to IIb fibers type) [46] and also by influencing the mRNA content of myosin of the treated muscles [48]. Unfortunately, due to the lack of clinical trials assessing repeated injections or higher doses of BoNT-A, the doubt if repeated injections could extend the mentioned adverse effects for a longer period of time remains.
It is well known that muscle size and muscular force, diminished by BoNT-A injections, are necessary factors to promote an appropriated muscle contraction and stimulate the apposition and resorption bone process [49]. Therefore, it would not be erroneous to cogitate that BoNT-A could have at least an indirect effect on bone tissue, due to the lack of stimulus coming from altered muscle function. None of the included studies reported or aimed to assess BoNT-A effects on bone tissue. Notwithstanding, experimental studies have reported less trabecular bone, high incidence of bony defects filled with active fibrocartilaginous tissue, higher bone porosity, bone loss in the alveolar region, trabecular bone loss in the condyle, and a decrease in bone volume after 1 to 3 months of BoNT-A injections [22, 24, 50].
Furthermore, some clinical studies that were not included in the present systematic review due to a lack of use of standardized diagnostic tools (RDC/TMD, DC/TMD, and ICHD beta version) have demonstrated a decrease in bone density of the mandibular condyle after multiple injections of BoNT-A [25] and a significant reduction in mandibular volume angle area after a second BoNT-A injection [26]. Based on these findings, it could be hypothesized that BoNT-A could have a direct toxicity effect on skeletal cells, or that the general inhibition of neurotransmitters release could affect another local signaling in the bone and cartilage [50]. However, it is also possible that these effects are merely consequences of modifying bone loading.
Clinically, BoNT-A therapeutic injections are usually given at intervals of 3 to 6 months, Severe side effects reported in this systematic review came mainly from studies performing one (experimental studies) or multiples (clinical studies) injection sessions of BoNT-A; based on that, it can be assume that patients treated with this drug could develop side effects in muscle and bone tissues. Therefore, it is important to analyze BoNT-A effectiveness for the conditions reported in this review. Even though BoNT-A reduced pain in almost all included studies, it was not superior when compared with placebo or other treatments, mainly due to the lack of standardized therapeutic protocols (doses and injection sessions) which are important for achieving an adequate effectiveness on any drug. It is important to consider the lack of evidence about BoNT-A effectiveness and the development of side effects before using this treatment.
To our knowledge, this may be the first systematic review dedicated to BoNT-A toxin adverse effects. The main purpose of any systematic review is to connect data from high-quality studies, synthesizing knowledge about a specific topic. The present review explored different databases to gather literature regarding BoNT-A adverse effects within an adequate search strategy and a strict criterion for the inclusion of papers. However, mainly due to the small sample size and an inadequate study design, the overall quality of the evidence was considered “poor,” owing to the risk of bias. Also, most of the studies included in this review did not assess objectively BoNT-A adverse effects, limiting to describe minor self-reported adverse effects (edema, itching, and pain at the injection site) that might be caused by the needle and/or clinician’s skills and not by the BoNT-A itself. As a final remark, we strongly recommend assessing the ratio between BoNT-A effectiveness and the possible development of adverse effects (mainly from multiple applications) before clinically using BoNT-A in the orofacial region.
Conclusion
Within the limitations of this review, it can be concluded that even though none of the included studies aimed to assess objectively BoNT-A adverse effects, this treatment in general was reported as well tolerated, since self-reported minor adverse effects with a spontaneous resolution were the most prevalent. Notwithstanding, it is recommended that future studies assess BoNT-A adverse effects mainly produced from multiple or high-dose applications, as well as the ratio between the effectiveness and the probability of developing adverse effects when this substance is the treatment choice.
References
Pellett S, Yaksh TL, Ramachandran R (2015) Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins (Basel) 7:4519–4563
Thenganatt MA, Fahn S (2012) Botulinum toxin for the treatment of movement disorders. Curr Neurol Neurosci Rep 12:399–409
Colhado OCG, Boeing M, Ortega LB (2009) Botulinum toxin in pain treatment. Braz J Anesthesiol 59:366–381
Dressler D, Adib Saheri F, Reis Barbosa E (2005) Botulinum toxin: mechanisms of action. Arq Neuropsiquiatr 63:180–185
Matak I, Lackovic Z (2014) Botulinum toxin a, brain and pain. Prog Neurobiol 119–120:39–59
Lora VRMM, Clemente-Napimoga JT, Abdalla HB, Macedo CG, de la Canales Torre G, Barbosa CMR (2017) Botulinum toxin type A reduces inflammatory hypernociception induced by arthritis in the temporomadibular joint of rats. Toxicon 129:52–57
Matak I, Tékus V, Bölcskei K, Lacković Z, Helyes Z (2017) Involvement of substance P in the antinociceptive effect of botulinum toxin type A: evidence from knockout mice. Neuroscience 358:137–145
Wu C-J, Lian Y-J, Zheng Y-K, Zhang H-F, Chen Y, Xie N-C, Wang LJ (2012) Botulinum toxin type A for the treatment of trigeminal neuralgia: results from a randomized, double-blind, placebo-controlled trial. Cephalalgia 32:443–450
Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14:162–173
Ranoux D, Attal N, Morain F, Bouhassira D (2008) Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol 64:274–283
De la Torre Canales G, Câmara-Souza MB, do Amaral CF, Garcia RCMR, Manfredini D (2017) Is there enough evidence to use botulinum toxin injections for bruxism management? A systematic literature review. Clin Oral Investig
Zhang H, Lian Y, Ma Y, Chen Y, He C, Xie N et al (2014) Two doses of botulinum toxin type a for the treatment of trigeminal neuralgia: observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J Headache Pain 15:1–6
Zúñiga C, Piedimonte F, Díaz S, Micheli F (2013) Acute treatment of trigeminal neuralgia with onabotulinum toxin A. Clin Neuropharmacol 36:146–150
Bohluli B, Motamedi MHK, Bagheri SC, Bayat M, Lassemi E, Navi F, Moharamnejad N (2011) Use of botulinum toxin A for drug-refractory trigeminal neuralgia: preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 111:47–50
Morra ME, Elgebaly A, Elmaraezy A, Khalil AM, Altibi AM, Vu TL, Mostafa MR, Huy NT, Hirayama K (2016 Dec) Therapeutic efficacy and safety of botulinum toxin A therapy in trigeminal neuralgia: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain 17(1):63
Hu Y, Guan X, Fan L, Li M, Liao Y, Nie Z, Jin L (2013) Therapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: a systematic review. J Headache Pain 14:72
Safarpour Y, Jabbari B (2018) Botulinum toxin treatment of pain syndromes –an evidence-based review. Toxicon. 147:120–128
Soares A, Andriolo RB, Atallah AN, da Silva EM (2014) Botulinum toxin for myofascial pain syndromes in adults. Cochrane Database Syst Rev (7):CD007533
Khalifeh M, Mehta K, Varguise N, Suarez-Durall P, Enciso R (2016) Botulinum toxin type A for the treatment of head and neck chronic myofascial pain syndrome: a systematic review and meta-analysis. J Am Dent Assoc 147(12):959–973
Turton K, Chaddock JA, Acharya KR (2002) Botulinum and tetanus neurotoxins: structure, function and therapeutic utility. Trends Biochem Sci 27:552–558
Coté TR, Mohan AK, Polder JA, Walton MK, Braun MM (2005) Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol 53:407–415
Kun-Darbois JD, Libouban H, Chappard D (2015) Botulinum toxin in masticatory muscles of the adult rat induces bone loss at the condyle and alveolar regions of the mandible associated with a bone proliferation at a muscle enthesis. Bone 77:75–82
Kim BW, Park GH, Yun WJ, Rho NK, Jang KA, Won CH, Chang SE, Chung SJ, Lee MW (2014) Adverse events associated with botulinum toxin injection: a multidepartment, retrospective study of 5310 treatments administered to 1819 patients. J Dermatol Treat 25:331–336
Rafferty KL, Liu ZJ, Ye W, Navarrete AL, Nguyen TT, Salamati A, Herring SW (2012) Botulinum toxin in masticatory muscles: short- and long-term effects on muscle, bone, and craniofacial function in adult rabbits. Bone 50:651–662
Raphael KG, Tadinada A, Bradshaw JM, Janal MN, Sirois DA, Chan KC, Lurie AG (2014) Osteopenic consequences of botulinum toxin injections in the masticatory muscles: a pilot study. J Oral Rehabil 41(8):555–563
Lee HJ, Kim SJ, Lee KJ, Yu HS, Baik HS (2017) Repeated injections of botulinum toxin into the masseter muscle induce bony changes in human adults: a longitudinal study. Korean J Orthod 47(4):222–228
Ho KY, Tan KH (2007) Botulinum toxin A for myofascial trigger point injection: a qualitative systematic review. Eur J Pain 11(5):519–527
Nixdorf DR, Heo G, Major PW (2002) Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain. 99(3):465–473
Khawaja SN, Scrivani SJ, Holland N, Keith DA (2017) Effectiveness, safety, and predictors of response to botulinum toxin type a in refractory masticatory myalgia: a retrospective study. J Oral Maxillofac Surg 75(11):2307–2315
Kurtoglu C, Gur OH, Kurkcu M, Sertdemir Y, Guler-Uysal F, Uysal H (2008) Effect of botulinum toxin- A in myofascial pain patients with or without functional disc displacement. J Oral Maxillofac Surg 66(8):1644–1651
Guarda-Nardini L, Manfredini D, Salamone M, Salmaso L, Tonello S, Ferronato G (2008) Efficacy of botulinum toxin in treating myofascial pain in bruxers: a controlled placebo pilot study. Cranio. 26(2):126–135
Abboud WA, Hassin-Baer S, Joachim M, Givol N, Yahalom R (2017) Localized myofascial pain responds better than referring myofascial pain to botulinum toxin injections. Int J Oral Maxillofac Surg 46(11):1417–1423
Ernberg M, Hedenberg-Magnusson B, List T, Svensson P (2011) Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: a randomized, controlled, double-blind multicenter study. Pain. 152(9):1988–1996
Guarda-Nardini L, Stecco A, Stecco C, Masiero S, Manfredini D (2012) Myofascial pain of the jaw muscles: comparison of short-term effectiveness of botulinum toxin injections and fascial manipulation technique. Cranio. 30(2):95–102
Piovesan EJ, Teive HG, Kowacs PA, Della Coletta MV, Werneck LC, Silberstein SD (2005) An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology. 65(8):1306–1308
Zhang H, Lian Y, Ma Y, Chen Y, He C, Xie N, Wu C. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J Headache Pain. 2014;15:65
Türk Börü Ü, Duman A, Bölük C, Coşkun Duman S, Taşdemir M (2017) Botulinum toxin in the treatment of trigeminal neuralgia: 6-month follow-up. Medicine (Baltimore) 96(39):e8133
Caldera MC, Senanayake SJ, Perera SP, Perera NN, Gamage R, Gooneratne IK (2018) Efficacy of botulinum toxin type an in trigeminal neuralgia in a South Asian Cohort. J Neurosci Rural Pract 9(1):100–105
Liu J, Xu YY, Zhang AL, Luo WF (2018) Efficacy and safety of botulinum toxin type a in treating patients of advanced age with idiopathic trigeminal neuralgia. Pain Res Manag 7365148:5
Wu CJ, Lian YJ, Zheng YK, Zhang HF, Chen Y, Xie NC, Wang LJ (2012) Botulinum toxin type A for the treatment of trigeminal neuralgia: results from a randomized, double-blind, placebo-controlled trial. Cephalalgia. 32(6):443–450
Shehata HS, El-Tamawy MS, Shalaby NM, Ramzy G (2013) Botulinum toxin-type A: could it be an effective treatment option in intractable trigeminal neuralgia? J Headache Pain. 14:92
Zhang H, Lian Y, Xie N, Chen C, Zheng Y (2017) Single-dose botulinum toxin type a compared with repeated-dose for treatment of trigeminal neuralgia: a pilot study. J Headache Pain 18(1):81
Ramirez-Castaneda J, Jankovic J, Comella C, Dashtipour K, Fernandez HH, Mari Z (2013) Diffusion, spread and migration of botulinum toxin. Mov Disord 28(13):1775–1783
Kim KS, Byun YS, Kim YJ, Kim ST (2009) Muscle weakness after repeated injection of botulinum toxin type A evaluated according to bite force measurement of human masseter muscle. Dermatol Surg 35(12):1902–1906
Park HU, Kim BI, Kang SM, Kim ST, Choi JH, Ahn HJ (2013) Changes in masticatory function after injection of botulinum toxin type A to masticatory muscles. J Oral Rehabil 40(12):916–922
Tsai CY, Lin YC, Su B, Yang LY, Chiu WC (2012) Masseter muscle fibre changes following reduction of masticatory function. Int J Oral Maxillofac Surg 41(3):394–399
Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W (2011) Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox). J Biomech 44(1):39–44
Gedrange T, Gredes T, Spassov A, Mai R, Kuhn DU, Dominiak M, Kunert-Keil C (2013) Histological changes and changes in the myosin mRNA content of the porcine masticatory muscles after masseter treatment with botulinum toxin A. Clin Oral Investig 17(3):887–896
Raadsheer MC, van Eijden TM, van Ginkel FC, Prahl-Andersen B (1999) Contribution of jaw muscle size and craniofacial morphology to human bite force magnitude. J Dent Res 78(1):31–42
Matthys T, Ho Dang HA, Rafferty KL, Herring SW (2015) Bone and cartilage changes in rabbit mandibular condyles after 1 injection of botulinum toxin. Am J Orthod Dentofac Orthop 148(6):999–1009
Funding source
Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (number 2017/21674-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix. CASP, Critical Appraisal Skills Programme
Appendix. CASP, Critical Appraisal Skills Programme
To access all 12 items of CASP quality assessment for cohort studies, click the following link:
https://casp-uk.net/wp-content/uploads/2018/03/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf
Rights and permissions
About this article
Cite this article
De la Torre Canales, G., Poluha, R.L., Lora, V.M. et al. Botulinum toxin type A applications for masticatory myofascial pain and trigeminal neuralgia: what is the evidence regarding adverse effects?. Clin Oral Invest 23, 3411–3421 (2019). https://doi.org/10.1007/s00784-019-03026-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03026-4