Abstract
Objectives
Botulinum toxin A (Botox) is increasingly used for treatment of muscle hyperfunction. For a better understanding of the possible morphologic and chewing changes in patients induced by a therapy with Botox, muscle fiber and myosin heavy chain (MyHC) mRNA alterations were examined in this animal study.
Materials and methods
The investigation was carried out on 14-week-old pigs (seven treated animals, eight controls; calculated animal size with a power of 0.5). To initialise the total immobilisation of the right masseter, the Botox injection was distributed into ten areas. After a 56-day period, muscle tissue was taken from the left and right side of the masseter (three regions), temporal (two regions), medial pterygoid and geniohyoid muscles using a standardized method. The muscle fiber cross sections were examined immunohistochemically. Fiber staining was accomplished with antibodies to specific MyHC isoforms. The MyHC mRNA changes were analysed using real-time RT-PCR.
Results
Muscles adapt to such stress by changing fiber types and MyHC mRNA content. Paralysed masseters display atrophic changes while other masticatory muscles show hypertrophic changes. The results indicated that the typical distributions of type IIa und IIb fiber types in masticatory muscles were increased in the masseter muscles due to Botox application. On the other hand, the masseters without Botox in the treated group showed a significant increase of type I MyHC.
Conclusions
Application of Botox may lead to uncontrolled structural changes in affected and unaffected muscles.
Clinical relevance
Treatment of muscle hypertrophy with Botox may cause muscle imbalance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The masticatory muscles are part of the strongest muscle groups in the human organism. The Musculus (M.) masseter and the M. temporalis play the decisive roles in chewing. The Mm. temporales are mainly responsible for the chopping movement of the lower jaw. The Mm. masseterici are substantially responsible for the grinding movement. It is known that muscle paralysis in very young patients can lead to changes in muscle structure and bone form. The muscle resection carried out by Schumacher [1] on young animals of different chewing types led to scoliosis with increasing age. Within the last years, extensive experimental studies have fundamentally enlarged our knowledge about the features of skull growth associated with changed muscle performance [2–5]. In this context, Fränkel [6] emphasizes that a balance between antagonistic muscles, which are inside or outside on the bone surface, is an absolute prerequisite for harmonious bone growth. He has not only taken into consideration the physiological function or dysfunction of the muscles but also the various tonicity of neighbouring muscle groups. Changes in masticatory muscle performance and the elimination of dysfunctions also facilitate orthopaedic corrections of malocclusions.
The force–velocity properties of muscle fibers, responsible for the differentiated muscle functions, are mainly dependent on their myosin heavy chain (MyHC) composition [7]. Myosin is a highly conserved, ubiquitous protein found in all eukaryotic cells, where it provides the motor function for diverse movements such cytokinesis, phagocytosis and muscle contraction [8]. There are several MyHC isoforms which can be classified according to their contraction speed in fast type (MyHC-2b, MyHC-2x and MyHC-2a) and slow type (MyHC-1) [7–9]. The genes coding for MyHC isoforms are both temporally and spatially regulated, and differential expression of MyHC isoforms contributes to the extensive diversity observed in skeletal muscle fiber types [9]. Skeletal muscle fibers can adapt to stress by changing their myosin heavy chain composition. Animal studies indicate differential expression of MyHC mRNA in muscles of mastication during functional advancement of the mandible [10, 11]. Furthermore, changes in the MyHC mRNA amounts were found after orthognathic surgery of patients with malocclusions [12, 13].
Stress and abnormal brain or spinal cord activity may lead to excessive or uncoordinated loading of muscles followed by non-physiological spasms of these muscles and often causing pain. Another kind of disturbance is insufficient muscle innervation. Botulinum toxin A (Botox A), a toxin produced by Clostridium botulinum, binds to the presynaptic cholinergic nerve terminals and inhibits the release of acetylcholine. Inhibition of acetylcholine produces chemical denervation and paralysis of the striated muscle. Botox A is used as a treatment for neuromuscular conditions such as dystonias and spasticity [14, 15]. Furthermore, Botox A is increasingly used in treatment of problems in the orofacial region [16, 17]. Locally administered botulinum toxin A is a good method for effective treatment of various movement disorders like bruxism [18, 19] or masseteric hypertrophy [20–22], musculoskeletal pain and temporomandibular joint disorders [23, 24]. Recently, it was shown that paresis of the right masseter of pigs after Botox A injection is followed by changes in the ionic content [25]. It is known that changes in the content of bound and diffusible ions in the muscle are related to muscle fiber composition [26]. Thus, the aim of the present animal study was to analyse the effect of Botox administration on muscle structure of paralysed and unparalysed masseter muscle of the pig. For this, the effect of unilateral paresis on the proportion of type I and type II muscle fibers and on type I and type II MyHC mRNA content was investigated using quantitative reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry.
Material and methods
The Saxon State government committee for animal research approved the protocol and all surgical procedures of the study (no. 24-9168.11-1-2002-1).
Animals
The experiments were carried out on 15 randomised pigs (race “Deutsches Landedelschwein”) as described previously [25]. All pigs were 13 weeks old and were acclimatized for 7 days in the holding facility. After this period, seven pigs were anesthetised before injection of 100 U of botulinum A (Botox®, Merz Pharma, Germany) into the exposed right masseter muscle. Eight pigs served as controls. In these animals, the skin was cut and the muscles were visualized, but no treatment was applied. The pigs were investigated, and the body weight was monitored at the 1st and 7th week. For identification of individual animals, the trovan® (Trovan Ltd. Company, Germany) identification system was used. A microchip sized 2.0 × 0.15 × 40 mm was injected subcutaneously in the dorsal region.
Masseter muscle denervation and muscle biopsy
Anesthesia was started by intramuscular injection of ketamine hydrochloride (10 mg/kg) and atropine sulfate 0.5 mg/1 ml (B. BRAUN, Germany). After placement of a venous catheter into the ear vein, anesthesia was maintained by injections of propofol (Abbott, Germany; 0.7 mg kg−1 h−1). In all animals, the right masseter muscle was surgically exposed. Seven pigs (treatment group) underwent injections of 10 ml with 100 U of botulinum A—into ten regions of the whole right masseter. In the control group, physiological sodium chloride solution was injected. After 56 days of investigation, muscle biopsies were carried out under anesthesia without resuscitation of the animal. Muscle tissue was collected from different regions of the masseter muscle (M1 = anterior, M2 = medial, M3 = posterior), of the temporal muscle (TP1 = anterior, TP2 = posterior), as well as from the medial pterygoid (PM), and the geniohyoid (GH) muscles (Fig. 1). A preliminary investigation revealed no significant differences between the right and left side masticatory muscles of healthy pigs. Therefore, the excision was performed on the right side in controls and treated pigs and additionally on the left side in treated pigs. After excision, each muscle sample was immediately divided into pieces for further investigation. The 56 control and 91 treated muscle samples were formaldehyde fixated for immunohistochemical fiber differentiation. Also, samples were frozen in liquid nitrogen and stored at −80°C for mRNA isolation. The animals were killed by an intravenous injection of T1 solution (B. BRAUN, Germany).
Immunohistochemistry
For immunohistochemical identification of myosin heavy chains in cross sections of muscles, the following methods were used: type I MyHC after Narusawa et al. [27] (mouse monoclonal anti-skeletal myosin antibody–MyHC I; clone: NOQ 7.5.4D, Sigma, Deisenhofen, Germany; dilution, 1:20), type IIa after Hughes et al. [28] (mouse monoclonal anti-skeletal myosin antibody–MyHC IIa, clone: A 4.74, Alexis, Swiss; dilution, 1:50), and type IIb monoclonal antibodies BF-F3 obtained from DSM (Deutsche Sammlung von Mikroorganismen und Zellkulturen).
For immunostaining of fibers, muscle samples were fixed in 4 % neutral buffered formaldehyde at room temperature (25°C). After 4 days of fixation, the samples were embedded in paraffin. Eight-micrometer cross sections were prepared at 20°C using a microtome (model 2055, Leica, Bensheim, Germany). Serial sections were collected on poly-l-lysine-coated glass slides, dried and stored at room temperature (RT) and then processed in parallel. Sections were preincubated in TRIS-buffered saline (TBS) and then exposed to 3.0 % hydrogen peroxide in 60 % methanol, 40 % buffered saline at room temperature for 20 min. To block non-specific binding of antibodies, the sections were incubated in 0.1 M TBS containing 1 % bovine serum albumin and 10 % normal serum at RT for 4 h. Primary antibodies were applied at 5°C for 12 h. After washing in TBS for 10 min, the serial sections were incubated for 30 min with a commercially available biotinylated goat anti-mouse antibody (Dako, Glostrup, Denmark; dilution, 1:100). Sections were then incubated for 30 min with avidin–biotinylated–peroxidase complex solution (Vectastain kit, Vector, Buvlingame, CA, USA). The brown colour was developed with diaminobenzidine (Aldrich Chemical Company, Inc., Milwaukee, USA), and the sections were counterstained with hematoxylin. For negative controls, the primary antibodies were replaced by TBS or irrelevant monoclonal IgG.
Morphometric analysis of the muscle tissue cross sections
The histological specimens were evaluated with an Olympus Imaging System device. Scanning was done with a CCD camera ColorView II (Soft Imaging System, Germany) installed at the BX-61 microscope. The total magnification of the microscope was × 100. The preparations were scanned at a 3.2-fold enlargement with 1,376 × 1,032 pixels of resolution. The fiber area was measured using a computer with a Pentium processor and the 3.2 Analysis Software (SIS, Germany). For area measurements, at least 25 % of each muscle sample was evaluated [11].
Determination of the protein content
The total protein content in the masticatory muscles was determined using the Biuret method as modified by Klinger and Müller [29]. Bovine serum albumin (Sigma, Germany) served as the protein standard.
RNA preparation and reverse transcription reaction
Forty milligrams of each muscle was homogenized with the TriPure™ Isolation Reagent (Roche, Germany) and by using a hand homogenizer (Merck, Germany). After adding chloroform, the muscle homogenates were centrifuged at 12,000 × g for 15 min at 4°C, and the RNA containing aqueous supernatant was transferred into a separate tube. The extracted RNA was precipitated with isopropanol and washed with 70 % ethanol. The RNA containing precipitate was dissolved in RNase-free water and mixed at 58°C for 15 min. The concentrations of RNA were determined by measuring the optical density by the absorbance of ultraviolet light at 260 nm and background compensation for the absorbance at 320 nm with a Photometer Smartspec (Bio-Rad, Germany). Quality and integrity of total RNA was checked on a 1 % agarose gel. Total RNA was stored at −80°C until quantification of the MyHC mRNA.
One microgram of each total RNA sample was used in reverse transcription reactions performed with the Clontech RT-PCR-Kit (USA) for synthesis of cDNA. The reaction was carried out at 42°C for 60 min. The reverse transcription reaction was inactivated by incubation for 5 min at 94°C.
Quantitative real-time RT-PCR
The expression of different types of MyHC mRNAs was evaluated by quantitative RT-PCR [30]. PCR reactions were performed using the Bio-Rad iCycler iQ system (Bio-Rad, Germany). The SYBR Green PCR core reagents kit (Applied Biosystems, USA) was used for the PCR reaction. The reaction contained 5 μl of SYBR reagent, 2 μl of diluted cDNA and 10 μM primers in a 50 μl volume. The thermal cycle conditions consisted of initial denaturation step at 95°C for 30 s, followed by 40 cycles of 95°C for 15 s and 60°C for 10 s. The fluorescence threshold value was calculated using the iCycle iQ system software. The transcript number of the pig β-actin was quantified as an internal RNA control, and each sample was normalized on the basis of its β-actin content. Additionally, the efficiency of the PCR was controlled by addition of the same mRNA sample in each PCR reaction. Relative quantification of the gene expression was obtained from the threshold cycle value (C T) which is the point where a significant increase of fluorescence is first detected. This method provided validation for the experiments performed (amplification efficiencies) between control and experimental groups as outlined for β-actin. The final data were normalized to β-actin and are presented as the molecules of transcript per molecules of β-actin × 100 (% β-actin). Results are expressed as means ± SE. All experiments were repeated at least twice on separate days to validate results.
Primers
PCR primers were designed between 170 and 367 bp in length using the BLAST program. The primer sequences for pigs MyHC of type I were as follows: CAGACTGAAGTGGAGGAGGC forward and reverse CTTCCGTCTGGTACGTGAGC (355 bp). The primer sequences for pigs MyHC of type IIa were as follows: GAAGCAGAGGCCGGTGGGACC forward and reverse TGGACGCGAGGAGCTTCTCAG (367 bp). The primer sequences for pigs MyHC of type IIb were as follows: GCCTTGAGCCTGCCACCGTC forward and reverse AAAGGATTCCTTGGGCTCCGCC (220 bp). The primer sequences for pigs MyHC of type IIx were as follows: AGGGTCTTTGACTGGGCTGCC forward and reverse CCCTCCTTCTCTGCTCTGGACA (211 bp). The primer sequences for muscle myosin light chains (LC) were as follows: probe, ATGTCCTTCAGTGCTGACCAG forward and reverse ATTGCTGGGGTTTCCCAGAAC (170 bp). The primer sequences for β-actin were as follows: CCGGCCATGTACGTGGCCATC forward and reverse CTCGGCCGTGGTGGTGAAGCT (227 bp).
Normalization of quantitative PCR
Two series of control samples were included in each assay. The first consisted of a series of five successive fivefold dilutions of an individual reverse transcription product from total RNA extracted from an untreated muscle. This series of controls allowed the estimation of the PCR efficiency by interpolating the slope of the curve relating the C T parameter obtained for each point with the relative concentration of cDNA (dilution of reverse transcription product). Theoretically, the PCR efficiency is 100 % when the number of copies doubles at each PCR cycle. After testing different couples of primer pairs in various magnesium concentrations, we kept the pair that gave the highest PCR efficiency (>90 %). The second series of controls consisted of five different RT products from various amounts of the pigs’ total skeletal muscle RNA (respectively, 1, 100, 10, 1 and 0.1 ng) mixed with an appropriate amount of total RNA from the worm Eisenia foetida andrei to keep the final amount of RNA in the RT mix to 1 μg. RNA from this worm was chosen because it is easy to obtain, and the gene sequences are divergent enough from the pigs’ gene sequences not to compete at the PCR level. With these controls, a standard curve was produced by associating the C T with its corresponding mRNA relative concentration.
To account for variations due to RNA extraction and the RT reaction, the measured levels of MyHC and β-actin mRNAs were correlated with those of β-actin mRNAs. Because the β-actin gene is ubiquitously expressed, we consider the mRNA level of β-actin to be proportional to the quantity of total RNA of all types of cells included in the sample. Results were expressed as the ratio of the mRNA level of each gene of interest (MyHC, LC) to the mRNA level of β-actin × 100 (% β-actin).
Statistical analysis
The sample size of n = 15 animals for statistical analysis was calculated with Sigma Plot version 11. The following elements were used for this calculation: alpha = 0.05; desired power = 0.9; expected difference in means = 1.3; expected standard deviation = 1.0.
Statistical evaluation was performed with the SPSS program. The results are given as arithmetic means ± standard deviation. For statistical analysis, Student’s t test was applied to evaluate differences in fiber types and biochemical data between controls and treated animals. Two by two comparisons were used in the same animal group, *p < 0.01, **p < 0.001; control n = 8 and treated animal group n = 7.
Results
Body and dental status
For all analyses, eight controls and seven treated animals were used. With these group sizes, the realized power of this study was 0.5. In all groups, a short-term increase of the chewing frequency occurred after surgical treatment. The increase of the body weight was similar in both experimental groups and corresponds to age (control group, pre-test: 32.1 ± 2.7 kg; after 8 weeks: 48.7 ± 4.0 kg and animals with Botox, pre-test: 33.0 ± 2.7 kg; after 8 weeks of treatment: 47.7 ± 3.9 kg).
Immunohistochemical changes in the muscle structure
In the present study, all histological used methods made possible a clear differentiation between type I, IIa and type IIb muscle fibers. At the staining with the type I antibody type I fibers turned brown. Type II fibers remained unstained (Fig. 2). The application of botulinum toxin led to a slight increase in the cross-sectional area of type I muscle fibers (Fig. 3) and an increase in the proportion of cross section areas of type I fibers. Beside M. geniohyoideus, all tested muscles showed a pronounced change of the muscle composition in the type I fiber area. Marked changes mainly occurred in the masseter muscle and smaller ones in the temporal muscle 8 weeks after Botox application. The injection of botulinum toxin is caused by a significant decrease of the fiber area in the whole masseter muscle in comparison to controls (p < 0.05) and in comparison to the left side (p < 0.001). The type I fiber area of pigs treated with Botox was even on the left side in contrast to a low increase in controls (p < 0.05). In contrast to the masseter, the right side of the temporal muscle (non-treatment) showed an increase of the type I fiber area in comparison to controls. The same proportion of increase was seen on the left side of temporal muscle. A further increase in the type I fiber area (control 21.2 % to right side 26.7 %; p < 0.01) was found in the medial pterygoid muscle (PM). No significant changes were found in the geniohyoid muscle (Table 1). Significant changes also occurred in the type IIa fiber area. In the three different regions of the masseter muscle (M1, M2, M3) a slight increase in the type IIa area was seen with botulinum toxin as compared to control. However, the differences were significant only for the left side of the masseter muscle. This difference was additionally caused by a decrease of the type IIa fiber area in the left side. The decrease of type IIa fibers was significant in M1 and M2 of the masseter muscle compared to controls (p < 0.05). The left side decrease of IIa fibers was significant in the anterior part of the temporal muscle (TP1) as compared to control. Treatment with botulinum toxin caused the smallest changes in type IIb fibers which occupied the smallest part of the whole muscle cross section (about 3 %). Significant differences were measured only in the masseter muscle. The injection of botulinum toxin induced an increase of the type IIb fiber area in the right masseter muscle in comparison to control (p < 0.05). In contrast to the right side, the left-sided masseter muscle showed a decreased type IIb area (p < 0.01). These changes of the fiber number were observed in the measured area and in the number of cells. The total number of fibers per measured area differs only in masseter muscles treated with Botox.
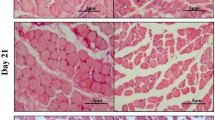
Immunohistochemistry of fiber type I in muscle cross sections of the anterior region of the masseter (M1). Myosin heavy chain protein distribution in masseter muscle was determined by indirect immunohistochemistry using a monoclonal anti-skeletal myosin heavy-chain type I antibody (Clone NOQ 7.5.4D, Sigma, Germany: dilution, 1:20). a Control animals, b Botulinumtoxin A treated animals, ride sight; c Botulinumtoxin A-treated animals, left sight
Percentage of fiber type numbers in overall muscle cross sections. M1 anterior region of the M. masseter, M2 medial region of the M. masseter, M3 lower posterior region of M. masseter, TP1 anterior region of the M. temporalis, TP2 posterior region of the M. temporalis, GH M. geniohyoideus and PM M. pterygoideus medialis. Arithmetic means ± standard deviation; t test; control = diamond and test group (treated animals), right side botox application = square; left side = triangle. Differences between control and right side: *p = 0.05; **p = 0.01; ***p = 0.001; control and left side: + p = 0.05; ++ p = 0.01; +++ p = 0.001; and right and left side: # p = 0.05; ## p = 0.01; ### p = 0.001. Encircled square = muscle probes with direct application of Botulinum toxin A
MyHC mRNA expression
Real-time RT-PCR analysis was used to quantify the relative levels of the MyHC mRNA expression. The differences between target gene and β-actin are given in (% β-actin). In these investigations, four types of MyHC mRNAs were evaluated. However, since the type IIx protein was not clearly detected, the type IIx mRNA was measured.
After 8 weeks of endurance stress on muscle structure by application of botulinum toxin in the right masseter muscle, only MyHC type I and IIb mRNA showed significant changes (Fig. 4). These molecular findings correspond with the histological results. The total masseter muscle (M1, M2 and M3) treated with botulinum toxin showed a significant decrease of the type I MyHC mRNA (p < 0.05) as compared to the control masseter muscle. On the other hand, a significant increase of the type I mRNA (p < 0.05) was observed in the left-sided masseter muscle as compared to the botulinum-treated and control masseter muscle. High rates of changes were also found between the right and left side of the total masseter muscle (p < 0.001). The changes showed similar proportions in the M1 (anterior), M2 (medial) and M3 (posterior) part of the masseter muscle (Fig. 4). Similar to the type I mRNA changes, type IIb MyHC mRNA showed significantly changed proportions only in the masseter muscle. In contrast to type I, the type IIb mRNA was significantly increased (p < 0.05) in the masseter muscle with botulinum toxin after 8 weeks of application. The increase of the mRNA level from the right side was similar to that on the control and left side. The left side masseter muscle showed no significant difference from control (Fig. 4). No changes in type I MyHC and type IIb MyHC were measured in the temporal, the medial pterygoid (PM) and in the geniohyoid muscle (GM) (Fig. 4). The levels of type IIa and IIx MyHC mRNA relative to β-actin showed no significant differences between the three groups of muscles (control, right and left side of treated animals; Table 1). Both types of mRNA (IIa and IIx), however, displayed a higher standard deviation than the type I and IIb mRNAs.
Content of MyHC type I and type IIb mRNA. M1 anterior region of the M. masseter, M2 medial region of the M. masseter, M3 lower posterior region of M. masseter, TP1 anterior region of the M. temporalis, TP2 posterior region of the M. temporalis, GH M. geniohyoideus and PM M. pterygoideus medialis. Arithmetic means ± standard deviation; t test; control = grey columns; test group (treated animals)–right side with botox application = checked column and left side without botox application = white columns. Differences between control and right side: *p = 0.05; **p = 0.01; ***p = 0.001; control and left side: + p = 0.05; ++ p = 0.01; +++ p = 0.001, and right and left side: # p = 0.05; ## p = 0.01; ### p = 0.001
Protein content
The total protein content in the masticatory muscles of all groups showed no changes after 8 weeks of treatment (Table 2).
Discussion
The present study demonstrates for the first time the examination of Botulinum toxin effects on the histology of muscle fiber and the mRNA content of myosins in various masticatory muscles. The pig was chosen as the animal model since the orofacial structures are similar to those of humans. Botulinum toxin A (Botox) has been increasingly used therapeutically in the masseter muscle. It was shown that intramuscular Botox application induces direct and significant alterations of the fiber composition [31]. The mRNA content of the respective myosins was influenced by masseter treatment. The histological changes compare and are identical to the molecular biological findings. Our findings are in agreement with former studies from Dood et al. [32]. In the injected plantaris and gastrocnemius muscle type I MyHC increased while type IIa/x decreased. Furthermore, it was shown that the botulinum toxin causes also shifts in MyHC composition in contralateral muscles [32]. Just as botulinum toxin injection chronic denervation is followed by changes of the fiber type composition and MyHC content in rat muscles [33, 34]. Parallel findings from histology and molecular biology are also reported in literature [10]. Although the alterations found in this study are not as marked as in complete muscle paresis, the former are still evident even after 56 days’ recovery. The mild influence in the masseter muscle may result from the permanent interplay of the masticatory muscles. Thus, Botox application has a chronic impact on the affected muscles and their structure [31]. Monkey masseter muscle showed few histological changes such as enlarged mitochondria in the region of the I-band and aberrations in the Z-line after Botulinum toxin A injection [35].
A closer analysis reveals that the largest changes of the mRNA content are limited to the masseter. However, the hypothesis that Botox effects are confined to the treated muscles cannot be supported. The histological findings show that treatment has an impact on the other chewing muscles as well. The medicinal influence of Botulinum toxin A exerted in the masseter induced a locally restricted paresis. However, Botox treatment of the masseter indirectly exerts stress and impact on the other masticatory muscles. The function of the contralateral masseter muscle is altered oppositely. Such changes point to heavy load to the muscle. Due to masseter treatment, the temporal muscle is subjected to extra load and is likely to take the paralyzed masseter’s functions. This state resembles that of humans who attempt to compensate for a disturbance by intentional chewing on the contralateral, unaffected side.
Similarly, fiber composition is altered following experimental impact. Recently, it was shown that endurance muscle training induced by sagittal advancement of the mandible induce an increase of type I fibers in pigs [10]. These fiber alterations are considered to indicate optimum training. The medial pterygoid muscle which, like the masseter muscle, plays an important role in adduction displays only slight alterations restricted to type I fibers. Obviously, the muscle functions not only as a coordinator in tooth clenching, but is actively involved in chewing as well.
Since application of Botox implies neither muscle extension nor compression, the changes required to develop greater force are relatively small. In addition, the other muscle groups took over the masseter functions. The force capacity of the masticatory muscle is very large. The masticatory muscles are permanently activated, not only during food ingestion, but also during rest or sleep [5, 36]. The load was distributed by the numerous muscles involved in chewing, thus minimizing the morphological and molecular biological changes. Moreover, the permanent movement produces a massage effect that bears on the Botox-treated masseter muscle. Probably, muscle perfusion is affected only slightly due to the permanent muscle movement. Altered perfusion is another factor that reflects muscle stress [37].
Using Botox, we have affected muscle function and altered the force systems. Muscle performance is affected also by biochemical, mechanical and structural parameters. According to the respective muscle function, the supply and transformation of chemical into mechanical energy (force or movement) need to be regulated and fitted to the respective requirements. This is effected using a muscle-specific range of proteins which combined form the “contractile apparatus” of a muscle. In a previous study, we examined the impact of Botox on the ion concentration in the muscle [25]. Despite a change of ion content, mRNA and the type of myosins in the muscles, the total protein content remains constant. Thus, the function and experimental Botox treatment only have a slight impact on the protein content per gram of tissue.
Application of Botox results in uncontrollable changes in the affected and unaffected muscle structures. These changes may cause a new imbalance. We used the geniohyoid muscle as a “control muscle”. Since the geniohyoid muscle is not directly involved in jaw abduction, it is not surprising that it fails to show any alteration. Hence, the two muscles, i.e. masseter and geniohyoid muscle, do not share the responsibility for the same function within the masticatory mechanism.
The applied methods and the low power of 0.5 need to be considered critically. The first issue refers to the point of time of muscle sampling. The mRNA response to stress is very rapid. Increased synthesis and accumulation of mRNA occur instantly, e.g. after loading. The mRNA content demonstrated represents a steady state rather than the current stress of the muscles. This study aimed to show the chronic effects of Botox because muscle remodelling is completed after 8 weeks of treatment as shown previously [38]. Protein synthesis and histologic remodelling reveal the full effects of Botox.
Also, the findings in pigs may be transferred to loading conditions in humans. However, the masticatory muscles of pigs are not directly comparable to the humans. The porcine masticatory muscles consist of 20 % type I fibers [10, 11]. Human masseter and temporal muscles have also heterogeneous fiber type composition, proportion of the fiber type 2 being generally smaller [39, 40]. However, our results imply heterogenous changes in anatomic structure of masticatory muscles due muscle paresis, which may influence their functional properties. This work adds some more information to how muscles adapt to these stress factor.
In summary, intramuscular Botox injections in the masticatory muscles alter the fiber composition and mRNA content of the respective myosins. Histological and molecular biological changes run parallel after Botox application. Assessment should be based on both finding categories combined. Botox application to one chewing muscle indirectly yields stress also to the others. The Botox effect may be compared to paresis, and thus, Botox application serves as a model for this disease pattern.
References
Schumacher GH (1968) Der maxillo-mandibuläre Apparat unter dem Einfluss formgestaltender Faktoren. Nova Acta Leopoldina 33:109–113
Alajbeg IZ, Valentic-Peruzovic M, Alajbeg I, Cifrek M (2006) The influence of age and dental status on elevator and depressor muscle activity. J Oral Rehabil 33:94–101
Herring SW, Rafferty KL, Liu ZJ, Marshall CD (2001) Jaw muscles and the skull in mammals: the biomechanics of mastication. Comp Biochem Physiol A Mol Integr Physiol 131:207–219
Teng S, Herring SW (1998) Compressive loading on bone surfaces from muscular contraction: an in vivo study in the miniature pig, Sus scrofa. J Morphol 238:71–80
van der Bilt A, Engelen L, Pereira LJ, van der Glas HW, Abbink JH (2006) Oral physiology and mastication. Physiol Behav 89:22–27
Frankel R (1980) A functional approach to orofacial orthopaedics. Br J Orthod 7:41–51
Bottinelli R, Canepari M, Pellegrino MA, Reggiani C (1996) Force–velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495(Pt 2):573–586
Weiss A, Leinwand LA (1996) The mammalian myosin heavy chain gene family. Annu Rev Cell Dev Biol 12:417–439
Schiaffino S, Reggiani C (1996) Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76:371–423
Gedrange T, Luck O, Hesske G, Buttner C, Seibel P, Harzer W (2001) Differential expression of myosin heavy-chain mRNA in muscles of mastication during functional advancement of the mandible in pigs. Arch Oral Biol 46:215–220
Gedrange T, Walter B, Tetzlaff I, Kasper M, Schubert H, Harzer W, Bauer R (2003) Regional alterations in fiber type distribution, capillary density, and blood flow after lower jaw sagittal advancement in pig masticatory muscles. J Dent Res 82:570–574
Gedrange T, Buttner C, Schneider M, Oppitz R, Harzer W (2005) Myosin heavy chain protein and gene expression in the masseter muscle of adult patients with distal or mesial malocclusion. J Appl Genet 46:227–236
Harzer W, Worm M, Gedrange T, Schneider M, Wolf P (2007) Myosin heavy chain mRNA isoforms in masseter muscle before and after orthognathic surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:486–490
Bhidayasiri R, Truong DD (2005) Expanding use of botulinum toxin. J Neurol Sci 235:1–9
Rudzinska M, Wojcik M, Szczudlik A (2010) Hemifacial spasm non-motor and motor-related symptoms and their response to botulinum toxin therapy. J Neural Transm 117:765–772
Gadhia K, Walmsley D (2009) The therapeutic use of botulinum toxin in cervical and maxillofacial conditions. Evid Based Dent 10:53
Hoque A, McAndrew M (2009) Use of botulinum toxin in dentistry. N Y State Dent J 75:52–55
Lee SJ, McCall WD Jr, Kim YK, Chung SC, Chung JW (2010) Effect of botulinum toxin injection on nocturnal bruxism: a randomized controlled trial. Am J Phys Med Rehabil 89:16–23
Tan EK, Jankovic J (2000) Treating severe bruxism with botulinum toxin. J Am Dent Assoc 131:211–226
Al-Ahmad HT, Al-Qudah MA (2006) The treatment of masseter hypertrophy with botulinum toxin type A. Saudi Med J 27:397–400
Arikan OK, Tan FU, Kendi T, Koc C (2006) Use of botulinum toxin type A for the treatment of masseteric muscle hypertrophy. J Otolaryngol 35:40–43
Kim HJ, Yum KW, Lee SS, Heo MS, Seo K (2003) Effects of botulinum toxin type A on bilateral masseteric hypertrophy evaluated with computed tomographic measurement. Dermatol Surg 29:484–489
Chang H (2005) Botulism toxin: use in disorders of the temporomandibular joint. Dent Today 24(48):50–51, quiz 51
Schwartz M, Freund B (2002) Treatment of temporomandibular disorders with botulinum toxin. Clin J Pain 18:S198–S203
Gedrange T, Mai R, Richter G, Wolf P, Lupp A, Harzer W (2005) X-ray microanalysis of elements in the masticatory muscle after paresis of the right masseter. J Dent Res 84:1026–1030
Wroblewski R, Arvidsson I, Eriksson E, Jansson E (1987) Changes in elemental composition of human muscle fibres following surgery and immobilization. An X-ray microanalytical study. Acta Physiol Scand 130:491–494
Narusawa M, Fitzsimons RB, Izumo S, Nadal-Ginard B, Rubinstein NA, Kelly AM (1987) Slow myosin in developing rat skeletal muscle. J Cell Biol 104:447–459
Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM (1993) Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol 158:183–199
Klinger W, Muller D (1974) The influence of age on the protein concentration in serum, liver and kidney of rats determined by various methods. Z Versuchstierkd 16:149–153
Heid CA, Stevens J, Livak KJ, Williams PM (1996) Real time quantitative PCR. Genome Res 6:986–994
Borodic GE, Ferrante R, Pearce LB, Smith K (1994) Histologic assessment of dose-related diffusion and muscle fiber response after therapeutic botulinum A toxin injections. Mov Disord 9:31–39
Dodd SL, Selsby J, Payne A, Judge A, Dott C (2005) Botulinum neurotoxin type A causes shifts in myosin heavy chain composition in muscle. Toxicon 46:196–203
Bobinac D, Malnar-Dragojevic D, Bajek S, Soic-Vranic T, Jerkovic R (2000) Muscle fiber type composition and morphometric properties of denervated rat extensor digitorum longus muscle. Croat Med J 41:294–297
DelGaudio JM, Sciote JJ (1997) Changes in myosin expression in denervated laryngeal muscle. Ann Otol Rhinol Laryngol 106:1076–1081
Capra NF, Bernanke JM, Porter JD (1991) Ultrastructural changes in the masseter muscle of Macaca fascicularis resulting from intramuscular injections of botulinum toxin type A. Arch Oral Biol 36:827–836
Wood WW (1987) A review of masticatory muscle function. J Prosthet Dent 57:222–232
Bauer R, Gedrange T, Bauer K, Walter B (2006) Intrauterine growth restriction induces increased capillary density and accelerated type I fiber maturation in newborn pig skeletal muscles. J Perinat Med 34:235–242
Kranjc BS, Sketelj J, D’Albis A, Erzen I (2001) Long-term changes in myosin heavy chain composition after botulinum toxin a injection into rat medial rectus muscle. Invest Ophthalmol Vis Sci 42:3158–3164
Eriksson PO, Thornell LE (1983) Histochemical and morphological muscle-fibre characteristics of the human masseter, the medial pterygoid and the temporal muscles. Arch Oral Biol 28:781–795
Korfage JA, Brugman P, Van Eijden TM (2000) Intermuscular and intramuscular differences in myosin heavy chain composition of the human masticatory muscles. J Neurol Sci 178:95–106
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gedrange, T., Gredes, T., Spassov, A. et al. Histological changes and changes in the myosin mRNA content of the porcine masticatory muscles after masseter treatment with botulinum toxin A. Clin Oral Invest 17, 887–896 (2013). https://doi.org/10.1007/s00784-012-0750-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0750-0