Abstract
Objective
The aim of the present in vitro study was to evaluate the remineralizing effects of NaF, AmF, KF gels and NaF toothpaste in combination with a potentially demineralizing saliva substitute (Glandosane; pH = 5.1) being widely used in Germany.
Methods
In each of 120 dentin specimens, three artificial lesions were created. One lesion was covered for analysis of pre-demineralization (ΔZB). Treatments during pH cycling (3 × 1 h demineralization/day [pH = 5.0] and 3 × 3 h Glandosane/day; 12 h 100%humidity) were as follows: no treatment (NT), application (5 min,2×/day) of 12.500 ppm F− [pH = 6.04] (NaF-gel1), 12.500 ppm F− [pH = 7.34] (NaF-gel2), 12.500 ppm F− [pH = 5.82] (AmF-gel), 1450 ppm F− [pH = 7.35] (KF-gel), and 5000 ppm F− [pH = 8.14]; (NaF-TP) for 7 days (E1). Subsequently, from each specimen, one lesion was covered, while the remaining lesion was cycled for another 7 days (E2). Differences in integrated mineral loss (ΔΔZE1/ΔΔZE2) were calculated between values before and after pH cycling.
Results
Mean (95%CI) ΔZB was 3851 (3762;3939) vol% × μm. Except for NaF-gel2 and NaF-TP, specimens of all other groups further demineralized. Only NaF-gel2 induced a significant gain in mineral content (p ≤ 0.004; paired t test). Significant differences in the change of mineral loss were found between NT and all fluoride groups for both ΔΔZE1 and for ΔΔZE2 (p < 0.05, Bonferroni post hoc test). However, only NaF-gel2 and NaF-TP induced remineralization.
Conclusion
Under the in vitro conditions chosen, all fluoride agents could significantly hamper the adverse effects of a demineralizing saliva substitute.
Clinical significance
In combination with a demineralizing saliva substitute, slight mineral gain was only observed for neutral NaF-gel2 and 5000 ppm F− toothpaste.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xerostomia is the sequel of several diseases and therapies, as for example head and neck radiotherapy [1] or Sjögren’s syndrome [2]. A decreased salivary flow leads to a reduced remineralizing potential of saliva resulting in reduced buffer capacity within cariogenic dental biofilms [3]. Therefore, patients suffering from xerostomia not only lack oral comfort but are also often afflicted with rampant caries—in particular in dentin [4,5,6].
Saliva substitutes are supposed to relieve the sensation of dry mouth [7] and focus on preventing lesion progression through improving patient’s oral hygiene. A number of artificial salivas with different chemical compositions demonstrated neutral or remineralizing effects on dental hard tissues [8]. One particular saliva substitute—being widely used in hospitals and care facilities in Germany—is Glandosane (Cell Pharm, Hannover, Germany) [9]. Glandosane is a carboxymethylcellulose (CMC)-based saliva substitute which is well-accepted by the patients because of pricing, taste, and handling [10]. However, in vitro, it has been observed that Glandosane induces demineralizing effects on enamel and dentin [8, 11, 12].
A systematic Cochrane review suggests that the application of highly concentrated fluoride gels results in a caries-inhibiting effect in healthy children and adolescents [13]. Moreover, for irradiated patients, highly fluoridated products such as gels are commonly recommended for caries prevention [14], since the topical use of these agents might trigger increased remineralization of enamel and dentin. Previous in vitro studies indicated that the additional use of fluoride agents in combination with a potentially demineralizing saliva substitute reduces mineral loss when compared with the saliva substitute alone [15]. The demineralizing effects of Glandosane were reduced by a mouthrinse containing AmF-SnF2 (250 ppm F−), a mouthrinse containing AmF-KF (250 ppm F−), a gel containing NaF (12,500 ppm F−), and a gel containing AmF (12,500 ppm F−) [15]. Furthermore, in another study, the application of a NaF-gel (12,500 ppm F−) and a highly fluoridated NaF toothpaste (5000 ppm F−) could significantly hamper the demineralizing effect of Glandosane [16]. Interestingly, brushing with the highly fluoridated toothpaste seems to result not only in a reminalization being several time higher compared to no brushing [11, 12, 17], the application of AmF-KF mouthrinse [11, 17], or brushing with AmF toothpaste [11, 12, 17] but also compared to the application of NaF-gel [16]. However, in the previous in vitro studies, specimens were solely stored in remineralizing solutions for either 5 or 10 weeks, respectively. No demineralization solution was used intermittently to simulate oral pH fluctuations that occur in the oral environment frequently.
Thus, the purpose of the present in vitro study was to compare NaF, AmF, KF gels and NaF toothpaste in combination with a potentially demineralizing saliva substitute in a net-demineralizing pH cycling model. We hypothesized that no significant differences in mineral loss would be observed between the fluoride agents but for all compared with the no treatment control.
Material and methods
Specimen preparation
Two hundred dentin specimens (6 × 4 × 4 mm3) were prepared from 50 extracted bovine incisors (negative BSE test) and stored in aqueous 0.08% thymol solution. Subsequently, all specimens were embedded in epoxy resin (Technovit 4071; Heraeus Kulzer, Wehrheim, Germany), and dentin surfaces were ground flat and hand-polished (waterproof silicon carbide papers, FEPA grit sizes: 1200 and 4000; Struers).
Lesion formation
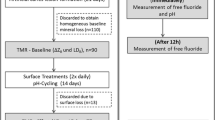
Half of the specimens were covered with acid-resistant nail varnish (Jet Set; L’oréal, Paris, France) (sound control windows; SC) (Fig. 1). Three artificial lesions were created by immersion in a solution of constant composition containing 47.6 μM NaF, 2.2 mM CaCl2 × 2H2O, 2.2 mM KH2PO4, 50 mM CH3COOH, and 10 mM KOH (Merck, Darmstadt, Germany) at pH 5.0 in an incubator (37 °C; BR 6000; Heraeus Kulzer) for 5 days [16] (demineralized treatment areas, windows DTB, DTE1, DTE2). The pH was monitored daily with a pH meter (GMH 3510; Greisinger, Regenstauf, Germany), and slight changes were either corrected with potassium hydroxide (1 M; Merck) or with hydrochloric acid (0.1 M; Merck). After 5 days of demineralization, six randomly chosen specimens were evaluated to control for similar and homogenous demineralization (± 150-μm depth) using transversal microradiography (TMR). Subsequently, one of these lesions (DTB) was covered with acid-resistant nail varnish for analysis of pre-demineralization (ΔZB).
Specimen preparation. a Frontal view of bovine front tooth. b Transversal section of bovine root. c Obtained specimens (6 mm × 4 mm × 4 mm). d Specimen covered with acid-resistant nail varnish (sound control windows SC). e pre-demineralized specimen (yellow, demineralized treatment area, windows DTB). f, g Control area and treatment area (blue, demineralized treatment areas, windows DTE1 and DTE2). h Obtainment of the 100-μm slices for baseline TMR analysis
pH cycling condition
Specimens were randomly allocated to six groups (n = 20) and pH cycled for 7 days (E1). Subsequently, from each specimen, one lesion was covered, while the remaining lesion was cycled for another 7 days (E2) (Fig. 1). Conditions were chosen with a daily schedule of 3 cycles where specimens were consecutively subjected to a demineralizing (1 h) and a potentially demineralizing (3 h) “saliva substitute” phase (Glandonsane; Cell Pharm, Hannover, Germany). In-between the cycles, specimens were rinsed with water (10 s). Overnight, all specimens were stored (12 h) in 100% humidity mimicking a neutral phase during bed time. The demineralizing solution contained 47.6 μM NaF, 2.2 mM CaCl2 × 2H2O, 2.2 mM KH2PO4, 50 mM CH3COOH, and 10 mM KOH (Merck, Darmstadt, Germany). The pH cycling solutions were refreshed every second day.
Surface treatment
Before the first and last saliva substitute phase of each day, the respective fluoride agents were applied without any force using either a toothpick (gel) or were brushed with the toothpaste slurry for 5 min: no treatment [NT], 12,500 ppm F− [AmF-gel], 12,500 ppm F− [NaF-gel1], 12,500 ppm F− [NaF-gel2], 5000 ppm F− [NaF-TP], and 1450 ppm F− [KF-gel] (Table 1). Gel treatments were not dissolved in water. Slurries were freshly prepared at each experimental day (one part toothpaste to three parts bi-distilled water, by weight).
Transversal microradiography
After pH cycling, thin sections (100 ± 10 μm) from all specimens were prepared using waterproof silicon carbide papers (SiC, grit sizes 1200 + 4000; Buehler, Düsseldorf, Germany) [18]. Exact section size was reevaluated with a digital micrometer (precision of 0.001 mm; Mitutoyo, Japan), and contact microradiographs of the dentin specimens were obtained (PW 1730/10; Philips, Eindhoven, Netherlands; 20 kV, 10 mA). The radiation source-to-film distance was 34 cm, with a 10-s exposure time, and a high resolution film (Motion picture fine grain positive film71337″; FUJIFILM, Japan) was used and developed under standardized conditions according to the manufacturer’s recommendations. Microradiographs were studied with a digital image-analyzing system (CCD video camera Modul XC77E; Sony, Japan) that was interfaced to a microscope (Axioplan; Zeiss, Oberkochen, Germany) and a computer [19]. Furthermore, graphics of mean mineral density profiles were prepared for all groups with the TMR/T-WIM Calculation Program (Version 2.0.27.2, Inspector Research System BV, Amsterdam, Niederlande) [20, 21].
Calculation of integrated mineral loss and lesion depth
The difference between the mineral content (vol%) in sound control and demineralized dentin over the total dimension of the lesion was calculated using TMR software. Differences in integrated mineral loss (ΔΔZE1 = ΔZB − ΔZE1 / ΔΔZE2 = ΔZB − ΔZE2) and lesion depth (ΔLDE1 = LDB − LDE1 / ΔLDE2 = LDB − LDE1) between values before and after pH cycling were calculated [22].
Statistical analysis
Data were analyzed with SPSS statistical software (SPSS 25.0; SPSS, Munich, Germany). Variables were tested for normal distribution (Shapiro-Wilk test). Changes in mineral loss and lesion depth before and after pH cycling were analyzed using two-tailed paired t tests [23]. One-way ANOVA and Bonferroni post hoc tests were used to detect differences in changes of mineral loss (ΔΔZE1 / ΔΔZE2) and lesion depth (ΔLDE1 / ΔLDE2) between the treatment groups. All tests were performed at a 5% level of significance.
Power calculation
The number of specimens per group was calculated based on previous studies (non-published data). The α-error was set at 5%. Considering the differences between NT and NaF1 [∆∆ZE1: mean difference of 800 (SD 500); ∆∆ZE2: mean difference of 2000 (SD 700)], the statistical power calculated for ∆∆ZE1 was > 80% and for ∆∆ZE2 > 80%. Dropout rate was assumed not to exceed 20% [24]. Approximately 20 specimens should have been enrolled into the study for analyses of at least 16 specimens per group. Since the retrospective power analysis with 14 specimens still provided a power of > 80% for ∆∆ZE1 (mean difference of 677 (SD 533)) and > 80% for ∆∆ZE2 (mean difference of 2341 (SD 785)), no additional specimens were included in the study.
Results
Mineral loss and lesion depth
For baseline mineral loss (ΔZB) and lesion depth (LDB), no significant difference between the groups could be observed (p > 0.05, ANOVA, Table 2). Mean (95%CI) ΔZB was 3851 (3762; 3939) vol% × μm, and LDB was 188 (182; 194) μm). Due to losses during preparation, TMR analysis was performed with 14–19 specimens per subgroup (Table 2).
After pH cycling specimens of NT, NaF-gel1, AmF-gel, and KF-gel showed signs of demineralization indicated by significantly higher ∆Z and LD values than before pH cycling (p ≤ 0.037, paired t test) except for LDE1 of AmF-gel. Contrastingly, specimens of NaF-gel2 and NaF-TP showed signs of remineralization indicated by lower ∆Z values. However, only NaF-gel2 induced a significant gain in mineral content after 7 and 14 days (p ≤ 0.025, t test, Table 2).
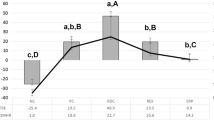
Significant differences in the change of mineral loss (ΔΔZ) and lesion depth (ΔLD) were found between NT and all fluoride groups after 7 as well as after 14 days (p ≤ 0.006, Bonferroni post hoc test). NaF-gel2 showed significantly higher values for ΔΔZE1 and ΔΔZE2 compared to NT, NaF-gel1, AmF-gel, and KF-gel. Furthermore, NaF-TP presented significantly higher values for ΔΔZE1 compared to NT, NaF-gel1, AmF-gel, and KF-gel (Fig. 2).
Means (95% confidence intervals) of the changes in mineral loss (ΔΔZE1/ΔΔZE2) and lesion depth (ΔLDE1/ΔLDE2). Different letters indicate significant differences between treatment groups for each storage time (uppercase letters ΔΔZE1/ΔLDE1; lowercase letters ΔΔZE2/ΔLDE2) (p < 0.05, Bonferroni post hoc test) [rhombus ΔΔZE1/ΔLDE1; square ΔΔZE2/ΔLDE2]
Mineral density of the lesion surface zone
The respective mineral distribution profiles of the lesions before and after pH cycling are shown in Fig. 3. After pH cycling, a second layer of demineralized tissue (lamination) could be observed in specimens treated in particular with KF-gel but also for NaF-TP.
Discussion
The present in vitro study evaluated the remineralizing effects of different highly concentrated fluoride agents in combination with a potentially demineralizing saliva substitute. Compared with the negative control, all fluoride agents could significantly hamper further demineralization. However, NaF-gel2 was significantly superior to the NaF-gel1, AmF-gel, and KF-gel. Furthermore, NaF-gel2 was the only agent inducing a slight remineralization of the dentin specimens. For this reason, our hypothesis was partially rejected.
Glandosane is supposed to relieve the sensation of dry mouth of patients suffering from xerostomia [10]. However, Glandosane has a rather low pH, and its demineralizing effect has already been demonstrated in several in vitro studies [11, 15]. Recently, it could be shown that the demineralizing effect of Glandosane could be nearly completely inhibited when NaF-gel1 (12,500 ppm F−) or NaF-TP (5000 ppm F−) were applied twice daily [16]. Nonetheless, in the previous studies, specimens were solely stored in Glandosane and no additional demineralization and nighttime period were established. Thus, so far, only the best case scenario (without demineralizing periods) was simulated. Contrastingly, in the present study, re- and demineralizing periods were included resulting in a more realistic model. Under these conditions, further demineralization was observed for the use of Glandosane alone as observed previously, as well. Thus, the demineralizing potential could even be induced with a rather short contact time and alongside neutral periods. This result should lead to rethinking of the widespread use of Glandosane.
In order to mimic the oral environment of patients suffering from xerostomia, the pH cycling protocol was slightly altered compared to previous pH cycling studies [25,26,27]. Firstly, the demineralizing phases were prolonged; instead of 30 min [26, 27], demineralization phases lasted 60 min. Secondly, specimens were consecutively subjected to a demineralizing and (potentially demineralizing) saliva substitute. Furthermore, to mimic a neutral phase during bedtime, specimens were stored in 100% humidity in the meantime. Although this design seems to be very extreme when compared to the in vivo situation, it simulates the reduced oral clearance rate of patients suffering from hyposalivation [28] since pH neutralization after a cariogenic attack and after the application a (potentially demineralizing) saliva substitute is slowed down. Therefore, it might also be speculated that effects of the fluoride agents might be less influential on mineralization when using a neutral or remineralizing saliva substitute.
Furthermore, the additional use of the tested fluoride agents significantly hampered further lesion progression when compared with no additional treatment. Interestingly, only the additional use of NaF-gel2 induced a significant gain in mineral content. Although NaF-gel2 was not tested in one of the previous models, the results seem to be in agreement with a previous in vitro study on erosion [29]. Under erosive conditions, NaF-gel2 (as well as AmF-gel) demonstrated a significantly higher anti-erosive effect compared with no treatment.
Several studies demonstrated that the effect of fluoride agents might be increased by reducing their pH [30,31,32]. For acidic fluoride agents, the formation of calcium fluoride is enhanced compared with neutral ones. Furthermore, the adsorption of mineral ions into the lesion increases with decreasing pH. Thus, the remineralizing effect of acidic agents is supposed to be significantly higher than the effect observed for neutral agents [30]. However, in the present study, the acidic gels (AmF-gel and NaF-gel1) could only hamper further demineralization. Contrastingly, only the neutral NaF-gel2 demonstrated a significant gain in mineral content. Therefore, it might be speculated that in the present study, the beneficial effect of the rather low pH of the fluoride agent (being supposed to increase mineral diffusion) was superimposed by the low pH of the saliva substitute Glandosane. Therefore, the rather low pH of a fluoride agent might not necessarily be required to increase mineral gain under these circumstance. In consequence, it might also be speculated that when acidic products are already used, neutral fluoride agents should be preferred.
After pH cycling, a significantly lower lesion progression was observed for specimens treated with NaF-gel1 when compared with no treatment. However, when compared to NaF-gel2 and NaF-TP, a less pronounced remineralizing effect was observed. The less pronounced remineralizing may, firstly, be based on the different pH values of the fluoride agents (as discussed above) or, secondly, caused by the Carbopol polymer (carbomer 956). Carbomer 956 is incorporated in NaF-gel1 but not in the other fluoride agents [11]. In NaF-gel1, it is used as thickening agent. Although the formation of calcium fluoride (CaF2) on the dentin surface has not been analyzed in the present study, it might be speculated that Carbomer 956 has also bound CaF2 just being incorporated in the enamel surfaces after applying the fluoride agent [33]. This “temporarily bound layer” was presumably removed during rinsing procedure, not being bioavailable during the following demineralization period. Consequently, this resulted in further surface mineral loss.
Glandosane in combination with the acidic AmF-gel presented a significantly lower lesion progression than Glandosane alone. This is in agreement with a previous in situ study [34]. After treatment with AmF-gel, the fluoride uptake was significantly higher when compared with a 5000 ppm F− gel and a fluoride-free placebo gel. Furthermore, two in vitro studies on erosion [29, 35] concluded that the additional use of a highly fluoridated acidic AmF-gel may protect enamel against erosion. Nevertheless, when compared to the NaF-gel2 and NaF-TP, a less pronounced mineral gain for the AmF-gel was observed. The low pH of the AmF-gel seemed to have no additional effect if demineralizing conditions predominate (as discussed above).
In the present study, NaF-TP and KF-gel significantly hampered further demineralization (KF-gel) or induce slight remineralization (NaF-TP). Furthermore, specimens of both agents showed an intact surface layer after pH cycling. Nevertheless, a second lesion body (lamination) after pH cycling for 7 as well as 14 days could be shown in both groups. In general, laminated (or layered) lesions present different surface zones with different mineral content [36]. The incorporation of fluorides seem to cause larger and less soluble crystallites [37]. Additionally, the fluorohydroxyapatites in the surface layer decrease the buffer capacity compared with hydroxyapatite [37]. Consequently, acids can easily pass the crystal structure of the original lesion without further neutralization [15, 26] resulting in a second lesion body. Interestingly, lamination has been observed in several pH cycling studies on enamel specimens [26, 27, 38] as well as dentin specimens [15]. However, lamination characteristics varied widely. In one pH cycling model, lamination was only observed for dentifrices containing 2800 ppm F− [38]. Contrastingly, an inverse correlation between fluoride concentration and severity of the lamination was observed in other models [15, 26, 27].
Within the limitations of this in vitro study, it can be concluded that all fluoride agents could significantly hamper the adverse effects of a demineralizing saliva substitute. However, slight mineral gain was only observed for the neutral NaF-gel2 (12,500 ppm F−) as well as 5000 ppm F− toothpaste. Further in vitro studies need to improve our knowledge about potentially demineralizing saliva substitute and the risks of long-term use.
References
Ahadian H, Yassaei S, Bouzarjomehri F, Ghaffari Targhi M, Kheirollahi K (2017) Oral complications of the Oromaxillofacial area radiotherapy. Asian Pac J Cancer Prev 18:721–725. https://doi.org/10.22034/APJCP.2017.18.3.721
Atkinson JC, Grisius M, Massey W (2005) Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin N Am 49:309–326. https://doi.org/10.1016/j.cden.2004.10.002
Almstahl A, Wikstrom M (2003) Electrolytes in stimulated whole saliva in individuals with hyposalivation of different origins. Arch Oral Biol 48:337–344
Tschoppe P, Wolgin M, Pischon N, Kielbassa AM (2010) Etiologic factors of hyposalivation and consequences for oral health. Quintessence Int 41:321–333
Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP (2003) Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14:199–212
Kielbassa AM, Hinkelbein W, Hellwig E, Meyer-Luckel H (2006) Radiation-related damage to dentition. Lancet Oncol 7:326–335. https://doi.org/10.1016/S1470-2045(06)70658-1
Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP (2003) Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med 14:213–225
Meyer-Lueckel H, Colfen H, Verch A, Tschoppe P (2010) Effects of carboxymethyl cellulose-based saliva substitutes with varying degrees of saturation with respect to calcium phosphates on artificial enamel lesions. Caries Res 44:127–134. https://doi.org/10.1159/000302901
Zimmermann JS, Niehoff P, Wilhelm R, Schneider R, Kovacs G, Kimmig B (1998) Prevention and therapy of acute radiation-related morbidity of the skin and mucosa. II, recommendations of the literature. Strahlenther Onkol 174:193–199
Momm F, Volegova-Neher NJ, Schulte-Monting J, Guttenberger R (2005) Different saliva substitutes for treatment of xerostomia following radiotherapy. A prospective crossover study. Strahlenther Onkol 181:231–236. https://doi.org/10.1007/s00066-005-1333-7
Zandim-Barcelos DL, Kielbassa AM, Sampaio JE, Tschoppe P (2015) Saliva substitutes in combination with high-fluoride gel on dentin remineralization. Clin Oral Investig 19:289–297. https://doi.org/10.1007/s00784-014-1264-8
Tschoppe P, Meyer-Lueckel H (2012) Effects of regular and highly fluoridated toothpastes in combination with saliva substitutes on artificial enamel caries lesions differing in mineral content. Arch Oral Biol 57:931–939. https://doi.org/10.1016/j.archoralbio.2012.02.010
Marinho VC, Worthington HV, Walsh T and Chong LY (2015) Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database Syst Rev:CD002280. doi: https://doi.org/10.1002/14651858.CD002280.pub2
Nieuw Amerongen AV, Veerman EC (2003) Current therapies for xerostomia and salivary gland hypofunction associated with cancer therapies. Support Care Cancer 11:226–231. https://doi.org/10.1007/s00520-002-0409-5
Tschoppe P, Meyer-Lueckel H (2011) Mineral distribution of artificial dentinal caries lesions after treatment with fluoride agents in combination with saliva substitutes. Arch Oral Biol 56:775–784. https://doi.org/10.1016/j.archoralbio.2011.01.002
Tschoppe P, Siegel A, Meyer-Lueckel H (2010) Saliva substitutes in combination with highly concentrated fluorides and brushing: in vitro effects on enamel subsurface lesions. Caries Res 44:571–578. https://doi.org/10.1159/000321656
Zandim DL, Tschoppe P, Sampaio JE, Kielbassa AM (2011) Effect of saliva substitutes in combination with fluorides on remineralization of subsurface dentin lesions. Support Care Cancer 19:1143–1149. https://doi.org/10.1007/s00520-010-0924-8
Wierichs RJ, Zelck H, Doerfer CE, Appel P, Paris S, Esteves-Oliveira M, Meyer-Lueckel H (2017) Effects of dentifrices differing in fluoride compounds on artificial enamel caries lesions in vitro. Odontology 105:36–45. https://doi.org/10.1007/s10266-016-0233-x
Arends J and ten Bosch JJ (1992) Demineralization and remineralization evaluation techniques. J Dent Res 71 Spec No:924–8
Wierichs RJ, Lausch J, Meyer-Lueckel H, Esteves-Oliveira M (2016) Re- and demineralization characteristics of enamel depending on baseline mineral loss and lesion depth in situ. Caries Res 50:141–150. https://doi.org/10.1159/000444537
Wierichs RJ, Stausberg S, Lausch J, Meyer-Lueckel H, Esteves-Oliveira M (2018) Caries-preventive effect of NaF, NaF plus TCP, NaF plus CPP-ACP, and SDF varnishes on sound dentin and artificial dentin caries in vitro. Caries Res 52:199–211. https://doi.org/10.1159/000484483
Meyer-Lueckel H, Wierichs RJ, Gninka B, Heldmann P, Dorfer CE, Paris S (2015) The effect of various model parameters on enamel caries lesions in a dose-response model in situ. J Dent 43:1261–1267. https://doi.org/10.1016/j.jdent.2015.08.003
Esteves-Oliveira M, Santos NM, Meyer-Lueckel H, Wierichs RJ, Rodrigues JA (2017) Caries-preventive effect of anti-erosive and nano-hydroxyapatite-containing toothpastes in vitro. Clin Oral Investig 21:291–300. https://doi.org/10.1007/s00784-016-1789-0
Wierichs RJ, Westphal S, Lausch J, Meyer-Lueckel H, Esteves-Oliveira M (2018) Influence of highly concentrated fluoride dentifrices on remineralization characteristics of enamel in vitro. Clin Oral Investig 22:2325–2334. https://doi.org/10.1007/s00784-018-2333-1
Wierichs RJ, Kogel J, Lausch J, Esteves-Oliveira M, Meyer-Lueckel H (2017) Effects of self-assembling peptide P11-4, fluorides, and caries infiltration on artificial enamel caries lesions in vitro. Caries Res 51:451–459. https://doi.org/10.1159/000477215
ten Cate JM, Exterkate RA, Buijs MJ (2006) The relative efficacy of fluoride toothpastes assessed with pH cycling. Caries Res 40:136–141. https://doi.org/10.1159/000091060
ten Cate JM, Buijs MJ, Miller CC, Exterkate RA (2008) Elevated fluoride products enhance remineralization of advanced enamel lesions. J Dent Res 87:943–947. https://doi.org/10.1177/154405910808701019
Duckworth RM, Gao XJ (2006) Plaque as a reservoir for active ingredients. Monogr Oral Sci 19:132–149. https://doi.org/10.1159/000090589
Wegehaupt FJ, Attin T (2010) The role of fluoride and casein phosphopeptide/amorphous calcium phosphate in the prevention of erosive/abrasive wear in an in vitro model using hydrochloric acid. Caries Res 44:358–363. https://doi.org/10.1159/000316542
Yamazaki H, Margolis HC (2008) Enhanced enamel remineralization under acidic conditions in vitro. J Dent Res 87:569–574. https://doi.org/10.1177/154405910808700612
Brighenti FL, Delbem AC, Buzalaf MA, Oliveira FA, Ribeiro DB, Sassaki KT (2006) In vitro evaluation of acidified toothpastes with low fluoride content. Caries Res 40:239–244. https://doi.org/10.1159/000092232
Alves KM, Pessan JP, Brighenti FL, Franco KS, Oliveira FA, Buzalaf MA, Sassaki KT, Delbem AC (2007) In vitro evaluation of the effectiveness of acidic fluoride dentifrices. Caries Res 41:263–267. https://doi.org/10.1159/000101915
Backfolk K, Lagerge S, Rosenholm JB, Eklund D (2002) Aspects on the interaction between sodium carboxymethylcellulose and calcium carbonate and the relationship to specific site adsorption. J Colloid Interface Sci 248:5–12. https://doi.org/10.1006/jcis.2001.8195
Altenburger MJ, Schirrmeister JF, Wrbas KT, Klasser M, Hellwig E (2008) Fluoride uptake and remineralisation of enamel lesions after weekly application of differently concentrated fluoride gels. Caries Res 42:312–318. https://doi.org/10.1159/000148164
Lennon AM, Pfeffer M, Buchalla W, Becker K, Lennon S, Attin T (2006) Effect of a casein/calcium phosphate-containing tooth cream and fluoride on enamel erosion in vitro. Caries Res 40:154–157. https://doi.org/10.1159/000091063
Lagerweij MD, ten Cate JM (2006) Acid susceptibility at various depths of pH-cycled enamel and dentine specimens. Caries Res 40:33–37. https://doi.org/10.1159/000088903
Brown WE, Gregory TM, Chow LC (1977) Effects of fluoride on enamel solubility and cariostasis. Caries Res 11(Suppl 1):118–141
Lippert F, Juthani K (2015) Fluoride dose-response of human and bovine enamel artificial caries lesions under pH-cycling conditions. Clin Oral Investig 19:1947–1954. https://doi.org/10.1007/s00784-015-1436-1
Acknowledgments
This study was conducted as part of the doctoral thesis of M.K.
Funding
This study was funded by the authors and their institution. Products were not provided by the manufacturers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Walther, C., Kreibohm, M., Paris, S. et al. Effect of NaF, AmF, KF gels and NaF toothpaste combined with a saliva substitute on dentin lesions in vitro. Clin Oral Invest 23, 2489–2496 (2019). https://doi.org/10.1007/s00784-018-2687-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2687-4