Abstract
Objectives
This bi-centric, placebo-controlled, randomized, evaluator-blinded, incomplete cross-over clinical phase II trial was initialized to identify the most appropriate concentration of octenidine dihydrochloride (OCT) in mouth rinses.
Materials and methods
Rinses of 0.10, 0.15, and 0.20% OCT were compared to a saline placebo rinse regarding the reduction of salivary bacterial counts (SBCs) in 90 gingivitis patients over 4 days. Changes in plaque (PI) and gingival index (GI), taste perception, and safety issues were evaluated.
Results
At baseline, the first OCT (0.10, 0.15, 0.20%) rinse resulted in a decrease of SBC (reduction by 3.63–5.44 log10 colony forming units [CFU]) compared to placebo (p < 0.001). Differences between OCT concentrations were not verified. After 4 days, the last OCT rinse again resulted in a significant SBC decrease (3.69–4.22 log10 CFU) compared to placebo (p < 0.001). Overall, SBC reduction between baseline and day 4 was significantly higher in OCT 0.15 and 0.20% groups compared to OCT 0.10% and placebo. Mean GI/PIs were significantly lower in OCT groups than in the placebo group (p < 0.001). Differences in GI/PI between OCT groups were not verified. Adverse effects increased with increasing OCT concentrations.
Conclusions
Considering antibacterial efficacy, frequency of adverse events, and user acceptance, 0.10% OCT was identified as the preferred concentration to be used in future clinical trials.
Clinical relevance
Due to its low toxicity and pronounced antibacterial properties, octenidine dihydrochloride (OCT) is a promising candidate for the use in antiseptic mouth rinses. OCT concentrations of 0.10% are recommended for future clinical trials evaluating the plaque-reducing properties of OCT mouth rinses.
(www.clinicaltrials.gov, NCT022138552)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In clinical practice, antiseptic mouthwashes are either used as an adjunct to improve the efficacy of mechanical oral hygiene or as the only measure of plaque control, e.g., in oral trauma patients, after oral surgical interventions but also in ventilated, long-term hospitalized, terminally ill patients [1, 2]. Antiseptics are primarily intended to reduce the overall bacterial load in the oral cavity but also the precipitation and proliferation of bacteria on non-shedding tooth surfaces. Antiplaque agents have an effect beyond antibacterial action and inhibit plaque growth and plaque-associated inflammation [3]. During a step-wise evaluation process of new formulations and products, the mode of action should be investigated as precisely as possible. Besides in vitro experiments, clinically controlled trials play a major role before regulatory approval for drugs is granted. A new formulation containing the same active ingredient as an already marketed product does not mean that efficacy is equivalent [4]. Therefore, each new formulation has to undergo the testing process.
Currently, chlorhexidine (CHX) is regarded the gold standard of antiplaque agents due to its efficacy and safety verified by a multitude of clinical studies over the past 45 years [5]. Nevertheless, adverse effects of CHX use like tooth discoloration, taste disturbance, and in rare occasions allergic reactions led to the search for an equivalent alternative [6, 7]. Octenidine dihydrochloride (OCT), a bispyridinamine, developed in the 1980s may qualify as a suitable candidate. It has been licensed as an antiseptic agent in 20 European countries since 1995. Like CHX, it unspecifically binds to negatively charged sites of bacterial cell walls as well as to all soft and hard tissue surfaces of the oral cavity due to its cationic nature. Binding to bacteria subsequently results in autolysis and cell death [8]. Thus, OCT is used as a preventive or therapeutic antiseptic for disinfecting skin, mucosa, and wound surfaces [9]. In the oral cavity, it proved to reduce the overall bacterial load [10, 11] by its broad spectrum efficacy affecting Gram-positive and Gram-negative organisms as well as yeasts [12]. Exerting a sustained antimicrobial effect made OCT also suitable for being used as an antiplaque agent [13]. Clinical trials verified its pronounced plaque and gingivitis-reducing properties. [13,14,15,16]. In therapeutic concentrations, OCT is well tolerated without relevant local or systemic toxicity and does not induce bacterial resistance [9].
Presently, 0.10% OCT in combination with 2% 2-phenoxyethanol is commercially available as a wound and mucosa disinfectant (octenisept®, Schülke & Mayr, Norderstedt, Germany) and as an antiseptic mouth rinse (octenimed® Gurgellösung, Schülke & Mayr, Zurich, Switzerland). Recently, an experimental mouth rinse containing only OCT plus flavoring agents showed promising results in an in vitro setting against a variety of human oral pathogens [17].
Aim
The aim of this clinical short-term phase II dose-finding study was to identify the most efficacious OCT concentration in experimental mouth rinses regarding salivary bacterial count (SBC) reduction, frequency of adverse events, and user acceptance over a 4-day application period in comparison to a placebo.
Materials and methods
Trial design
This investigation was performed as a bi-centric phase II study with a placebo-controlled, randomized, evaluator-blinded, 4-day, incomplete cross-over design [18].
After screening, patients were randomly assigned to one of three treatment groups resulting in six treatment sequences: OCT 0.10%-placebo, placebo-OCT 0.10%, OCT 0.15%-placebo, placebo-OCT 0.15%, OCT 0.20%-placebo, placebo-OCT 0.20%. Between both treatment periods (placebo or OCT), a wash-out period of 14–21 days was performed (Fig. 1).
Before study initiation, ethical approval was granted by the institutional review board of the Technische Universität Dresden (EK 292082013). The trial was conducted in accordance with the Declaration of Helsinki and met GCP criteria. It was registered at www.clinicaltrials.gov (NCT022138552) and at the European Clinical Trials Database (EudraCT-No. 2013-002708-14).
All examinations and measurements were performed at the Department of Periodontology of the Faculty of Medicine Carl Gustav Carus Dresden and at the Division of Periodontology of the University Hospital Würzburg between October 2013 and February 2014. All microbiological analyses were conducted at the Institute for Hygiene and Microbiology of the University of Würzburg and the Institute of Medical Microbiology and Hygiene at the Technische Universität Dresden.
Study population
Study subjects were recruited from the resident population by local advertisements or from patients entering the departments of periodontology of Dresden or Würzburg for a routine, prophylactic checkup.
Eligibility criteria
Inclusion criteria were age ≥ 18 years, presence of mild gingivitis (mean gingival index [19] score between 0.2 and 1.0), and the existence of the Ramfjord teeth (16, 21, 24, 36, 42, 44) or their replacements [20].
Exclusion criteria were the manifestation of severe systemic diseases (e.g., diabetes, hepatitis, HIV, tuberculosis, cancer); the requirement of endocarditis prophylaxis before dental treatments; untreated caries; gingival hyperplasia or other severe oral diseases; the presence of orthodontic appliances or removable dentures; antibiotic therapy less than 3 months and mouth rinse use less than 8 weeks prior to the baseline examination; chronic treatment with steroids; the manifestation of xerostomia; smoking of more than 10 cigarettes per day; hypersensitivity or allergy to the test product, its ingredients, or to medications that have a similar chemical structure; pregnancy or breastfeeding; intellectual inability to assess essence and consequences of study participation; and evidence suggesting lack of compliance.
All volunteers willing to participate and meeting the aforementioned eligibility criteria were comprehensively informed about the aims and risks of study in a face-to-face interview and gave their written informed consent.
OCT mouth rinses
All study products used in the study were delivered by NextPharma GmbH (Göttingen, Germany). The study medication consisted of the test rinse containing OCT in three different concentrations: 0.10, 0.15, and 0.20%. As negative control, a 0.9% saline solution (placebo) was applied. Study medication was delivered in identical white boxes. OCT mouth rinse was provided in 250-ml bottles with a measuring cup. The placebo was packaged in 12 single 10-ml containers. A measuring cup was added in the box. Bottles were weighed before delivery and after return.
In total, 10 doses of the test product and 10 doses of the placebo were applied per patient. Subjects rinsed twice daily in the morning and in the evening with 10 ml for 30 s. The exact rinsing time was measured with a stop watch. The use of the mouthwash was documented by the patients on an identification card.
Screening visit
After informed consent was obtained, eligibility criteria were checked, demographic data and concomitant medication were documented, and the oral cavity was inspected for abnormalities. Plaque index [21] and gingival index [19] were recorded on the Ramfjord teeth (16, 21, 24, 36, 41, 44) [22] or their replacement teeth (17, 11, 25, 37, 31, 45) at four sites per tooth: distovestibular, vestibular, mesiovestibular, and oral.
First study period
Baseline examination (visit 1, V1)
At baseline, an examination of the oral cavity was performed, GI [19] was assessed, and general health status and concomitant medication were updated by the “evaluating physician” (EP).
Independently, the “treating physician” (TP) handed out a supply of the experimental mouth rinses to the study subjects and instructed and supervised them to properly rinse with the allocated mouth rinse. Immediately before and 1 min after the first supervised rinse, study subjects rinsed their mouth with 5 ml of sterile water for 30 s. The water/saliva mixture was spit in a sterile lockable container, kept in the refrigerator at 4–6 °C, and sent to the microbiological laboratory for further SBC analysis within 4 h after sampling.
Finally, all subjects received a professional tooth cleaning comprising the biofilm removal of all surfaces of the teeth using a rubber cup and a professional cleaning paste. The study subjects were instructed to rinse their mouth twice daily with the assigned rinsing solution for the next 4 days and to refrain from any oral hygiene or the use of any oral antiseptics during this period.
Reevaluation visit 2 (V2)
After 4 days of rinsing, the study patients returned and PI and GI were recorded. Safety and tolerability were assessed by the occurrence of adverse events (AEs) and inspection of the oral cavity by the EP. The TP supervised the last rinse. Pre- and post-rinsing saliva samples were taken, and a professional tooth cleaning similar to V1 was repeated. A questionnaire to evaluate taste, freshness, aftertaste, and the overall impression of the mouth rinses was given out. All study subjects judged on a scale from 0 (not at all, very poor) to 10 (very strong, very good) and returned the questionnaire to the study nurse at the same visit. Study medication was taken back and counted.
After V2, a wash-out phase of 14 or 21 days, respectively, followed. During this time, subjects performed their habitual tooth brushing, but furthermore, the use of any mouthwash was not allowed.
Second study period
Baseline examination visit 3 (V3)
At V3, the same procedures were applied as at V1. The participants again received study medication for home use and returned to the study center 4 days later for visit 4 (V4).
Reevaluation Visit4 (V4)
At V4, exactly the same procedures as at V2 were executed. Study medication was taken back and counted.
At V1 and V4, a pregnancy test (One Step®, GEDPHARM Ltd. and Co. KG, Viersen, Germany) was performed in women of childbearing age.
Visit 5
Fourteen to 21 days later, patients were phone-called to follow up possible AEs that might have occurred after the last rinse. After visit 5 (V5), the study was finished. Study procedures are summarized in Fig. 1.
Microbiological analysis
Six tenfold serial dilution saliva samples were prepared in neutralization solution. From each dilution and a neutralization solution control, 2× 0.5 ml were inoculated on blood agar plates (Columbia agar + 5% sheep blood; bioMérieux SA, Marcy l’Etoile, France) and incubated at 37 °C for 48 h. Colony forming units (CFU) grown on both plates were counted to determine the number of CFU/ml for the respective dilution. The number of CFU per 1 ml was determined.
Calibration and blinding
All clinical examiners met for examiner training [23]. Blinding was assured in each center by splitting up responsibilities among two examiners, an EP and a TP and a study nurse. The EP recorded all oral parameters and the general health status, and the TP handled the study medication, collected the saliva samples, and performed the professional tooth cleaning. EP and TP worked in separate rooms to prevent the EP from having insight in the rinsing allocation. The microbiological laboratories did not have any knowledge of the sample allocation.
Randomization
Patients were consecutively assigned to screening numbers according to their chronological entry into screening. At V1, selected patients were assigned a consecutive randomization number according to chronological entry into the study at baseline. The randomization list was computer-generated by the provider of the study medication (NextPharma GmbH).
Sample size calculation
Sample size calculation was based on preliminary evaluation available from 20 subjects using decadic logarithm values of SBC. Results of the evaluation 1 min after rinsing with 0.10% OTC showed a mean SBC reduction of − 2.96 ± 0.91 log units (Schülke and Mayr, internal data, unpublished). For non-active controls, mean reductions of 0.14–0.30 log units were described with an estimated standard deviation of 0.38–0.60 log units. A strong effect was assumed when the mean reduction factor was at least 1.5 log units [10]. Aiming for robust efficacy and safety estimations, n = 24 participants were calculated per treatment group, resulting in a total number of n = 72. Considering a drop-out rate of 20%, at least n = 90 subjects were calculated to be randomized.
Statistical analysis
Statistical analyses were based on the intention to treat (ITT) population; all patients were analyzed by the treatment sequence (OCT-placebo or placebo-OCT). The per-protocol analysis included all patients who completed the study without major protocol deviations. For all safety analyses, the ITT population was applied. A level of significance of 5% was defined. All calculations for change in SBC were carried out using decadic logarithm of the absolute numbers of bacteria. The primary endpoint, SBC reduction after single OCT application at V1 and V3, and SBC reduction at V2 and V4 were analyzed using a separate ANOVA model for each of the three OCT concentrations including the effects “sequence” (placebo followed by OCT/OCT followed by placebo), “period” (treatment period 1/treatment period 2), and “treatment” (placebo/OCT). SBC reductions in saliva between V1/V2 and V3/V4, mean PI at V2 and V4, change in mean GI between V1/V2 and V3/V4, and evaluation of taste and flavor were analyzed by ANOVA with factor treatment (placebo/0.10% OCT/0.15% OCT/0.20% OCT). Multiple comparisons of means were performed by Student-Newman-Keuls (SNK) test (α ≤ 0.05). Questionnaire results (taste, general impression) were listed as descriptive statistics. Adverse events were listed and assigned to the different treatment groups and periods. All statistical evaluations and analyses were performed using SAS V9.2 (SAS, Cary, NC, USA) and SPSS V21.0.0.0 (IBM, Chicago, IL., USA).
Primary research question
The primary endpoint was SBC reduction after a single application of OCT in comparison to placebo application at V1 and V3.
Secondary research questions
-
SBC reduction after use of OCT in comparison to placebo mouthwash at V2 and V4
-
SBC before mouthwash application at V1 compared to V2
-
SBC before mouthwash application at V3 compared to V4
-
Mean PI at V2 and V4
-
Change in mean GI between V1/V2 and V3/V4, respectively
-
Evaluation of taste and flavor (questionnaire)
-
Safety evaluation (adverse events, serious adverse events)
Results
Assignment and compliance
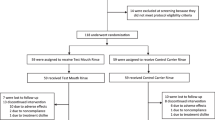
Overall, 90 (32 males, 58 females) healthy participants, aged 19 to 75 years (28.4 ± 11.0 years), meeting the inclusion criteria were recruited at both study centers (Table 1). The flow of participants is shown in Fig. 2. No major protocol deviations occurred with exception of one patient who did not appear at visits 3 and 4 and another patient who did not appear at visit 2. Because the numbers of missing data are minimal, no per-protocol analysis was performed. All 90 patients were included in the ITT analysis.
Salivary bacterial counts
Primary endpoint: After a single application of 0.10, 0.15, and 0.20% OCT rinsing formulations, mean SBCs were reduced at V1 and at V3. SBC remained almost unchanged after the placebo rinse. There were statistically significant treatment effects in SBC reduction after application of OCT mouth rinses compared to placebo (ANOVA, p < 0.001, SNK). There were no period effects and no sequence effects (Table 2).
At V2 and at V4, a single application of 0.10, 0.15, and 0.20% OCT led to a reduction of mean SBC, too. SBC remained almost unchanged after the placebo rinse. There were significant treatment effects in SBC reduction after application of OCT compared to placebo (ANOVA, p < 0.001, SNK). With exception of OCT 0.15% (period effect, p = 0.0363), there were no period effects and no sequence effects (Table 3).
After 4 days of mouth rinse application (10 doses), significant differences in SBC reduction between placebo (increase) and all OCT concentrations (decrease) were found (ANOVA p < 0.001, SNK). Additionally, with OCT 0.15% and OCT 0.20%, a statistically significantly higher SBC reduction was achieved than with OCT 0.10% (Table 4).
Plaque index
At baseline, mean PI scores in all rinsing groups and the placebo group did not differ significantly from each other (p > 0.05; Table 1). Participants showed a mean baseline PI of 0.54 ± 0.24 before professional cleaning. The plaque index increased in all groups during the rinsing period. However, the increase in the placebo group was significantly more than three times higher than in the OCT groups (ANOVA, p < 0.001, SNK). No differences were observed between the different OCT concentrations (ANOVA, p > 0.001, SNK; Table 5).
Gingival index
Participants showed a mean baseline GI of 0.57 ± 0.19. Data per group are listed in Table 1. No differences existed between groups (p > 0.05). While the GI increased on average by 0.24 in the placebo group, it slightly decreased in the three OCT groups over the four-day treatment period. This difference between placebo and OCT was statistically significant (ANOVA, p < 0.001, SNK). No differences existed between the three OCT concentration groups (ANOVA, p > 0.001, SNK; Table 5).
Taste and general impression
All three OCT concentrations were rated to have a bitter taste; means varied from 5.03 ± 3.54 to 5.28 ± 3.17. Freshness of breath was judged good (6.87 ± 2.23 to 7.60 ± 1.16), and a strong long-lasting aftertaste was observed (7.59 ± 2.63 to 8.32 ± 2.04). The general impression of OCT mouth rinses ranged from 6.03 ± 2.36 to 6.34 ± 2.01 on average. No concentration-dependent order could be detected, and no significant differences between OCT concentrations existed (ANOVA, SNK). A re-use rate of OCT rinses of 68.9% was reported. The lowest mean rate of re-use concerned 0.20% OCT (54.9%) while with 0.10% OCT (75.8%) and 0.15% OCT (76.7%), it was considerably higher.
Safety
No serious adverse events and 71 AEs occurred. In 46 cases (65%), a “probable” or “definite” relation to OCT use was noted. Reversible mild tooth and tongue discoloration as most frequent AEs occurred in 12 subjects who rinsed with 0.10%, in 11 subjects with OCT 0.15%, and in 18 subjects with OCT 0.20%.
Discussion
This phase II study served as dose-finding study for a newly developed octenidine mouth rinse formulation. In this early stage of product evaluation, we primarily wanted to investigate the antibacterial effect in saliva after a one-time rinse with this antiseptic [3, 24]. Testing antibacterial action was the first step during the in vitro evaluation process of this antiplaque mouth rinse. At the same time, the appropriate concentration could be identified. Plaque-inhibiting effects and effects on gingivitis were studied here as secondary outcomes and will be focused on in upcoming studies.
From previous studies with OCT containing solutions, it is known that OCT has a comparable antibacterial and antiplaque mode of action like CHX. However, in this first study in men, the appropriate OCT dose should be investigated rather than the equivalence or superiority to CHX. This is why only a negative control was chosen in this study instead of CHX as positive control.
The experimental plaque model has proven its suitability in previous studies [25,26,27,28,29]. As before, a cross-over design was applied to compare between test and negative control. Possible carry-over effects were excluded by a sufficient wash-out period of at least 2 weeks between treatment periods.
Antibacterial action
The test formulations showed a clinically relevant and statistically significant antibacterial effect. This was proven after a single rinse both at baseline and at day 4. The mean magnitude of this effect ranged from 3.63 to 5.44 and from 3.69 to 4.22 log units, respectively. If reduction factors of ≥ 1.5 correlate with a “strong” effect [10], a very strong effect was achieved in this study with OCT. A comparable study on CHX mouth rinse revealed smaller differences of 1.9 ± 0.9 log units 10 min after rinsing and of 1.3 ± 0.5 log units after 7 days [30]. Other 4-day plaque re-growth studies that tested OCT in comparison to CHX showed a similar or even stronger effect of OCT on SBC reduction. SBC could be reduced by OCT from about 7.13 log units to 2.70 log units 15 min after rinsing. With CHX 0.12%, SBCs were reduced from 7.44 to 6.48 log units. At the fourth day of daily rinsing, median SBCs were 3.40 for OCT and 5.54 for CHX. Here, OCT exerted the strongest antibacterial effect [31]. A recent study by Welk and co-workers showed a comparable antibacterial efficacy of OCT and CHX both after a single rinse and after 4 days of rinsing [15].
The SBC reduction over 4 days can be interpreted as a sustained effect of OCT on total bacterial load in saliva. To the contrary, the expected increase in bacterial load was detected in the placebo group due to the omission of any oral hygiene.
Plaque and gingival inflammation
Without oral hygiene, plaque developed in all groups. On average, an increase in PI by 0.5 was seen independently from the OCT concentration applied. This effect is known from CHX as even the most potent antiseptic was not able to inhibit plaque growth completely when oral hygiene was ceased [26, 32, 33]. Plaque inhibition was clinically meaningful and sufficient to prevent the development of gingivitis. When rinsing with a placebo, mean plaque accumulation during 4 days was about three times higher compared to OCT.
Taste and safety
Despite the efforts to mask the bitter taste with menthol oils, the majority of participants rated the taste of OCT as bitter. However, freshness was judged as good with a long aftertaste. The general impression for all OCT rinses was “medium” with no preference for a certain OCT concentration. About 70% of participants would re-use OCT mouth rinse with the lowest rate for 0.20% OCT.
During OCT rinse use, only mild AEs occurred. Twenty-nine “definitely related” and 17 “probably related” AEs occurred in the OCT groups including tooth and tongue staining. Numbers of staining cases increased with increasing OCT concentration. Staining propensity is known from previous studies on OCT and CHX [13, 33]. In the majority of cases, tooth staining was mild and only detected by the investigator. Therefore, it can be summarized that the OCT mouth rinses were well perceived and tolerated and were safe for use.
Octenidine dihydrochloride containing mouth rinses as applied in this 4-day plaque re-growth study were able to statistically and clinically significantly depress salivary bacteria after single and multiple applications. In addition, OCT was capable to inhibit clinically relevant plaque re-growth and gingivitis. Concerning the primary study parameter, all of the OCT concentrations were effective. Thereby, OCT 0.10% as the lowest of the three OCT concentrations showed a statistically significant and clinically meaningful reduction in SBC. Furthermore, OCT 0.10% was the formulation with the least side effects and highest re-use rate. Therefore, it is recommended for further clinical studies.
References
Addy M, Moran JM (1997) Clinical indications for the use of chemical adjuncts to plaque control: chlorhexidine formulations. Periodontol 2000 15:52–54
Ozcaka O, Basoglu OK, Buduneli N, Tasbakan MS, Bacakoglu F, Kinane DF (2012) Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: a randomized clinical trial. J Periodontal Res 47:584–592. https://doi.org/10.1111/j.1600-0765.2012.01470.x
Addy M, Moran JM (1997) Evaluation of oral hygiene products: science is true; don't be misled by the facts. Periodontol 2000 15:40–51
Li W, Wang RE, Finger M, Lang NP (2014) Evaluation of the antigingivitis effect of a chlorhexidine mouthwash with or without an antidiscoloration system compared to placebo during experimental gingivitis. J Investig Clin Dent 5:15–22. https://doi.org/10.1111/jicd.12050
Varoni E, Tarce M, Lodi G, Carrassi A (2012) Chlorhexidine (CHX) in dentistry: state of the art. Minerva Stomatol 61:399–419
Gurgan CA, Zaim E, Bakirsoy I, Soykan E (2006) Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse used as an adjunct to non-surgical periodontal treatment: a double-blind clinical study. J Periodontol 77:370–384. https://doi.org/10.1902/jop.2006.050141
Van Strydonck DA, Slot DE, Van der Velden U, Van der Weijden F (2012) Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. J Clin Periodontol 39:1042–1055. https://doi.org/10.1111/j.1600-051X.2012.01883.x
Assadian O (2016) Octenidine dihydrochloride: chemical characteristics and antimicrobial properties. J Wound Care 25:S3–S6. https://doi.org/10.12968/jowc.2016.25.Sup3.S3
Hubner NO, Siebert J, Kramer A (2010) Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin Pharmacol Physiol 23:244–258. https://doi.org/10.1159/000314699
Pitten FA, Kramer A (1999) Antimicrobial efficacy of antiseptic mouthrinse solutions. Eur J Clin Pharmacol 55:95–100
Kramer A, Hoppe H, Krull B, Pitten FA, Rosenau S (1998) Antiseptic efficacy and acceptance of Octenisept computed with common antiseptic mouthwashes. Zentralbl Hyg Umweltmed 200:443–456
Koburger T, Hubner NO, Braun M, Siebert J, Kramer A (2010) Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J Antimicrob Chemother 65:1712–1719. https://doi.org/10.1093/jac/dkq212
Beiswanger BB, Mallatt ME, Mau MS, Jackson RD, Hennon DK (1990) The clinical effects of a mouthrinse containing 0.1% octenidine. J Dent Res 69:454–457
Patters MR, Nalbandian J, Nichols FC, Niekrash CE, Kennedy JE, Kiel RA, Trummel CL (1986) Effects of octenidine mouthrinse on plaque formation and gingivitis in humans. J Periodontal Res 21:154–162
Welk A, Zahedani M, Beyer C, Kramer A, Muller G (2015) Antibacterial and antiplaque efficacy of a commercially available octenidine-containing mouthrinse. Clin Oral Investig 20:1469–1476. https://doi.org/10.1007/s00784-015-1643-9
Patters MR, Anerud K, Trummel CL, Kornman KS, Nalbandian J, Robertson PB (1983) Inhibition of plaque formation in humans by octenidine mouthrinse. J Periodontal Res 18:212–219
Radischat N (2013) In-vitro efficacy experiments of different OCT concentrations by quantitative suspension test on bactericidal and fungicidal effects according to relevant DIN norms for dose range selection. Schülke & Mayr, internal data
Addy M, Willis L, Moran J (1983) Effect of toothpaste rinses compared with chlorhexidine on plaque formation during a 4-day period. J Clin Periodontol 10:89–99
Loe H (1967) The gingival index, the plaque index and the retention index systems. J Periodontol 38(Suppl):610–616. https://doi.org/10.1902/jop.1967.38.6.610
Ramfjord SP (1959) Indices for prevalence and incidence of periodontal disease. J Periodontol 30:51–59
Silness J, Loe H (1964) Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 22:121–135
Ramfjord SP (1987) Maintenance care for treated periodontitis patients. J Clin Periodontol 14:433–437
Hefti AF, Preshaw PM (2012) Examiner alignment and assessment in clinical periodontal research. Periodontol 59:41–60. https://doi.org/10.1111/j.1600-0757.2011.00436.x
Lorenz K, Bruhn G, Netuschil L, Heumann C, Hoffmann T (2009) How to select study designs and parameters to investigate the effect of mouthrinses? Part I: rationale and background. J Physiol Pharmacol 60(Suppl 8):77–83
Arweiler NB, Henning G, Reich E, Netuschil L (2002) Effect of an amine-fluoride-triclosan mouthrinse on plaque regrowth and biofilm vitality. J Clin Periodontol 29:358–363
Arweiler NB, Netuschil L, Reich E (2001) Alcohol-free mouthrinse solutions to reduce supragingival plaque regrowth and vitality. A controlled clinical study. J Clin Periodontol 28:168–174
Singh A, Daing A, Dixit J (2013) The effect of herbal, essential oil and chlorhexidine mouthrinse on de novo plaque formation. Int J Dent Hyg 11:48–52. https://doi.org/10.1111/j.1601-5037.2012.00556.x
Jenkins S, Addy M, Newcombe R (1989) Toothpastes containing 0.3% and 0.5% triclosan. I. Effects on 4-day plaque regrowth. Am J Dent 2 Spec No:211–214
McClanahan SF, Bollmer BW, Court LK, McClary JM, Majeti S, Crisanti MM, Beiswanger BB, Mau MS (2000) Plaque regrowth effects of a triclosan/pyrophosphate dentifrice in a 4-day non-brushing model. J Clin Dent 11:107–113
Dahlen G (1984) Effect of antimicrobial mouthrinses on salivary microflora in healthy subjects. Scand J Dent Res 92:38–42
Dogan AA, Cetin ES, Hussein E, Adiloglu AK (2009) Microbiological evaluation of octenidine dihydrochloride mouth rinse after 5 days’ use in orthodontic patients. Angle Orthod 79:766–772. https://doi.org/10.2319/062008-322.1
Moran J, Addy M, Wade W, Milson S, McAndrew R, Newcombe RG (1995) The effect of oxidising mouthrinses compared with chlorhexidine on salivary bacterial counts and plaque regrowth. J Clin Periodontol 22:750–755
Rosin M, Welk A, Kocher T, Majic-Todt A, Kramer A, Pitten FA (2002) The effect of a polyhexamethylene biguanide mouthrinse compared to an essential oil rinse and a chlorhexidine rinse on bacterial counts and 4-day plaque regrowth. J Clin Periodontol 29:392–399
Acknowledgements
The authors would like to thank Olaf Böhm for sample size planning and for generating the randomization list and Uta Schwanebeck for performing the statistical analysis.
Funding
The conduction of this study was funded by Schülke and Mayr GmbH, Norderstedt, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Lorenz, K., Jockel-Schneider, Y., Petersen, N. et al. Impact of different concentrations of an octenidine dihydrochloride mouthwash on salivary bacterial counts: a randomized, placebo-controlled cross-over trial. Clin Oral Invest 22, 2917–2925 (2018). https://doi.org/10.1007/s00784-018-2379-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2379-0