Abstract
Objectives
To evaluate and compare the efficacy of prepared propolis mouth rinse with Chlorhexidine mouthwash on oral pathogens and also the plaque and gingival index scores.
Material and methods
A triple-blind, concurrent parallel randomized controlled trial was conducted on 120 participants randomized to 4 mouth rinse study groups: (1) Hot Ethanolic Propolis extract; (2) Cold Ethanolic Propolis extract; (3) Chlorhexidine and (4) Distilled water. After a washout period of two weeks, oral prophylaxis and polishing was performed. Participants rinsed twice a day for 3 months. Saliva was collected at baseline, 5 min and 1 h for microbiological analysis. Plaque and Gingival index were recorded at baseline, 15 days, 1 month and 3 months. Repeated measures ANOVA with Bonferroni post hoc tests were used for statistical analysis.
Results
A decline in the concentration of S. mutans was observed in samples collected after the use of mouth rinse (p < 0.05). In comparison with baseline, L. acidophilus and S. mutans count decreased simultaneously when exposed to Hot Ethanolic mouthwash group (5.5 × 102) and Chlorhexidine mouthwash (5.8 × 102) respectively. At the end of 3 months, similar reduction in plaque scores was found in Chlorhexidine (0.45), Cold Ethanolic (0.46), Hot Ethanolic (0.47) mouthwash groups.
Conclusion
Propolis was found to be as efficient as Chlorhexidine in reducing plaque, gingivitis and dental caries pathogens.
Clinical relevance
Common microorganisms implicated in oral disease are S. mutans and L. acidophilus. There is great paucity of information on antimicrobial activity of propolis, against these microorganisms. Hence, the present study has been taken up to assess the effects of propolis on these oral pathogens.The effects of propolis on oral health have been proved which is obviously a new finding of significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the aeon of proliferation, there is an evident shift in health care approaches. The critical approach is focused on research toward evolving innovative health care research and practices. The base of this movement is fixed towards producing a product that could be applicable of carrying a label on a global scale — eco-friendly. This thought has led therapeutic researchers to develop and experiment with properties of various plants and herbal products to enhance pharmaceutical products.
Oral health is integral to general health; mouth is swarming with bacteria most of them harmless. However, without proper oral hygiene, increased bacterial levels might lead to oral infections. A number of controlled trials have demonstrated that using herbal products reduces supragingival plaque, antimicrobial activity and gingivitis [1, 2].
Propolis being one such product, has been used for centuries by the Egyptian and Greek civilization which recognized its healing qualities. More recently, propolis has been used for treating different diseases and inflammatory conditions as both local and systemic applications [3]. Propolis is a sticky substance that bees make which is also known as “bee glue”. Bees use propolis as an antiseptic barrier covering invaders with the sticky substance to prevent hive contamination [4].
In the past decades, health professionals have found Propolis to boost the effects of other antibiotics like penicillin and expand the immune system [4]. This resinous substance is composed of amino acids, minerals, vitamins A, B complex, E and the highly active bio-chemical substance known as bioflavenoid (Vitamin P) [5, 6]. Flavonoids are well-known plant compounds which have antimicrobial, antioxidant and anti-inflammatory effects. It is this property of propolis that has been found to be very effective against gram positive bacteria and against gram negative bacteria [7].
Propolis is available in the world markets in different medicinal forms and is recently been introduced into the list of toothpastes. Propolis has long been used also for healing oral ulcers [8] and its antibacterial, antifungal, antiviral, antioxidative, antitumor, and anti-inflammatory properties have been proven [9, 10]. Thus, the objectives of the present study were to assess and compare the effect of propolis mouth rinse with Chlorhexidine on two dental caries pathogens and also the dental plaque and gingival index scores.
Material and methods
The study consisted of two phases:
Phase 1: In-vitro experimental study carried out to estimate the zone of inhibition and Minimal Inhibitory Concentration (MIC) of various propolis extracts against Streptococcus mutans and Lactobacillus acidophilus
Phase 2: In-vivo concurrent parallel study in which the effect of propolis mouth rinse on dental plaque, gingiva and dental caries pathogens was compared with a positive control (0.2% Chlorhexidine) and a negative control (distilled water).
Study design, study area and study population
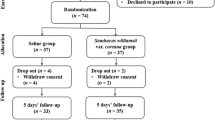
A triple-blind, concurrent parallel randomized controlled trial was carried out among 120 dental students of the Pacific Dental College and Hospital, Udaipur, India. Systemically healthy subjects in the age group of 18 to 22 years who agreed to cooperate and were willing to participate in the study were included. Those subjects currently using any mouthwash, were on antibiotic therapy within the last 2 months, had a history of hypersensitivity to any product used, who underwent a recent tooth extraction, had oral lesions, periodontal disease or orthodontic appliances and who were under the influence of oral tissue abusive habits like alcohol, smoking, tobacco chewing, etc. were excluded.
Ethical clearance and official permission
The study protocol was reviewed by the Ethical committee of Pacific Dental College and Hospital, Udaipur. An official permission was obtained from the principal of Pacific Dental College and Hospital, Udaipur. Prior to the commencement of the study, a detailed procedure of the study and written informed consent were obtained from all the subjects.
Phase 1
Crude sample of propolis was obtained from Apis mellifera bee hive of the western part of Maharashtra region (Nasik). The aqueous and ethanol extracts of propolis were used for the antimicrobial screening using the agar well diffusion method. The media was punched with 7-mm diameter wells and were filled with various concentrations of the extracts 2 μg/ml, 5μg/ml, 10μg/ml, 25μg/ml, 50μg/ml and 75μg/ml. Incubation of the plates was done at 37°C for 24 h. The zone of growth of inhibition between the edges of the lawn for each extract was measured in millimeters by using a special scale designed for their purpose.
Determination of MIC
Six dilutions of each extract were done with brain heart infusion (BHI) broth micro-dilution. In the initial tube 20 ml of extract was added into the 380 ml of BHI broth. For further dilutions 200 ml of BHI broth was added to subsequent 6 tubes. Then, from the 380-ml tube 200 ml was transferred to the first tube containing 200 ml of BHI broth. This was considered as 10−1 dilution. From 10−1 diluted tube, 200 ml was transferred to the second tube to make 10−2 dilution. The serial dilution was repeated up to 10−6 dilution for each extract.
Formulation of mouth rinse
The propolis was cleaned free of wax, paint, wood etc. Later on, propolis was cut into small pieces.
Preparation of hot propolis mouth rinse
The mouthwash was made by dissolving raw propolis into 70% ethyl alcohol. Propolis (100 gm) was mixed with 1000 ml of solvent ethyl alcohol (99.97 grade) by hot continuous method using Soxhlet apparatus. The temperature during the method was maintained between 40 and 50°C for a period of 3 to 4 h. The filtrate was concentrated to yield 5 g of semisolid extract. This extract was further diluted to a concentration of 5 μg/ml, according to the required MIC values.
Preparation of cold propolis mouth rinse
Mouth rinse was also made in a similar manner by dissolving raw propolis into 70% ethyl alcohol. Propolis (100 g) was mixed with 1000 ml of solvent ethyl alcohol (99.97 grade) at room temperature and was then later cooled in a refrigerator at 10–20°C for a period of 3 to 4 h and further diluted to a concentration of 5 μg/ml according to the required MIC values.
Preparation of distilled water propolis mouth rinse
Distilled water propolis mouth rinse was prepared by dissolving raw propolis in 1 l of distilled water. The solvent was stored at room temperature for a period of 3 to 4 h, extract was further diluted to a concentration of 5 μg/ml according to the required MIC values. Distilled water propolis extract was found to be ineffective at all concentrations against S. mutans and therefore was not included in the study.
Coloring and flavoring agents were added to the three prepared formulas to improve the acceptability of the mouthwash ingredients. All the preparations were made to look alike and were delivered to the participants in similar bottles marked “A”, “B” or “C”.
Random allocation of the test products and instructions
Subjects were randomly allocated by lottery method into 4 study groups with 30 participants in each group. Each participant was asked to take a slip from a box containing 120 slips with equal number of four different codes for the products and they were allotted to that group. The subjects were designated to the groups by a person not involved in the examination. Subjects received the products according to the specified code. The 4 study groups were:
-
Group 1 - Hot ethanolic propolis extract
-
Group 2 - Cold ethanolic propolis extract
-
Group 3 - Chlorhexidine gluconate 0.2%
-
Group 4 - Placebo (distilled water)
Placebo acted as a negative control instead of distilled water propolis group.
Training and calibration
One trained examiner performed all the clinical examination. Gingival inflammation was determined by Löe and Silness (1963) [11], and dental plaque indexes were assessed according to Silness and Löe (1964) [12]. Four surfaces per tooth (buccal, lingual, mesial and distal) were examined in every permanent tooth (except for third molars). Plaque and gingival indexes were calculated separately for every participant. The intra examiner reliability was assessed using Kappa statistics, which was found to be 90% each for both the indices.
Phase 2
Method of collection of saliva
Participants were instructed not to eat or drink anything for at least one hour before the collection of saliva sample. To control the biological variations, samples were collected between 8:00 am and 9:00 am. To avoid contamination of saliva with food debris, the subjects were asked to rinse their mouth with distilled water. The collected samples were transferred into 5-ml sterile disposable vials. Samples were collected at baseline, 5 min and 1 h. The samples were transported to the laboratory, within 2 h of saliva collection.
Laboratory procedure
The colony count was done using electron microscope for S. mutans and L. acidophilus and was expressed as number of CFU per ml of saliva.
Methodology
Before starting the study, oral prophylaxis and polishing were performed to remove all calculus, plaque and extrinsic stains. All subjects were given Colgate Regular Flavour toothpaste (Non-fluoridated) and a Colgate soft manual tooth brush to brush twice daily for the washout period (two week) and to continue using the standardized products throughout the course of the study. Standardized brushing technique was demonstrated by the investigator to the participants to ensure that they fully understood the brushing instructions. Subjects were also instructed not to use any other oral hygiene aids during the course of the study. They were also asked to abstain from any other mouth rinse than those provided for the study.
After 15 days (washout period), subjects reported to the Department of Public Health Dentistry, oral prophylaxis was performed. For a period of 3 months all the students were instructed to rinse twice daily with 10 ml of mouth wash for 1 min (before breakfast and after dinner) and avoid eating or drinking thereafter. At the beginning, twice daily rinsing was supervised throughout the week. Unsupervised rinsing took place after a period of 1 week in the hostel rooms after the examiner was satisfied that the participants had understood the procedure of rinsing and followed it effectively.
Prior to the commencement of the study, the subjects were also informed not to eat or drink anything one hour prior to saliva collection. After the instructions were given, the participants were made to swish with 10 ml of assigned test products for 1 min followed by saliva collection and baseline plaque and gingival indices were recorded. Plaque and gingival scores were recorded. Then, the patients were asked to follow the given instructions regarding the oral hygiene practices and rinsing procedure. Plaque and gingivitis scores were then recorded at 15 days, 1 month and 3 months from baseline. All the subjects were provided with 140 ml of test mouth rinse for a period of 1 week which had to be refilled on every Mondays during the course of study. A beaker was also provided to measure 10 ml of the rinse.
Statistical analysis
Statistical analysis was performed using SPSS 19 (SPSS Inc., Chicago, IL, USA) software. Repeated measure ANOVA was used to compare the effectiveness of the mouth rinses between the 4 groups at baseline, 15 days, 1 month, 3 months. Post hoc Bonferroni test was used for difference between the mouth rinses. One-way ANOVA was used for the comparison between the groups. Level of significance was set at p < 0.05.
Results
At low concentration (5 μg/ml) hot ethanolic propolis extract (8.25 ± 1.20) showed significant (p < 0.05) greater mean zone of inhibition than cold ethanolic propolis extract (7.78 ± 1.15) against S. mutans. L. acidophilus showed a greater mean zone of inhibition at all concentrations compared to other extracts. Distilled water propolis extract was found to be ineffective at all concentrations against S. mutans. However, it was effective against L. acidophilus. Mean zone of inhibition of all extracts increased (in mm) as the concentration of the extract increased (Table 1).
MIC values of all extracts against test pathogens mostly ranged between 4 and 5 μg/ml as shown in Table 2. No significant difference was observed at baseline among the different groups in mean salivary colony counts of L. acidophilus and S. mutans (p > 0.05). At the end of 5 min and 1 h intervals the mean L. acidophilus and S. mutans colony count decreased significantly within the groups. However, water group showed no significant difference (Table 3 and Table 4).
NNo significant difference was observed on comparing the mean plaque scores at baseline (p = 0.60). At the end of 15 days, 1 month and 3 months intervals the mean plaque score decreased significantly in groups (Table 5). However, there was no significant difference observed in the mean plaque scores of water group throughout the study. No significant difference in the mean plaque score was observed among the test groups and positive control group at the end of different time interval respectively.
Table 6 illustrates the mean gingival scores between the mouth rinses. At baseline there was no-significant difference in the mean gingival scores of all the groups (p = 0.60). A statistically significant reduction in mean gingival score within the groups at different intervals was seen among the three groups (Hot ethanolic propolis, Cold ethanolic propolis and Chlorhexidine groups). No significant difference in the mean gingival score was observed between the test groups and positive control. The water group showed no change at time intervals.
Discussion
The microbial composition can vary depending on the different sites of the tooth surface. These microorganisms interact with each other in a dynamic and concerted polymicrobial synergy to form a cariogenic biofilm (that is, a biofilm that can cause caries) within which the community changes as caries progress from early onset (initial demineralization) to deeper lesions with dentin exposure. Biofilm control to prevent diseases is not based on killing the bacteria; instead, the biofilm control is related to maintain the symbiosis (or equilibrium), and factors that modulate the environment (e.g., diet and saliva) are crucial [13].
The realization that bacteria can be reduced by preventing plaque formation has been a hallmark for preventive dentistry in reducing caries and periodontal problems. Despite hygiene products and mechanical procedure such as tooth brushing and inter-dental aids, clinical experts and population-based studies have demonstrated that such methods are not being employed sufficiently [10]. Chemical aids compared to mechanical plaque control are considered to be less technique sensitive alternatives [14]. Propolis is a well-known resinous material collected by bees; more than 300 components have been identified in propolis, revealing that its composition is dependent upon the plant source and local flora [15].
Antimicrobial efficacy is usually determined by examining MIC and MBC [16]. In the present study, the cultural method used was well diffused agar method which offered added advantage for selective quantification of microorganisms [16]. MIC of propolis extracts against S. mutans and L. acidophilus was around 4.5 to 5 μg/ml. Although several investigations have evaluated antimicrobial properties of propolis, it is very demanding to compare the results due to various methods used [17]. According to Bankova, et al. [18], the chemical composition of propolis is complex; it depends on the flora in the areas where it is collected from. It is generally recognized that gram-positive bacteria are more susceptible to the antibacterial action of propolis than gram-negative bacteria [17, 19, 20].
It is well documented that the bacteria of the genera Streptococcus, Lactobacillus are normal flora of the mouth and can as well cause dental caries [21]. In the present study, there was a major drop in the colony count of L. acidophilus from baseline to 5 min and 5 min to 1 h which was seen in the three groups but no shift in the colony count was seen in the water group.
According to Silva et al. [22] and Trusheva et al. [23] the antimicrobial activity of propolis towards S. mutans is accounted to the high content of flavonoids present.
Plaque scores
In our study, no statistically significant difference was found between test rinse and positive control at different intervals; it implies that test mouth rinse was effective in inhibiting plaque formation as Chlorhexidine. Similar results were obtained by Anauate-Netto et al. [24] in which propolis mouth rinse showed similar efficacy in inhibiting plaque as compared to Chlorhexidine. In contrast Ozan et al. [25] used propolis mouth rinse and found it to be less effective on antiplaque activity compared to Chlorhexidine. In another study Hidaka et al. [20] showed that propolis reduced the rate of amorphous calcium phosphate transformation into hydroxyapatite and concluded that it had a potential as an anticalculus and antiplaque agent in toothpastes and mouthwashes.
Gingival scores
Hot ethanolic propolis mouth rinse and Cold ethanolic propolis mouth rinse were equally effective in inhibiting gingival inflammation as compared to Chlorhexidine. The results of the present study clearly demonstrate that exemplified propolis rinse was effective in reducing gingival inflammation. The reason why propolis extract could produce a pro gingival effect could be that propolis flavonoids possess direct anti-oxidant properties, such as radical scavenging ability and indirect anti-oxidant effects such as induction of endogenous anti-oxidant enzymes. Flavonoids are familiar plant compounds which have antimicrobial, antioxidant and anti-inflammatory proprieties [26]. Hayacibara et al. [27] found that the insoluble glycan synthesis and glucosyltransferase activity were inhibited by multiple action of propolis.
Limitations
A crossover design should have been implicated over a concurrent parallel randomized controlled clinical trial. A follow-up after the intervention would have helped to know the duration of sustainability of the mouth rinses in the oral cavity for its effectiveness after the intervention was over. However, no allergic reactions or burning sensation were reported by any of the study participants.
Conclusions
Our data showed that propolis mouth rinse was as effective as Chlorhexidine on plaque accumulation and gingival inflammation and dental caries pathogens. A few more herbal products should be tested alongside with propolis to know their efficacy.
References
George J, Hegde S, Rajesh KS, Kumar A (2009) The efficacy of a herbal-based toothpaste in the control of plaque and gingivitis: a clinico-biochemical study. Indian J Dent Res 20(4):480–482
Pannuti CM, Mattos JP, Ranoya PN, Jesus AM, Lotufo RF, Romito GA (2003) Clinical effect of a herbal dentifrice on the control of plaque and gingivitis: a double-blind study. Pesqui Odontol Bras 17(4):314–318
Park YK, Alencar SM, Aguiar (2002) Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem 50(9):2502–2506
Paroliya A, Thomas MS, Kundabala M, Mohan M (2010) Propolis and its potential uses in oral health. Int J Med Med Sci 2(7):210–215
Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH (2002) Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother 46(5):1302–1309
Ophori EA, Eriagbonye BN, Ugbodaga P (2010) Antimicrobial activity of propolis against Streptococcus mutans. Afr J Bio 9(31):4966–4969
Bascones A, Morante S, Mateos L, Mata M, Poblet J (2005) Influence of additional active ingredients on the effectiveness of non-alcoholic chlorhexidine mouthwashes: a randomized controlled trial. J Periodontol 76(9):1469–1475
Nazeri R, Ghaiour M, Abbasi S (2019) Evaluation of antibacterial effect of propolis and its application in mouthwash production. Front Dent 16(1):1–12
Daugsch A, Moraes CS, Fort P, Park YK (2008) Brazilian red propolis—chemical composition and botanical origin. Evid Based Complement Alternat Med 5(4):435–441
Ozan F, Sümer Z, Polat ZA, Er K, Ozan U, Deger O (2007) Effect of mouthrinse containing propolis on oral microorganisms and human gingival fibroblasts. Eur J Dent 1(4):195–201
Löe H, Silness P (1963) Periodontal disease in pregnancy I. Acta Odontol Scand 21:533–551
Silness P, Löe H (1964) Periodontal disease in pregnancy II. Acta Odontol Scand 22:121–126
Bowen WH, Burne RA, Wu H, Koo H (2018) Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol 26(3):229–242
Drago L, Mombelli B, De Vecchi E, Fassina MC, Tocalli L, Gismondo MR (2000) In vitro antimicrobial activity of propolis dry extract. J Chemother 12(5):390–395
Sforcin JM (2016) Biological properties and therapeutic applications of propolis. Phytother Res 30(6):894–905
Wagh VD, Borkar RD (2012) Indian propolis: a potential natural antimicrobial and antifungal agent. Int J Pharm Pharm Sci 4(4):12–17
Sforcin JM, Fernandes A Jr, Lopes CA, Bankova V, Funari SR (2000) Seasonal effect on Brazilian propolis antibacterial activity. J Ethnopharmacol 73(1-2):243–249
Bankova VS, de Castro SL, Marcucci MC (2000) Propolis: recent advances in chemistry and plant origin. Apidologie 31(1):3–15
Ncube NS, Afolayan AJ, Okoh AI (2008) Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr J Biotechnol 7(12):1797–1806
Hidaka S, Okamoto Y, Ishiyama K, Hashimoto K (2008) Inhibition of the formation of oral calcium phosphate precipitates: the possible effects of certain honeybee products. J Periodontal Res 43(4):450–458
Ansorge S, Reinhold D, Lendeckel U (2003) Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-beta1 production of human immune cells. Z Naturforsch C J Biosci 58(7-8):580–589
Silva BB, Rosalen PL, Cury JA, Ikegaki M, Souza VC, Esteves A, Alencar SM (2008) Chemical composition and botanical origin of red propolis, a new type of Brazilian propolis. Evid Based Complement Alternat Med 5(3):313–316
Trusheva B, Trunkova D, Bankova V (2007) Different extraction methods of biologically active components from propolis: a preliminary study. Chem Cent J 1:13
Anauate-Netto C, Anido-Anido A, Leegoy HR, Matsumoto R, Alonso RC, Marcucci MC, Paulino N, Bretz WA (2014) Randomized, double-blind, placebocontrolled clinical trial on the effects of propolis and chlorhexidine mouthrinses on gingivitis. Braz Dent Sci 17(1):11–15
Ozan F, Polat ZA, Er K, Ozan U, Deger O (2007) Effect of propolis on survival of periodontal ligament cells: new storage media for avulsed teeth. J Endod 33(5):570–573
Ozaki F, Pannuti CM, Imbronito AV, Pessotti W, Saraiva L, de Freitas NM, Ferrari G, Cabral VN (2006) Efficacy of a herbal toothpaste on patients with established gingivitis — a randomized controlled trial. Braz Oral Res 20(2):172–177
Hayacibara MF, Koo H, Rosalen PL, Duarte S, Franco EM, Bowen WH, Ikegaki M, Cury JA (2005) In vitro and in vivo effects of isolated fractions of Brazilian propolis on caries development. J Ethnopharmacol 101(1-3):110–111
Acknowledgements
The authors would like to thank the study participants and their parents for their participation and kind cooperation throughout the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author SB declares that he has no conflict of interest. Author RN declares that he has no conflict of interest. Author GR declares that she has no conflict of interest. Author KB declares that she has no conflict of interest.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bapat, S., Nagarajappa, R., Ramesh, G. et al. Effect of propolis mouth rinse on oral microorganisms — a randomized controlled trial. Clin Oral Invest 25, 6139–6146 (2021). https://doi.org/10.1007/s00784-021-03913-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-03913-9