Abstract
Objectives
This study aims to evaluate the color change and tooth sensitivity (TS) caused by at-home bleaching in patients with sound and with restored teeth.
Methods
Forty patients were selected according to the inclusion and exclusion criteria and divided into two groups: So (patients with six caries-free maxillary anterior teeth) and Re (patients with at least one restoration in the six maxillary anterior teeth). Both groups were bleached with 10% carbamide peroxide (CP) at-home bleaching. The color change (CIELab [ΔE*ab], CIEDE00 [ΔE00], and whiteness index [∆WID]) were assessed using a spectrophotometer at baseline, 2 weeks, and 1 and 3 months after bleaching. Patients recorded their TS using a numeric rating scale (0–4). Data of color change were submitted to Student’s T-test. The absolute risk and intensity of TS were compared using Fisher’s and the Mann–Whitney tests, respectively (α = 0.05).
Results
Higher ΔE*ab, ΔE00, and ∆WID values were observed for So in relation to Re after all recall rate (p < 0.0001). No significant differences were observed regarding of bleaching-induced TS (p > 0.9).
Conclusions
At-home dental bleaching with 10% CP generated the same pattern of TS in patients with or without restorations. However, in patients with restored teeth, it produced a lower color change after 2 weeks of bleaching.
Clinical significance
After 2 weeks of at-home bleaching, a lower whitening effect was observed in patients with anterior restorations when compared with patients with sound teeth.
Trial registration
ClinicalTrials.gov identifier RBR-52j6gmg
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental bleaching is considered the treatment of choice to reduce dissatisfaction of patients with tooth discoloration, due to its noninvasive approach and low cost when compared with other procedures in dentistry, besides being an effective and safe treatment [1, 2]. Among dental bleaching techniques, at-home bleaching with customized trays is more indicated than in-office techniques, as it is an easy protocol and is cheaper and requires less chair time than in-office bleaching [3]. Furthermore, at-home bleaching produces the same bleaching results as in-office dental bleaching [4,5,6,7]. In addition, this technique provides lower gingival irritation and tooth sensitivity (TS) risk due to the lower concentrations of hydrogen peroxide (HP) used [8, 9]. Gingivitis that tends to be present at the end of treatment regresses spontaneously after 24–48 h [6]. However, one of the most commonly reported adverse effects following all bleaching techniques was bleaching-induced TS [1, 2, 10]. Although TS after bleaching is reported as mild and transient, sometimes it can be severe and irritating, leading the patient to give up the bleaching treatment [11]. Meanwhile, in some cases, patients submitted to bleaching still report TS after the procedure [7, 9, 12, 13].
It is accepted that the bleaching-induced TS occurs because peroxides penetrate through dental structures and reach the pulp, producing an inflammatory reaction [14], with release of inflammation mediators responsible for local vasodilation and pain. The rate of HP penetration is different between bleaching agents and depends on their concentration and time of application [15,16,17]. However, the presence of adhesive interface restorations or enamel craze lines may generate greater facilitation of HP reaching the pulp chamber [18,19,20]. Unfortunately, despite that in daily clinical practice, tooth bleaching is often performed on teeth with adhesive restorations or with the presence of cracks, only a few clinical studies have evaluated the effect of bleaching procedures in these special conditions [18, 21,22,23].
For instance, Bonafé et al. [21] demonstrated that patients with adhesive restorations had a higher TS index when compared with patients with sound teeth. Another study [22] conducted on patients with enamel craze lines demonstrated that TS in patients with sound teeth was lower when compared with patients with enamel craze lines. However, in all clinical studies, the authors evaluated in-office dental bleaching using higher concentrations of HP [18, 21,22,23]. It is worth mentioning that although bleaching in restored teeth produces higher TS, the literature demonstrated that the degree of whitening observed in the restored teeth was similar to that observed in the sound teeth [21, 22].
Patients with adhesive restorations often come to the dental office requesting tooth bleaching treatment. However, the few clinical trials that have evaluated the effect of tooth whitening on color change and TS of patients with restored teeth have been carried out in-office with 35% hydrogen peroxide [21, 22] and have report a high risk of TS. To the extent of the authors’ knowledge, no clinical study has been performed evaluating the efficacy of at-home bleaching and the experience of TS in patients with adhesive restorations. Therefore, the aims of this single-blind, controlled, and parallel, randomized clinical trial were to evaluate the color change and risk and intensity of TS following at-home bleaching performed with 10% carbamide peroxide (CP) in patients with sound teeth in comparison with patients with adhesive restorations.
Materials and methods
Ethics approval and protocol registration
This clinical investigation was approved by the ethics committee (CAAE, 45,800,815.1.0000.5188) of the local university, and it was registered in the Brazilian clinical trials registry under the identification number RBR-52j6gmg. We prepared this article using the protocol established by the Consolidated Standards of Reporting Trials statement [24].
Trial design, settings, and locations of data collection
This was a controlled, parallel, randomized, single-blind clinical trial, in which the patient was blinded about the group criteria assignment and bleaching agent tested. This study was performed between August 2016 and December 2017 in the clinics of the school of dentistry at the local university.
Recruitment
Two weeks before the bleaching procedures, all volunteers, who were patients and students seeking treatment at the clinic of the dental school, received dental prophylaxis with pumice and water in a rubber cup and signed an informed consent form. Recruitment was performed by placing a written advertisement on the university walls.
Eligibility criteria
Patients included in this clinical trial were at least 18 years old and had good general and oral health. The participants had six maxillary anterior teeth with shade A2 or darker based on value-oriented shade guide (Vitapan Classical, Vita Zahnfabrik, BadSackingen, Germany) classification that was provided by a spectrophotometer (Vita Easyshade Advance, Vita Zahnfabrik, BadSackingen, Germany). These participants should have only sound anterior teeth (sound group) or at least one restored tooth with at maximum 25% of the vestibular face restored with esthetic restorations (restored group). These restorations must be classified as very good or good for all parameters according to the FDI criteria [25]. One experienced and calibrated investigator performed this evaluation.
Participants with dental prosthesis, orthodontics apparatus, or severe internal tooth discoloration (tetracycline stains, fluorosis, and purple teeth) were not included in the study. In addition, pregnant/lactating women, participants with any other pathology that could cause sensitivity (such as recession, dentin exposure, or the presence of visible cracks in the teeth), smokers, bruxers, or participants who had previously undergone tooth whitening procedures were also excluded.
Sample size calculation
The primary outcome of this study was color change (∆E*ab), based on the study of Bonafé [21, 22]. A minimum of 40 participants was required to exclude a mean difference of 2.6 in ∆E*ab, with a power of 90% and an alpha of 5%, for a standard deviation of ∆E*ab of approximately 2.4. To consider the possible loss of patients, 10% was added to the sample. Therefore, the final calculation was 40 participants. This limit of equivalence (difference of means) was based on the fact that only ΔE values greater than 2.6 are considered clinically perceptible.
Study intervention
In the first appointment, alginate impressions (Jeltrate, Dentsply, Petropolis, RJ, Brazil) were made of each participant’s maxillary arch, and after disinfection, these were filled with dental stone (Asfer, Asfer Indústria Química Ltda, São Caetano do Sul, SP, Brazil). Then, a reservoir was created in the dental stone (six anterior superior teeth) [3]. A 1.0-mm soft vinyl material (Whiteness Placas para Moldeiras, FGM Dental Products, Joinville, SC, Brazil) was used to fabricate the custom-fitted tray that would hold the whitening gel in the Plastvac P7 (BioArt, São Carlos, SP, Brazil). The excess material from the labial and lingual surfaces was trimmed to 1 mm from the gingival junction.
In the second appointment, all participants were instructed to wear the tray with the bleaching agent (Polanight 10%, SDI Limited, Bayswater, Victoria, Australia) 4 h a day for 2 weeks at night and to remove the tray after each bleaching period, wash it with water, and brush their teeth as usual. At this time, both trays were tested, and the clinical procedures (amount of bleaching gel and cleaning the trays) were demonstrated to each patient. The identification seals were removed from each bleaching agent syringe to mask the product used. As a measure of adherence to the experimental protocol, each syringe was weighed in an analytical balance before and 1 week after each session (M245A, Bel Ltda, Piracicaba, SP, Brazil). If participants had worn the bleaching tray for 2 weeks with the correct amount of gel, this would result in 100% adherence to the protocol.
Color measurement
One experienced and calibrated examiner evaluated the color at baseline (T0), during treatment (after 2 weeks; T1), and 1 (T2) and 3 (T3) months after the end of bleaching treatment. The color evaluation was performed with the use of a spectrophotometer (Vita Easyshade Advance, Vita Zahnfabrik, BadSackingen, Germany) [9]. For this purpose, an impression of both arches was taken with dense silicone paste (Speedex Putty, Coltene, Rio de Janeiro, RJ, Brazil). The impression was extended to the premolars and served as a standard color measurement guide for the spectrophotometer. For all six maxillary anterior teeth to be evaluated, a window was created on the labial surface of the molded silicone guide using a metal device with a radius of 6 mm and well-formed borders. The shade was determined using the parameters of the Easyshade device, indicating the values L*, a*, and b*, where L* represented the value from 0 (black) to 100 (white) and a* and b* represented the shade, where a* was the measurement along the red-green axis and b* was the measurement along the yellow-blue axis. The average of the L*, a*, and b* values of all six maxillary anterior teeth for each participant was recorded. The ΔL*, Δa*, and Δb* before (T0) and after treatment (T3, T2, and T1) were evaluated. Additionally, the color change was calculated using the following formulas [26,27,28,29]:
and
Tooth sensitivity
Each participant was asked to keep a daily record of whether they experienced sensitivity. The patient was asked to indicate the numerical value of the degree of sensitivity using a 5-point numeric rating scale (NRS) (where 0 = none, 1 = mild, 2 = moderate, 3 = considerable, and 4 = severe) [3, 9, 30, 31]. The worst NRS score during all bleaching treatments was considered for statistical purposes, so that only a single value was taken from the 2-week treatment. The values were arranged into two categories: absolute risk of TS, which represented the percentage of patients who reported TS at least once during treatment, and the overall TS intensity.
Statistical analysis
The statistical analysis followed the intention-to-treat protocol and included all of the participants, who were randomly allocated. All data were checked for normal distribution using the Shapiro–Wilk test of normality. Means and standard deviations of color change (∆L*, ∆a*, ∆b*, ∆E*ab, ∆E00, and ∆WID) between baseline and assessment times (2 weeks, 1 and 3 months), color coordinates (L*, a*, and b*), and WID showed a normal distribution, as well as for ∆weight of bleaching agents; therefore, the differences between groups were calculated using Student’s T-test for independent samples.
The absolute risk of TS was compared using Fisher’s exact test. The relative risk and confidence interval for the effect size were also calculated. The distribution of TS intensity was non-normal, and the data were compared using the nonparametric Mann–Whitney test (between treatment groups) and Friedman test (within the same group). The chi-square test was used to compare the differences between treatment groups regarding participants’ descriptive characteristics (gender and profession). In all statistical tests, the significance level was 5%.
Results
Characteristics of included participants
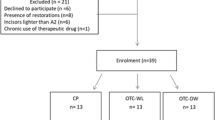
A total of 137 participants were examined according to the inclusion and exclusion criteria (Fig. 1), but only 40 remained for the clinical trial. The number of tooth restorations present in each individual was one restoration (n = 6), two restorations (n = 8), three restorations (n = 3), and four restorations (n = 3). The main characteristics of the included participants are described in Table 1. The baseline color of the participants’ teeth was similar in terms of L*, a*, and b*, and their mean age in years and the gender distribution in both groups were quite similar (Table 1). No hypothesis testing was performed for baseline features because any difference in these features was attributed to chance alone.
Adherence to the protocol
The adherence to protocol was 100% for both groups, meaning that all patients used the bleaching tray during the 2-week protocol. However, two participants (one from each group) did not attend the 1-month recall visit. For these participants, the last observation was carried forward for statistical purposes to keep the intention-to-treat analysis [24]. Figure 1 depicts the participant flow in the different phases of the study design. The final syringe weight showed that, for both groups, a similar amount of bleaching gel was used (Table 1).
Color evaluation
Significant whitening was observed in both study groups under all evaluation parameters (p < 0.01). However, lower Δb* and higher ΔE*ab, ΔE00, and ∆WID values were observed for the sound group in relation to the restored group after the end of treatment and at the 1- and 3-month recalls (p < 0.0001; Table 2).
It is worth mentioning that a slight regression of the color change occurred for both groups after the 3-month recall (p < 0.04; Table 2). However, after 3 months, a ∆E*ab higher than 6.4 and a ΔE00 higher than 4.0 units were observed for both groups. These values are superior to the 50:50% perceptibility/acceptability threshold [28] for sound and restored teeth (Table 2).
Tooth sensitivity
Regarding the absolute risk of bleaching-induced TS, no significant difference was observed between groups, as seen in Table 3 (p = 1.0). The risk ratio, along with the 95% confidence interval, was also evidence that there was no significant risk of bleaching-induced TS when both groups were compared. Regarding the TS intensity, no significant differences between the two groups were observed (Table 4; p = 0.9), showing lower intensity of TS during 2 weeks of bleaching treatment. In fact, due to the low intensity of TS in both groups, no volunteers needed oral medication.
Discussion
Bleaching-induced TS is the most common adverse effect reported by patients undergoing a bleaching procedure, even when at-home bleaching is applied [5, 7, 9, 12, 13, 30]. It occurs due to the low molecular mass of HP, which favors its fast penetration through dental structures and entering the pulp chamber [18,19,20]. As a result, an inflammatory reaction occurs [14], which is experienced by the patients as TS. Therefore, it was expected that the presence of adhesive restorations, as evaluated in the restored group of the present study, would show more TS when compared with sound teeth [18,19,20]. However, in this study, the number of patients who reported TS in the group with adhesive restorations did not differ statistically from those with sound teeth, contrary to previous published clinical studies [21, 22]. This seems to be explained by the lower concentration of CP for at-home bleaching used in contrast with the higher concentration of HP for in-office bleaching used in Bonafé et al.’s studies [21, 22].
The amount of HP reaching the pulp is higher in restored teeth when compared with sound teeth, whether at-home or in-office bleaching techniques were used [18,19,20, 32]. However, when 10% CP was evaluated, the amount of HP observed inside the pulp chamber for the restored teeth was similar to that observed for sound teeth when 35% HP in-office bleaching was applied [18, 32]. This means that, even with adhesive restorations, the amount of HP inside the pulp chamber is lower when 10% CP for at-home bleaching is used.
Some factors such as pH, gel viscosity, HP concentration, enamel, and dentin thickness may affect the HP diffusion to pulp cavity [8, 10, 16,17,18, 33]. In fact, the amount of HP inside 10% CP gel is around 3.5%. On the other hand, in studies conducted by Bonafé [21, 22], 35% HP was applied, which represent a concentration 10 times higher than that used in the present study. Therefore, greater TS intensity is experienced during in-office dental bleaching [10, 21, 22] due to the higher penetration of peroxides inside the pulp chamber and production of an inflammatory process [15, 34].
Although more HP could be found in the restored group in comparison with the sound group [19,20,21], the lower HP concentration in at-home bleaching was sufficient for the pulp tissue to produce enough defense cells to protect the pulp from the damage caused by HP [35], and, consequently, no significant increase in the TS was observed. This could also be confirmed for the evaluation of intensity of TS. In the present study, the majority of patients experienced mild TS intensity, which is in line with other studies that performed at-home dental bleaching [5, 9, 12, 30]. On the other hand, in Bonafé et al.’s studies [21, 22], moderate TS was found when restored and sound teeth were evaluated. It is worth mentioning that only three patients, two in the sound and one in the restored group, reported a considerable and severe TS intensity according to the NRS. Nevertheless, none of the patients requested the application of a desensitizing agent in an attempt to reduce the TS intensity, which demonstrated that the intensity of TS during at-home dental bleaching is mild and bearable the majority of the time.
Regarding color change, significant bleaching was observed in both study groups for all evaluation parameters. However, lower values of ΔE*ab, ΔE00, and ∆WID were found in the group of restored teeth in relation to the group of sound teeth after the bleaching procedure. A factor that may have contributed to this was that in some patients, especially those who had lateral incisors restored, the active tip of the spectrophotometer (6 mm in diameter) involved both the dental structure and the margins of the restoration, and, in this way, less whitening was expected to be observed in restored teeth, because the structure of the resin matrix is different from that observed in the dental tissues. When in contact with the dental surface, diffusion of HP through enamel and dentin occurred, followed by breaking the double bonds of organic and inorganic components within the dentinal tubules [36].
On the other hand, the HP action in the composite restorations only occurs on the surface [37]. Some studies showed that the application of CP at-home bleaching gel decreased the microhardness and increase the superficial roughness of resin composite [38,39,40]. Therefore, the use of at-home bleaching gel is expected to be able to remove only extrinsic stains from composite restorative materials [39, 41]. In fact, although some whitening effect could be measured, the use of 10% CP gel led to slight color changes of composite resins [42,43,44]. However, even 10% CP bleaching agents were able to remove extrinsic stains from composite restorative materials [39, 41]. In fact, as in the restored group, the teeth with restorations also had part of the dental structure; the lower color change in the restored group after bleaching when compared with the sound teeth group was probably due to the non-alteration of the intrinsic color of the restored teeth.
Unlike what was observed in our study, the studies conducted by Bonafé et al. [21, 22] showed no significant difference in color between restored and sound teeth, which indicates that the restored teeth obtained the same level of bleaching as the sound teeth. This probably did not occur in our study due to the low concentration of CP used. According to Monaghan et al. [45, 46] and Andrade et al. [40], highly concentrated in-office bleaching gels can affect the color of composite resin more significantly than lower concentrations of at-home bleaching gels. The clinical studies conducted by Bonafé et al. [21, 22] used 35% HP, which explains the greater whitening effect on restored teeth when compared with the results of the present study.
To evaluate the color change of bleaching procedures, spectrophotometers are commonly used, analyzing the L*a*b* values according to the CIELab system [47,48,49]. In this study, the CIE00 [26, 27] and WID [50,51,52,53] systems were also used. Despite the color rebound observed after 3 months, the values of color observed in the restored group were superior to the 50:50% perceptibility/acceptability threshold (∆E*ab > 6.4; ΔE00 > 4.0), which indicates effectiveness of bleaching in both groups of this study [28].
Finally, regarding the limiting factors of the study, most of the participants were young adults, which could affect the generalization of the study results for populations of other ages. In this way, future studies should be conducted in order to evaluate the effect of higher CP concentrations used in at-home bleaching technique on tooth color change and TS in older adults. Due to the fact that patients with adhesive restorations reported the same TS level as patients with sound teeth, 10% CP at-home bleaching should be indicated for the former. However, it is indicated that professionals should examine restorations meticulously before starting a bleaching procedure and renew insufficient restorations as necessary prior to bleaching to achieve an optimal seal of the cavities and thus reduce the risk of TS [37]. Still, it should be considered that the use of 10% CP at-home bleaching leads to less color change for patients with adhesive restorations. Therefore, the dentist must advise the patients that, after at-home bleaching, it would be necessary to replace adhesive restorations for complete esthetic satisfaction.
Conclusions
Although, the presence of esthetic restorations did not influence the TS when 10% CP was used, 2 weeks of at-home bleaching were not enough to promote a similar result in terms of color change when patients with esthetic restorations were compared with patients with sound teeth.
References
Haywood VB, Sword RJ (2020) Tray bleaching status and insights. J Esthet Restor Dent: 1–12. https://doi.org/10.1111/jerd.12688
Kielbassa AM, Maier M, Gieren A-K, Eliav E (2015) Tooth sensitivity during and after vital tooth bleaching: a systematic review on an unsolved problem. Quintessence Int 46:881–897. https://doi.org/10.3290/j.qi.a34700
Martini EC, Favoreto MW, Coppla FM, Loguercio AD, Reis A (2020) Evaluation of reservoirs in bleaching trays for at-home bleaching: a split-mouth single-blind randomized controlled equivalence trial. J Appl Oral Sci 28:1–11. https://doi.org/10.1590/1678-7757-2020-0332
De Geus JL, Wambier LM, Kossatz S, Loguercio AD, Reis A (2016) At-home vs in-office bleaching: a systematic review and meta-analysis. Oper Dent 41:341–356. https://doi.org/10.2341/15-287-LIT
Basting RT, Amaral FLB, França FMG, Flório FM (2012) Clinical comparative study of the effectiveness of and tooth sensitivity to 10% and 20% carbamide peroxide home-use and 35% and 38% hydrogen peroxide in-office bleaching materials containing desensitizing agents. Oper Dent 37:464–473. https://doi.org/10.2341/11-337-C
Fiorillo L, Laino L, Stefano R, D’Amico C, Bocchieri S, Amoroso G, Isola G, Cervino G (2019) Dental whitening gels: strengths and weaknesses of an increasingly used method. Gels 5:35. https://doi.org/10.3390/gels5030035
Tay LY, Kose C, Daniel RH, Reis A, Loguercio AD (2012) Long-term efficacy of in-office and at-home bleaching : a 2-year double-blind randomized clinical trial. Am J Dent 25:200–204
Rezende M, Loguercio AD, Kossatz S, Reis A (2016) Predictive factors on the efficacy and risk/intensity of tooth sensitivity of dental bleaching: a multi regression and logistic analysis. J Dent 45:1–6. https://doi.org/10.1016/j.jdent.2015.11.003
Meireles SS, Heckmann SS, Leida FL, Santos IS, Della Bona Á, Demarco FF (2008) Efficacy and safety of 10% and 16% carbamide peroxide tooth-whitening gels: a randomized clinical trial. Oper Dent 33:606–612. https://doi.org/10.2341/07-150
Pontes M, Gomes J, Lemos C, Leaõ R, Moraes S, Vasconcelos B, Pellizzer E (2020) Effect of bleaching gel concentration on tooth color and sensitivity: a systematic review and meta-analysis. Oper Dent 45:265–275. https://doi.org/10.2341/17-376-L
Parreiras SO, Szesz AL, Coppla FM, Martini EC, Farago PV, Loguercio AD, Reis A (2018) Effect of an experimental desensitizing agent on reduction of bleaching-induced tooth sensitivity: a triple-blind randomized clinical trial. J Am Dent Assoc 149:281–290. https://doi.org/10.1016/j.adaj.2017.10.025
Maran BM, Vochikovski L, Hortkoff DRA, Stanislawczuk R, Loguercio AD, Reis A (2018) Tooth sensitivity with a desensitizing-containing at-home bleaching gel — a randomized triple-blind clinical trial. J Dent 72:64–70. https://doi.org/10.1016/j.jdent.2018.03.006
De La Peña VA, Ratón ML (2014) Randomized clinical trial on the efficacy and safety of four professional at-home tooth whitening gels. Oper Dent 39:136–143. https://doi.org/10.2341/12-402-C
Silva-Costa RSG, Ribeiro AEL, Assunção IV, AraújoJúnior RF, Araújo AA, Guerra GCB (2018) In-office tooth bleaching with 38 % hydrogen peroxide promotes moderate / severe pulp inflammation FGF-2 and osteocalcin in rats. J Appl Oral Sci 26:1–9
Soares DG, Basso FG, Pontes ECV, Garcia LDFR, Hebling J, de SouzaCosta CA (2014) Effective tooth-bleaching protocols capable of reducing H2O2 diffusion through enamel and dentine. J Dent 42(3):351–358. https://doi.org/10.1016/j.jdent.2013.09.001
Balladares L, Alegría-Acevedo LF, Arana-Montenegro A, Arana-Gordillo LA, Pulido C, Salazar-Gracez MT, Reis A, Loguercio AD (2019) Effects of pH and application technique of in-office bleaching gels on hydrogen peroxide penetration into the pulp chamber. Oper Dent.https://doi.org/10.2341/18-148-l
Marson FC, Gonçalves RS, Silva CO, Cintra LTÂ, Pascotto RC, Dos Santos PH, Briso ALF (2015) Penetration of hydrogen peroxide and degradation rate of different bleaching products. Oper Dent 40:72–79. https://doi.org/10.2341/13-270-L
Benetti AR, Valera MC, Mancini MNG, Miranda CB, Balducci I (2004) In vitro penetration of bleaching agents into the pulp chamber. Int Endod J 37:120–124. https://doi.org/10.1111/j.0143-2885.2004.00761.x
Briso ALF, Lima APB, Gonçalves RS, Gallinari MO, Dos Santos PH (2014) Transenamel and transdentinal penetration of hydrogen peroxide applied to cracked or microabrasioned enamel. Oper Dent 39:166–173. https://doi.org/10.2341/13-014-L
Patri G, Agnihotri Y, Rama Rao S, Lakshmi N, Das S (2013) An in vitro spectrophotometric analysis of the penetration of bleaching agent into the pulp chamber of intact and restored teeth. J Clin Diagn Res 7:3057–3059. https://doi.org/10.7860/JCDR/2013/7589.3852
Bonafé E, Bacovis CL, Iensen S, Loguercio AD, Reis A, Kossatz S (2013) Tooth sensitivity and efficacy of in-office bleaching in restored teeth. J Dent 41:363–369. https://doi.org/10.1016/j.jdent.2013.01.007
Bonafé E, Loguercio AD, Reis A, Kossatz S (2014) Effectiveness of a desensitizing agent before in-office tooth bleaching in restored teeth. Clin Oral Investig 18:839–845. https://doi.org/10.1007/s00784-013-1055-7
Özcan M, Abdin S, Sipahi C (2014) Bleaching induced tooth sensitivity: do the existing enamel craze lines increase sensitivity? A clinical study. Odontology 102:197–202. https://doi.org/10.1007/s10266-013-0104-7
Schulz KF, Altman DG, Moher D (2011) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 9:672–677. https://doi.org/10.1016/j.ijsu.2011.09.004
Hickel R, Roulet JF, Bayne S, Heintze SD, Mjör IA, Peters M, Rousson V, Randall R, Schmalz G, Tyas M, Vanherle G (2007) Recommendations for conducting controlled clinical studies of dental restorative materials. Clin Oral Investig 11:5–33. https://doi.org/10.1007/s00784-006-0095-7
Luo MR, Cui G, Rigg B (2001) The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res Appl 26:340–350. https://doi.org/10.1002/col.1049
Sharma G, Wu W, Dalal EN (2005) The CIEDE2000 color-difference formula: implementation notes, supplementary test data, and mathematical observations. Color Res Appl 30:21–30. https://doi.org/10.1002/col.20070
Paravina RD, Ghinea R, Herrera LJ, Bona AD, Igiel C, Linninger M, Sakai M, Takahashi H, Tashkandi E, DelMarPerez M (2015) Color difference thresholds in dentistry. J Esthet Restor Dent 27:S1–S9. https://doi.org/10.1111/jerd.12149
Pérez MDM, Ghinea R, Rivas MJ, Yebra A, Ionescu AM, Paravina RD, Herrera LJ (2016) Development of a customized whiteness index for dentistry based on CIELAB color space. Dent Mater 32:461–467. https://doi.org/10.1016/j.dental.2015.12.008
Chemin K, Rezende M, Loguercio AD, Reis A, Kossatz S (2018) Effectiveness of and dental sensitivity to at-home bleaching with 4% and 10% hydrogen peroxide: A randomized, triple-blind clinical trial. Oper Dent 43:232–240. https://doi.org/10.2341/16-260-C
Poubel LAC, Gouvea CVD, Calazans FS, Dip EC, Alves WV, Marins SS, Barcelos R, Barceleiro MO (2019) Pre-operative use of dexamethasone does not reduce incidence or intensity of bleaching-induced tooth sensitivity. A triple-blind, parallel-design, randomized clinical trial. Clin Oral Investig 23:435–444. https://doi.org/10.1007/s00784-018-2452-8
Gökay O, Yilmaz F, Akin S, Tunçbilek M, Ertan R (2000) Penetration of the pulp chamber by bleaching agents in teeth restored with various restorative materials. J Endod 26:92–94. https://doi.org/10.1097/00004770-200002000-00008
Vaz MM, Lopes LG, Cardoso PC, de Souza JB, Batista AC, Costa NL, Torres ÉM, Estrela C (2016) Inflammatory response of human dental pulp to at-home and in-office tooth bleaching. J Appl Oral Sci 24:509–517. https://doi.org/10.1590/1678-775720160137
Mena-Serrano AP, Parreiras SO, do Nascimento EMS, Borges CPF, Berger SB, Loguercio AD, Reis A (2015) Effects of the concentration and composition of in-office bleaching gels on hydrogen peroxide penetration into the pulp chamber. Operative Dentistry 40(2):E76–E82. https://doi.org/10.2341/13-352-L
Soares DG, Basso FG, Hebling J, de Souza Costa CA (2014) Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent 42:185–198. https://doi.org/10.1016/j.jdent.2013.10.021
Kwon SR, Wertz PW (2015) Review of the mechanism of tooth whitening. J. Esthet. Restor. Dent. 27:240–257. https://doi.org/10.1111/jerd.12152
Attin T, Hannig C, Wiegand A, Attin R (2004) Effect of bleaching on restorative materials and restorations - A systematic review. Dent. Mater. 20:852–861. https://doi.org/10.1016/j.dental.2004.04.002
de Andrade ICGB, Basting RT, Rodrigues JA, do Amaral FLB, Turssi CP, França FMG (2014) Microhardness and color monitoring of nanofilled resin composite after bleaching and staining. Eur J Dent 8:160–165. https://doi.org/10.4103/1305-7456.130586
Esmaeili B, Zenouz G, Khazaei F, Daryakenari G, Bizhani A (2018) Effect of different concentrations of carbamide peroxide on the staining susceptibility of resin composites. J Conserv Dent 21:500. https://doi.org/10.4103/jcd.jcd_59_18
de Andrade ICGB, Basting RT, Lima-Arsati YB de O, do Amaral FLB, Rodrigues JA, França FMG (2011) Surface roughness evaluation and shade changes of a nanofilled resin composite after bleaching and immersion in staining solutions. Am J Dent 24:245–249. http://europepmc.org/abstract/MED/22016920
Villalta P, Lu H, Okte Z, Garcia-Godoy F, Powers JM (2006) Effects of staining and bleaching on color change of dental composite resins. J Prosthet Dent 95:137–142. https://doi.org/10.1016/j.prosdent.2005.11.019
Amengual-Lorenzo J, Montiel-Company JM, Bellot-Arcís C, Labaig-Rueda C, Solá-Ruiz MF (2019) Effect of two whitening agents on the color of composite dental restorations. J Clin Exp Dent 11:e15–e20. https://doi.org/10.4317/jced.55450
Hubbezoglu I, Akaoǧlu B, Dogan A, Keskin S, Bolayir G, Özçelik S, Dogan OM (2008) Effect of bleaching on color change and refractive index of dental composite resins. Dent Mater J 27:105–116. https://doi.org/10.4012/dmj.27.105
Anagnostou M, Chelioti G, Chioti S, Kakaboura A (2010) Effect of tooth-bleaching methods on gloss and color of resin composites. J Dent 38:e129–e136. https://doi.org/10.1016/j.jdent.2010.06.006
Monaghan P, Lim E, Lautenschlager E (1992) Effects of home bleaching preparations on composite resin color. J Prosthet Dent 68:575–578. https://doi.org/10.1016/0022-3913(92)90368-K
Monaghan P, Trowbridge T, Lautenschlager E (1992) Composite resin color change after vital tooth bleaching. J Prosthet Dent 67:778–781. https://doi.org/10.1016/0022-3913(92)90581-T
Maran BM, Vochikovski L, Hortkoff DRA, Stanislawczuk R, Loguercio AD, Reis A (2020) Bleaching sensitivity with a desensitizing in-office bleaching gel: a randomized double-blind clinical trial. Quintessence Int 51:788–797. https://doi.org/10.3290/j.qi.a45173
Martini EC, Parreiras SO, Szesz AL, Coppla FM, Loguercio AD, Reis A (2019) Bleaching-induced tooth sensitivity with application of a desensitizing gel before and after in-office bleaching: a triple-blind randomized clinical trial. Clin Oral Investig.https://doi.org/10.1007/s00784-019-02942-9
Rezende M, da Silva KL, Miguel TC, Farago PV, Loguercio AD, Martins LD, Reis A (2020) Prior application of 10% potassium nitrate to reduce postbleaching sensitivity: a randomized triple-blind clinical trial. J Evid Based Dent Pract 20:101406. https://doi.org/10.1016/j.jebdp.2020.101406
Tao D, Smith RN, Zhang Q, Sun JN, Philpotts CJ, Ricketts SR, Naeeni M, Joiner A (2017) Tooth whitening evaluation of blue covarine containing toothpastes. J Dent 67:S20–S24. https://doi.org/10.1016/j.jdent.2017.10.014
Westland S, Luo W, Li Y, Pan Q, Joiner A (2017) Investigation of the perceptual thresholds of tooth whiteness. J Dent 67:S11–S14. https://doi.org/10.1016/j.jdent.2017.09.013
Lilaj B, Dauti R, Agis H, Schmid-Schwap M, Franz A, Kanz F, Moritz A, Schedle A, Cvikl B (2019) Comparison of bleaching products with up to 6% and with more than 6% hydrogen peroxide: whitening efficacy using BI and WID and side effects – an in vitro study. Front Physiol 10:919. https://doi.org/10.3389/fphys.2019.00919
Costacurta AO, Borges CEP, Centenaro C, Correr GM, Kaizer M da R, Gonzaga CC (2020) The bleaching efficacy of carbamide peroxide gels containing potassium nitrate desensitizer. J Clin Exp Dent 12:e644–e649. https://doi.org/10.4317/JCED.56917
Funding
The work was partially supported by the National Council for Scientific and Technological Development (CNPq/ Brazil) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior — Brazil (CAPES) — Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This clinical trial was approved (CAEE, 45800815.1.0000.5188) by the ethics and research committee of the Federal University of Paraiba. It was registered in the Brazilian Clinical Trials Registry (REBEC) under registration number RBR-52j6gmg. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The participants who meet the eligibility criteria signed an informed consent form before enrollment in the study. Details that might disclose the identity of the subjects under study were omitted.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meireles, S.S., de Oliveira, R.D.B., Barbosa, M.T.G. et al. Efficacy and tooth sensitivity of at-home bleaching in patients with esthetic restorations: a randomized clinical trial. Clin Oral Invest 26, 565–573 (2022). https://doi.org/10.1007/s00784-021-04035-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04035-y