Abstract
Objectives
To evaluate CPP-ACP effect on colour change and tooth sensitivity (TS) associated with at-home vital tooth bleaching using 20% carbamide peroxide (CP).
Methods
A randomised double-blind placebo-controlled clinical trial was conducted to measure the TS and tooth colour change of 24 patients at 3-day, 7-day, 14-day and 30-day periods. The participants were instructed to apply 20% CP (7 days—04 h each) followed by the application of either CPP-ACP or non-active placebo paste, delivered by the bleaching custom tray (7 days—30 min each). Lightness (L*), redness (a*) and yellowness (b*) were measured using a digital spectrophotometer and the overall colour changes ∆E were calculated. ∆E and TS values were statistically analysed. The level of statistical significance was established at p = 0.05.
Results
No significant differences were detected between CPP-ACP and placebo groups regarding the ∆E. The ∆E measurements presented significant differences within CPP-ACP groups between 3-day vs. 14-day and 30-day measurements. The CPP-ACP application reduced significantly the TS reported by the participants at 3-day when compared with the placebo group.
Conclusion
The application of CPP-ACP paste during at-home tooth bleaching with 20% CP was beneficial since its use reduced the TS and presented no deteriorating effect on the colour change.
Clinical relevance
The current findings are of importance for clinicians to manage TS reported by patients when a high CP bleaching agent is used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tooth bleaching is advocated wherever possible to treat patients who seek dental appearance improvements as it presents a minimally invasive approach when compared with other invasive aesthetic treatments [1]. The most used approach to manage discoloured teeth is at-home dentist supervised tooth bleaching with custom trays [2]. The use of a high concentration bleaching agent revealed similar tooth bleaching to that obtained using lower concentration after 1 week, but with faster and major changes in lightness and chroma [3]. An increase in the enamel surface porosity and irregularities were reported following tooth bleaching [4, 5]. These alternations can facilitate the peroxide penetration within the tooth structure which might explain the tooth sensitivity (TS) occurrence [6].
Casein phosphopeptides-amorphous calcium phosphate (CPP-ACP) is a protein remineralization technology whereby the solubility of ACP system is controlled by a milk protein, casein phosphopeptide (CPP), added to regulate the ACP solubility and to inhibit the early transformation into crystalline forms [7]. Enamel surfaces treated with CPP-ACP exhibited an increased HA crystals size and a decreased surface roughness compared to the negative control samples [8]. The daily use of CPP-ACP for 7 days during bleaching regimen resulted in a recovered enamel surface with no regressive effect in the colour change in vitro [9, 10]. To the best of our knowledge, the use of CPP-ACP during at-home vital tooth bleaching using 20% carbamide peroxide (CP) bleaching agent was not evaluated in vivo. Therefore, the aim of this randomised controlled study was to evaluate the effect of CPP-ACP on the tooth sensitivity (TS) and bleaching effectiveness using 20% CP. The two null hypotheses investigated were (a) using CPP-ACP during tooth bleaching with 20% CP has no effect on TS and (b) this therapy has no deteriorating effect on the colour improvement.
Materials and methods
The design of this double-blind randomised, parallel, placebo-controlled trial with an equal allocation rate followed the guidelines published by Consolidated Standards of Reporting Trials (CONSORT) [11]. This study was reviewed and approved by the Health Research Ethics Board at the Damascus University, registration number: 820, 9/01/2017.
Sample size calculation
Sample size was calculated using Minitab 17 software by choosing the t-student statistical test for two independent samples. It was necessary to recruit eight participants per group to detect a significance of a clinical difference of at least 2.5 increase, assuming a standard deviation of 1.2 in accordance with a previous study [12]. Twelve participants per group were recruited to avoid possible loss to follow-up.
Inclusion and exclusion criteria
The inclusion criteria in this study are the following: be at least 18 years old, six maxillary anterior teeth were present with no restorations or carious lesions on their buccal surfaces, colour shade A2 or darker on the shade guide (Vitapan Classical, Vita Zahnfabrik) and no history of tooth sensitivity or use of a desensitising agent or desensitising toothpaste in the past 3 months. The exclusion criteria are the following: previous tooth-bleaching procedures, chronic therapeutic drug history, orthodontic appliance use, periodontal disease or active carious lesions, pregnancy or lactation, severe internal tooth discolouration, allergies to bleaching agent or tray material, and smoking. Written consents were obtained from all selected participants after verbal and written explanation of the trial.
Intervention
Participants were randomly assigned into two experimental groups (n = 12) by dragging a paper showed the code of the material used after bleaching procedures (CPP-ACP or Placebo). This procedure was accomplished by a staff member who was not involved in the trial; therefore, the participants and the examiner were both blinded to group assignment. Each participant received a treatment kit containing (1) his custom tray (fabricated using a 1-mm-thick soft vinyl material through a vacuum-formed process and trimmed on the gingival margin), (2) the bleaching gel syringe (Opalescence® PF™, Ultradent Products Inc. USA—20% carbamide peroxide), (3) a white plastic coded container containing either CPP-ACP (Tooth Mousse, GC International, Itabashi-ku, Tokyo, Japan) or non-active placebo paste and (4) a toothbrushes with a standardised non-tooth-whitening toothpaste (Colgate Total®). The participants were instructed to use the bleaching gel for 4 h a day, to wash/dry the tray and to apply the studied material in the tray for 30 min once a day after bleaching procedure. This protocol was repeated for 7 days.
Shade evaluation
Digital colour images of the upper anterior teeth were taken before treatment as a baseline measurement using a digital spectrophotometer (Vita Easyshade Advance 4.0 ®; Vita, Germany) on the middle third of the labial surface of the maxillary right central incisors. Shade evaluation was performed at 3-day, 7-day, 14-day, and 30-day periods. The spectrophotometer was calibrated against the calibration block prior each colour measurement. The digital spectrophotometer measures the shade of teeth based on the CIE L*a*b* colour space system, allowing the determination of colour in a three-dimensional space. The colour difference between the colour coordinates is calculated as ΔE* = [(ΔL*)2 + (Δa*)2+ (Δb*)2]1/2. Three readings were taken, and the average value was calculated and recorded.
Tooth sensitivity evaluation
Participants were instructed to record tooth sensitivity (TS) daily for 7 days using a visual analogue scale (VAS) ranked from 0 ‘no sensitivity’ to 10 ‘severe sensitivity’.
Statistical analysis
The statistical analysis was conducted using SPSS statistical package (version 23, SPSS Inc./IBM, Chicago, IL). The data was tested for normality using Histogram/Q-Q plots/Shapiro-Wilk tests. As ∆E and TS data had a normal distribution, repeated measures two-way ANOVA was conducted.
Results
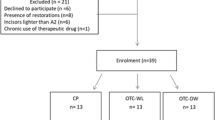
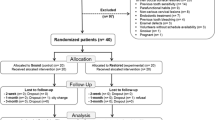
Fifty subjects were examined for potential inclusion in this trial, from those 24 participants were enrolled in this study (Fig. 1). The means ± SE of the ∆E values in the CPP-ACP and placebo groups are presented in Fig. 2a. The application of CPP-ACP paste reduced the ∆E values when compared with the placebo groups. However, these reductions were not statistically significant at any measurement point (p > 0.05). The ∆E values exhibited significant differences within CPP-ACP groups between 3-day (6.6 ± 1.5, means ± SE) vs. 14-day measurements (10.7 ± 1.3, p = 0.014) and 30-day measurements (10.7 ± 1.7, p = 0.009). In the placebo groups, the ∆E value at 3-day (9.3 ± 1.2) showed no significant differences comparing to the measurements at 14-day (12.4 ± 1.2, p > 0.05) and 30-day (12.8 ± 0.9, p > 0.05).
Figure 2b presents the means ± SE of the TS values in the CPP-ACP and placebo groups at different point of measurements. Overall, the use of CPP-ACP resulted in significantly less TS when compared with the placebo groups (p = 0.002). The CPP-ACP application during the bleaching procedure reduced significantly the TS reported by the participants at 3-day (0.4 ± 0.2) when compared with the placebo group (3.7 ± 0.9, p = 0.002). The TS was less in the CPP-ACP group (0.5 ± 0.3) at 7-day than those reported by the participants in the placebo group (1.9 ± 0.7), but with no statistical difference (p > 0.05). The TS was not reported at 14-day and 30-day measurements in the CPP-ACP groups, whilst two and one participant reported TS at 14-day and 30-day respectively in the placebo groups.
Discussion
This clinical trial utilised a high concentration of CP for at-home tooth bleaching which was associated clinically with a colour change improvement and a significant TS [2, 3, 13]. It has been shown that 71.4% of the participants who used 20% CP at-home bleaching agent reported TS [14]. Enamel surfaces treated with 16% CP at-home bleaching agent for 14 days presented an increased surface roughness and a decreased microhardness measurement [5]. Those surface changes may promote the diffusion of the bleaching agent inside the pulp chamber to activate the pulpal sensory afferents, resulting in TS [15]. In this study, the first null hypothesis was rejected as the use of CPP-ACP in the custom bleaching tray significantly reduced the TS during at-home tooth bleaching with 20% CP. The TS reduction is in accordance with the findings of previous studies which showed that the application of CPP-ACP reduced significantly the TS associated with in-office vital tooth bleaching using hydrogen peroxide gel [16,17,18].
The second null hypothesis investigated in this clinical trial was rejected as the use of CPP-ACP paste during the bleaching procedure exhibited a negative effect on the colour change at 3-day measurements. However, the colour change was not significant to that in the placebo groups at 7-day, 14-day and 30-day postbleaching periods. It has been shown that the use of a combination of CPP-ACP with 16% CP did not affect tooth bleaching efficacy in vitro [19]. The use of a blend of CPP-ACP and 16% CP (1:1) increased significantly postbleaching enamel microhardness and did not adversely affect bleaching efficacy [20]. The daily use of CPP-ACP following tooth bleaching with 38% hydrogen peroxide improved significantly enamel microhardness [21]. This microhardness increase may suggest a mineral deposition on enamel when treated with CPP-ACP during the bleaching protocol. The microscopy analysis of the samples treated with CPP-ACP after bleaching with 40% hydrogen peroxide showed the presence of amorphous deposits at the surface with a reduced porosity implying a surface remineralization [22].
Evaluating the potential role of CPP-ACP in reducing TS associated with at-home tooth bleaching using 20% CP was not reported previously in the dental literature. The delivery of the experimental paste using the bleaching custom tray for 30 min daily permitted the retention of the paste on the tooth surface for a standard period for all participants. The results of this study showed that the colour change was maintained in the CPP-ACP and the placebo groups after 1 month. The lightning effect obtained using 16% CP at-home bleaching agent at 7-days was maintained after 6 months [23]. However, there is no previous investigation regarding the long-term colour stability when the CPP-ACP is used with the 20% CP at-home bleaching agent, implying a further study with a longer post-treatment evaluation is recommended.
Conclusion
The application of CPP-ACP paste during at-home tooth bleaching with 20% CP was beneficial since its use reduced significantly the TS and presented no deteriorating effect on the colour change after 14 days when compared with the placebo groups.
References
Alani A, Kelleher M, Hemmings K, Saunders M, Hunter M, Barclay S, Ashley M, Djemal S, Bishop K, Darbar U (2015) Balancing the risks and benefits associated with cosmetic dentistry–a joint statement by UK specialist dental societies. Br Dent J 218(9):543–548. https://doi.org/10.1038/sj.bdj.2015.345
Meireles S, Heckmann S, Leida F, Santos I, Bona Á, Demarco F (2008) Efficacy and safety of 10% and 16% carbamide peroxide tooth-whitening gels: a randomized clinical trial. Oper Dent 33(6):606–612. https://doi.org/10.2341/07-150
Braun A, Jepsen S, Krause F (2007) Spectrophotometric and visual evaluation of vital tooth bleaching employing different carbamide peroxide concentrations. Dent Mater 23(2):165–169. https://doi.org/10.1016/j.dental.2006.01.017
Schemehorn B, González-Cabezas C, Joiner A (2004) A SEM evaluation of a 6% hydrogen peroxide tooth whitening gel on dental materials in vitro. J Dent 32:35–39
Polydorou O, Scheitza S, Spraul M, Vach K, Hellwig E (2018) The effect of long-term use of tooth bleaching products on the human enamel surface. Odontology 106(1):64–72. https://doi.org/10.1007/s10266-017-0308-3
Pintado-Palomino K, Peitl Filho O, Zanotto ED, Tirapelli C (2015) A clinical, randomized, controlled study on the use of desensitizing agents during tooth bleaching. J Dent 43(9):1099–1105. https://doi.org/10.1016/j.jdent.2015.07.002
Reynolds E, Cain C, Webber E, Black C, Riley P, Johnson I, Perich J (1995) Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat. J Dent Res 74(6):1272–1279. https://doi.org/10.1177/00220345950740060601
Zhou C, Zhang D, Bai Y, Li S (2014) Casein phosphopeptide–amorphous calcium phosphate remineralization of primary teeth early enamel lesions. J Dent 42(1):21–29. https://doi.org/10.1016/j.jdent.2013.11.005
Machado C, Borges B, Vasconseloes A, Cunha A, Vitoriano J, Alves-Junior C, Santos A (2012) Tooth bleaching with blends of peroxides and a CPP-ACP paste. Dent Mater 28:e50. https://doi.org/10.1016/j.dental.2012.07.117
Francci C, Gomes M, Devito-Moraes A, Rodrigues F, Yamazaki L, Silva L, Fróes-Salgado N, Nishida A (2010) Structural analysis of the enamel subjected to pre-and post-bleaching agents. Dent Mater 26:e71. https://doi.org/10.1016/j.dental.2010.08.160
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux P, Elbourne D, Egger M, Altman DG (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Bmj 340:c869. https://doi.org/10.1136/bmj.c869
Hyland BW, McDonald A, Lewis N, Tredwin C, Petrie A, Hall S, Todd C, McCaughan B, Callan JF (2015) A new three-component formulation for the efficient whitening of teeth (carbamide plus). Clin Oral Investig 19(6):1395–1404. https://doi.org/10.1007/s00784-014-1352-9
Ontiveros JC, Eldiwany MS, Paravina R (2012) Clinical effectiveness and sensitivity with overnight use of 22% carbamide peroxide gel. J Dent 40:e17–e24. https://doi.org/10.1016/j.jdent.2012.08.009
Basting RT, Amaral F, França F, Flório F (2012) Clinical comparative study of the effectiveness of and tooth sensitivity to 10% and 20% carbamide peroxide home-use and 35% and 38% hydrogen peroxide in-office bleaching materials containing desensitizing agents. Oper Dent 37(5):464–473. https://doi.org/10.2341/11-337-C
Markowitz K (2010) Pretty painful: why does tooth bleaching hurt? Med Hypotheses 74(5):835–840. https://doi.org/10.1016/j.mehy.2009
Singh M, Mahajan P, Monga P, Mahajan S, Singla D, Kaur N (2017) Comparative evaluation of effectiveness of sodium fluoride and casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) in treating postoperative sensitivity associated with in-office vital tooth bleaching: a clinical study. Endodontology 29(1):26. https://doi.org/10.4103/endo.endo_118_16
Alexandrino LD, Alencar CdM, SILVEIRA ADSd, Alves EB, Silva CM (2017) Randomized clinical trial of the effect of NovaMin and CPP-ACPF in combination with dental bleaching. J Appl Oral Sci 25 (3):335–340. doi: https://doi.org/10.1590/1678-7757-2016-0408
Maghaireh G, Alzraikat H, Guidoum A (2014) Assessment of the effect of casein phosphopeptide–amorphous calcium phosphate on postoperative sensitivity associated with in-office vital tooth whitening. Oper Dent 39(3):239–247. https://doi.org/10.2341/12-527-C
de Vasconcelos A, Cunha A, Borges B, Machado C, Dos Santos A (2012) Tooth whitening with hydrogen/carbamide peroxides in association with a CPP-ACP paste at different proportions. Aust Dent J 57(2):213–219. https://doi.org/10.1111/j.1834-7819.2012.01683.x
Borges B, Borges J, De Melo C, Pinheiro I, Ad S, Braz R, Montes M (2011) Efficacy of a novel at-home bleaching technique with carbamide peroxides modified by CPP-ACP and its effect on the microhardness of bleached enamel. Oper Dent 36(5):521–528. https://doi.org/10.2341/11-013-L
Bayrak S, Tunc E, Sonmez IS, Egilmez T, Ozmen B (2009) Effects of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) application on enamel microhardness after bleaching. Am J Dent 22(6):393–396
Coceska E, Gjorgievska E, Coleman NJ, Gabric D, Slipper IJ, Stevanovic M, Nicholson JW (2016) Enamel alteration following tooth bleaching and remineralization. J Microsc 262(3):232–244. https://doi.org/10.1111/jmi.12357
Meireles S, Heckmann S, Santos I, Della Bona A, Demarco F (2008) A double blind randomized clinical trial of at-home tooth bleaching using two carbamide peroxide concentrations: 6-month follow-up. J Dent 36(11):878–884. https://doi.org/10.1016/j.jdent.2008.07.002
Funding
The work was supported by the Department of Restorative Dentistry, Damascus University, Syria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yassin, O., Milly, H. Effect of CPP-ACP on efficacy and postoperative sensitivity associated with at-home vital tooth bleaching using 20% carbamide peroxide. Clin Oral Invest 23, 1555–1559 (2019). https://doi.org/10.1007/s00784-018-2574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2574-z