Abstract
Objectives
The objective of this study is to evaluate the effects of treatment modalities on titanium surface characteristics and surrounding tissues.
Materials and methods
Eighteen participants each had four titanium healing caps (HC) attached to four newly inserted implants. After healing, each HC was randomly assigned to either (1) titanium curettes (TC), (2) stainless steel ultrasonic tip (PS), (3) erythritol air-polishing powder (EP), or (4) only rubber cup polishing (CON). Probing depths (PD), bleeding on probing (BOP), matrix metalloproteinase 8 (MMP-8), and periopathogens were recorded before and 3 months following instrumentation. After final assessments, HCs were removed, cleaned, and subjected to (a) bacterial colonization (Streptococcus gordonii, 24 h; mixed culture, 24 h) and (b) gingival fibroblasts (5 days). HC surfaces were analyzed with a scanning electron microscope (SEM).
Results
No significant differences between the groups were evident before or after instrumentation for PD and BOP (except TC showed a significant decrease in PD; p = 0.049). MMP-8 levels and bacterial loads were always very low. MMP-8 decreased further after instrumentation, while bacteria levels showed no change. No significant differences (p > 0.05) were evident in bacterial colonization or fibroblast attachment. A comparison of the overall mean SEM surface roughness scores showed a significant difference between all groups (p < 0.0001) with the lowest roughness after EP.
Conclusions
All treatments performed yielded comparable outcomes and may be implemented safely.
Clinical relevance
Clinicians may fear implant surface damage, but all instrumentation types are safe and non-damaging. They can be implemented as needed upon considering the presence of staining and soft and hard deposits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As more and more dental implants are being used to replace missing teeth, peri-implant diseases are increasing [1], which represent a current and future challenge for dentists as well as patients. Similar to natural teeth, there might be a smooth transition from healthy peri-implant tissues to mucositis and then to peri-implantitis. Since periodontal diseases (gingivitis and periodontitis) and peri-implant diseases (mucositis and peri-implantitis) are biofilm-derived infections, it is increasingly important to evaluate teeth as well as implants at regular intervals and to effectively treat sites that show signs of inflammation and infection. Therapy through biofilm management can improve the long-term functionality of dental implants [2, 3].
Various types of instruments have been investigated regarding their effects on implant surfaces. In general, it was concluded that the regular use of cleaning instruments during maintenance therapy can affect the integrity of the titanium surface [4, 5]. Thus, if implant surfaces become rougher due to repeated instrumentation, they allow easier and faster bacterial attachment and plaque retention [6,7,8]. While practitioners fear roughening through instrumentation and consequently enhanced biofilm formation, it should be considered that the surface condition is also a critical factor for the attachment of fibroblasts, among other cells, in order to establish a biological soft tissue seal around the implant [9, 10]. A surface that is too smooth may weaken the soft tissue attachment to the implant surface as indicated by Bollen et al. [9]. The authors recommend a good balance between bacterial adhesion and soft tissue sealing and conclude an ideal surface roughness of R a = 0.2 μm [9]. While this is a value coming from brand new implants or abutments, there is a need to evaluate both the effect of instrumentation on bacterial adhesion and the attachment of fibroblasts. Up until now, only few studies have investigated bacterial adhesion or fibroblast attachment on different implant surfaces (one in vitro co-culture study by Zhao et al. [11], one in vitro study by Guillem-Marti et al. [12]). However, neither study investigated any type of instrumentation on the implant surface.
When considering instrumentation modalities, it has to be differentiated between approaches in mucositis and peri-implantitis therapy [13, 14]. Similarly, recommendations for regular biofilm management during maintenance therapy, comparable to a prophylactic professional tooth cleaning, must be considered. In maintenance therapy, which is repeated up to four times per year, the focus should be set on “as little as possible, as much as necessary.”
In light of the increasing number of inserted implants and the need for prophylactic measurements to avoid peri-implant diseases, the establishment of a clear therapeutic concept for optimal biofilm removal during regular re-care appointments is essential to maintain peri-implant tissue health and long-term implant success.
Thus, the aim of this study was to investigate the peri-implant tissues before and after instrumenting titanium healing caps (HCs) using various treatment modalities through assessment of intraoral parameters and the effects of these different treatment modalities on the HC surfaces through several laboratory procedures.
The primary null hypothesis was that any treatment modality will cause no difference (within or between groups) 3 months after instrumentation in peri-implant probing depths (PD). Secondary null hypotheses included (part 1) any treatment modality will cause no difference (within or between groups) 3 months after instrumentation in (1) implant BOP percentages, (2) implant MMP-8 levels, and (3) implant bacterial load; and (part 2) there will be no difference between any treatment modality with respect to the amount of (4) biofilm formation and (5) fibroblast attachment to the implant surface as well as (6) surface roughness.
Materials and methods
This was a monocentric, prospective, randomized, clinical, controlled study to evaluate the effects of different types of instruments on specially constructed titanium HCs before and 3 months after instrumentation with respect to clinical and laboratory parameters.
The protocol of the study was reviewed and approved by the Medical Ethics Committee, Philipps-University Marburg in Germany (no. 159/12). Once approval was received, the study was conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and Good Clinical Practice guidelines as well as the Declaration of Helsinki.
Sample size estimate and power calculation
To the authors’ knowledge, the reported study was the first of its type, and therefore, a power calculation based upon mean (± SD) outcomes from pilot work was not possible for instrumentation outcomes. Since the study was peer reviewed for a grant, a large group of experts recommended a sample size of 20 subjects corresponding to 80 implants. With regard to possible dropouts, a sample size of n = 22 (88 implants) was determined and sequentially recruited.
Patient recruitment and study population
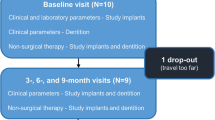
Study participants were recruited to the Department of Periodontology at Philipps-University, Marburg, Germany, and included patients from the Philipps-University Dental Clinic as well as some private dental practices in Germany. Twenty-nine potential participants took part in an initial screening visit to assess their eligibility and their need of and interest in four titanium dental implants after being informed about all treatment options. In addition to needing four dental implants, patients were required to be older than 18 years of age, in good health, have no allergies to the products used in the study, be non-smokers (less than five cigarettes per day), have been clarified of the clinical study, and have received the participant information and signed an informed consent form. The exclusion criteria were as follows: diabetes mellitus, smoker (more than five cigarettes per day), untreated periodontal disease, systemic antibiotic therapy within the last 6 months, or pregnancy. Finally, 22 participants were included and informed about the necessary study visits. The study flow is presented in Fig. 1.
Pre-operative appointment and implant insertion
Microbial analysis was completed at the first examination to ensure a good periodontal condition prior to implant insertion. A commercial bacteria test (qPCR; Bioscientia, Berlin, Germany) was applied to detect 11 periodontal pathogens (Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Treponema denticola, Parvimonas micra, Fusobacterium sp., Campylobacter rectus, Eubacterium nodatum, Eikenella corrodens, and Capnocytophaga gingivalis) by inserting four paper points into the sulcus of four periodontally compromised teeth for 20 s and then sending the paper points in a plastic tube to an external laboratory (Bioscientia, Berlin, Germany) for a pooled sample analysis.
Each patient was treated with four dental implants (3.8 or 4.3 mm diameter, Screw-Line Promote®, Camlog, Wimsheim, Germany). Eleven of the included participants required dental bone regeneration (Geistlich Bio-Gide®/Geistlich Bio-Oss®, Geistlich Biomaterials, Baden-Baden, Germany) and one participant required soft tissue regeneration (Geistlich Mucograft®, Geistlich Biomaterials, Baden-Baden, Germany) in the area of implant insertion. Of the 22 participants, 13 underwent subgingival (covered) healing while 9 underwent transgingival (open) healing. Three participants each had one implant that did not osseointegrate, and thus, required a second implantation, all of which proved to be successful.
To mimic the later clinical implant neck, cylindrical HCs (Camlog, Wimsheim, Germany) were specially constructed of the same grade 4 titanium as implants (i.e., pure titanium with machined surface; R a = 0.2 μm) with height 3 or 4 mm and were attached to the implants for healing for a minimum of 5 months. For the cases undergoing covered healing (n = 13), HCs were attached to the implants after a 12-week healing period and uncovering of the implant. Directly following insertion of the implants and HCs, patients received instruction to rinse with 10 ml of 0.2% chlorhexidine mouthrinse (CHX) every morning and evening for 14 days, a routine procedure following implant placement. After these 2 weeks, the gingival crevicular fluid (GCF) in each implant sulcus was tested for matrix metalloproteinase-8 (MMP-8) levels at two sites per implant. This was done using special GCF collection strips (dentognostics GmbH, Jena, Germany) that were inserted for 30 s into the sulcus and sent in plastic tubes to an external laboratory (Bioscientia, Berlin, Germany) for site-specific analysis. Sutures were then removed and patients received instruction to use a soft manual toothbrush around the HCs during their oral hygiene home care.
Part 1. Clinical evaluation of parameters before and after instrumentation
Measurement of clinical indices and collection of immunological and bacterial parameters
At baseline (2 months after implant insertion) and 3 months later (5 months after insertion of implants and HCs), one sample each of MMP-8 and a microbial diagnostic of 11 periodontal pathogens (both Bioscientia, Berlin, Germany), as described earlier, were taken for each implant. The GCF strips (for the MMP-8 test) and paper points (for the bacteria test) were inserted into approximal areas on opposite sides of the implant so that no site was sampled twice. The same sampling sites were used for each test at both time points.
Peri-implant PDs (mm) and BOP (%) were noted at six sites per implant (mesiobuccal, buccal and distobuccal, mesiolingual, lingual and distolingual) using a 0.2-N pressure-sensitive probe (DB764R UNC 15, Aesculap, Tuttlingen, Germany). BOP was recorded as being positive if 30 s after probing bleeding was evident and negative if bleeding was absent within 30 s of probing. All clinical measurements were made by the same calibrated and experienced examiner who was not aware of the type of treatment rendered.
Instrumentation of healing caps
After baseline measurements, participants attended a regular maintenance appointment that included instrumentation of each HC separately with a different treatment:
-
(1)
Titanium curettes (Ergoplant universal curettes, Aesculap, Tuttlingen, Germany); TC
-
(2)
Stainless steel ultrasonic tip (Instrument PS, EMS, Munich, Germany) using an ultrasonic device (Air Flow® Master Piezon, EMS); PS
-
(3)
Air-Flow® Plus (EMS) erythritol powder using an air-polishing device (Air Flow® Master Piezon, EMS); EP
-
(4)
No instrumentation except for polishing with a rubber cup (Prophy Cup, KerrHawe SA, Bioggio, Switzerland) and low Relative Dentin Abrasion (RDA)-level prophylaxis paste (Proxyt RDA 7, Ivoclar Vivadent, Schaan, Liechtenstein); CON
In order to randomize instrumentation, HCs were numbered clockwise (beginning in the first quadrant) from 1 to 4 per patient, so that allocation of the treatment could follow a randomization scheme prior to patient recruitment.
Each instrument in groups (1)–(3) was used to treat each HC for 2 min according to the manufacturer’s instructions and subsequently rinsed with 5 ml 0.2% CHX (Chlorhexamed forte, GSK, Bühl, Germany). In group (4), each HC was subsequently rinsed with 5 ml 0.9% sodium chloride (NaCl) solution instead of CHX.
Three months following instrumentation (i.e., 5 months following HC insertion), the same parameters (PD, BOP, MMP-8, and bacteria test) were assessed again.
After clinical assessment, the titanium HCs were carefully removed for further laboratory investigation and analysis (part 2 of the study) and replaced with conventional HCs until attachment of the prosthodontic superstructure.
Part 2. Laboratory evaluation of instrumented healing caps
Each HC underwent various laboratory steps (Fig. 2) to analyze bacterial colonization and attachment of fibroblasts to the altered titanium surface. Surface roughness was investigated under a SEM.
Biofilm formation on instrumented healing caps
Bacterial colonization followed a similar, but slightly deviated procedure described in Schmidt et al. [15]. Prior to and in between each colonization period, HCs were cleaned with 70% alcohol and autoclaved to ensure sterility prior to the next incubation cycle. HCs underwent two different cycles of bacterial colonization for 24 h, with Streptococcus gordonii (ATCC 10558) and with a mixed anaerobic culture containing S. gordonii (ATCC 10558), Actinomyces naeslundii (ATCC 12104), Fusobacterium nucleatum (ATCC 25586), P. gingivalis (ATCC 33277), and T. forsythia (ATCC 43037). Bacterial strains were pre-cultured 2 to 3 days (S. gordonii 1 day) on Tryptic Soy Agar (TSA) plates (BioMerieux, Marcy l’Etoile, France) (plus 5% sheep blood for the mixed culture) and incubated at 37 °C in 5% CO2 and anaerobically, respectively. Bacterial suspensions (20 ml for the single strain experiment and 5 ml of each of the mixed culture bacteria) were made for each of the bacterial strains in dH2O with a turbidity of 3 to 4 following McFarland’s standard. Taken from their corresponding 5 ml suspension mixture were 1500 μl S. gordonii, 2250 μl A. naeslundii, and 4500 μl of the three other bacterial strains. Fifteen milliliters of this newly created mixed bacterial suspension was then combined with 15 ml of doubled-concentrated brain heart infusion medium (containing 750 μl of blood) (Oxoid, Thermo Scientific, UK). Each 100 μl/well of that bacterial suspension was given into 96-well plates. HCs were submerged in the suspension and incubated at 37 °C for 24 h for both S. gordonii and the anaerobic mixture at the respective atmospheric conditions. Following the incubation period, HCs were carefully removed, dipped shortly into 0.9% NaCl solution, and transferred to tubes containing 500 μl of 0.9% w/v NaCl. After extensive vortexing and a 10-min exposure to ultrasonication, aliquots were taken, diluted, and spread on TSA containing 8% sheep blood. Following a 24-h incubation period (anaerobic mixture 5 days), the number of colony-forming units (CFUs) was counted. Each cycle of bacterial colonization (single culture and mixed culture) was completed twice.
Attachment of fibroblasts to instrumented healing caps
The protocol by Eick et al. [16] was followed for the adhesion of human periodontal ligament fibroblasts to each of the HCs. Informed and signed consent was received from two patients from whom fibroblasts were harvested (the fibroblasts of one donor were used for the first ten HCs while the fibroblasts from the second donor were used for the remaining HCs). In short, autoclaved HCs were dipped into collagen R solution (SERVA, Heidelberg, Germany) for 10 min. After a drying period (1 h), the HCs were transferred to a suspension of fibroblasts in the third passage (20,000 cells/ml) containing Dulbecco’s modified Eagle medium (Life Technologies/Invitrogen, Paisley, UK) and 10% fetal calf serum (Life Technologies/Invitrogen) in 96-well plates. The fibroblasts were given a period of 5 days at 37 °C in a 5% CO2 atmosphere to attach to the HCs. Attached fibroblasts were fixed and stained with 4′,6-diamidine-2′-phenylindole dihydrochloride or DAPI (Roche Diagnostics GmbH, Mannheim, Germany) and then counted under a fluorescent microscope (Olympus BX51, Tokyo, Japan).
Scanning electron microscopy analysis and visual assessment
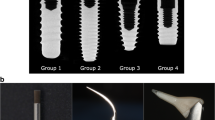
Surface changes of the HCs were observed under a SEM (Phenom-World, Eindhoven, Netherlands). HCs were first carefully washed in a soap solution (Zack Spülmittel, August Wencke OHG, Bremen, Germany), rinsed with water, and air-dried prior to looking at each surface of the HC under an optical microscope (Leica MS5, Wetzlar, Germany) at magnification ×160 to detect the areas of the HCs with the most surface change (if any). This ensured that the intended surface that was to be examined was correctly placed face up. The stub with the HC was carefully positioned on the microscope’s sample holder and placed in the SEM, where four photographs of the surface were randomly taken at magnification ×500 and ×1000 for each HC in order to analyze and compare the surfaces between the four treatment modalities (Fig. 3). Images at ×1000 magnification were used for the following image analysis. The images of the HCs per study participant were blindly assessed independently by four examiners (KS, TA, NA, and SE), which means that none of the examiners knew the treatment group except for the images of the CON HCs. The examiners objectively rated the surface alterations of the HCs by comparing the four images of each of the instrumented groups of one participant to the four images of the CON of that same participant using a categorical rating scale described by Bain [17], which was also used in a previous in vitro study [15]. The process of scoring each of the SEM images was completed twice by each examiner, leaving a period of 2 weeks between scoring. The scores for evaluating the surface roughness are as follows: 1 = smoother (less rough) than the control, 2 = the same as control, 3 = rougher than the control, and 4 = much rougher than the control [17].
Statistical analysis
All data were collected in pseudonymous form and statistical analyses were performed blindly by a statistician (HC) using PASW 21.0 (IBM SPSS Statistics, Chicago, IL, USA).
After decoding the data, the mean values and standard deviations of PDs (mm), BOP (%), and MMP-8 (ng) were calculated for each instrumentation group. The results of the bacteria test from the laboratory were graded from < 104, = 104, < 105, < 106, > 106, and > 107 and converted to a ranking system from 0, 1, 2, 3, 4, and 5, respectively, for all of the bacteria except for A. actinomycetemcomitans, which had a lower reference area and was therefore ranked as follows: 0:< 103, 1:< 104, 2:< 105, 3:< 106, 4:> 106, and 5:> 107). The primary outcome was PD after 3 months.
A within-group analysis between the two time points (i.e., before and 3 months after instrumentation) was completed using a paired t test while a between-group analysis (i.e., comparison between the different instrument types) was completed for each parameter using linear mixed models with the groups as fixed effects and subject specific random effects to adjust for possible within patient correlations.
In the laboratory part of this study, mean log10 values of the CFUs and standard deviations were calculated for both cycles of each treatment group and bacterial colonization mixture (i.e., single and mixed cultures). The overall mean and standard deviation was calculated with respect to data for the number of fibroblasts attached to the surface as well as the two scoring periods for the surface roughness. A between-group analysis for bacterial colonization was completed using pairwise comparisons of linear mixed models with instrument group as fixed effects (and again subject-specific random effects). For the fibroblast attachment, only a between-group analysis (pairwise comparisons based on a linear mixed model) was performed as only one attachment cycle was conducted. For the SEM scoring, a within-group analysis between the 2-week scoring periods was completed using a paired t test while a between-group analysis was completed using ANOVA. The CON group was set as 2.0 according to the classification by Bain [17]. Additionally, intra- and inter-rater agreement for the SEM image analysis was evaluated using a repeatability measure based on the two replications of each examiner and the kappa statistics as inter-rater agreement measures, respectively (kappa- and gamma-analysis).
For all analyses, adjustment for pairwise comparisons among the means was completed using the Bonferroni correction method and a difference was considered significant at a confidence interval of 95% (α = 0.05).
Results
Of the 22 participants recruited, four had to be excluded part way through the study due to either receiving their prosthodontic superstructures prior to the end of the study, were smoking heavily (both violation of the protocol), or had implants that failed. These incidents were discovered while the study was in progress. Thus, a total of 18 patients (13 female and 5 male) with a mean age of 51.9 ± 14.9 years (range 22 to 73 years) could be included in the analysis corresponding to 72 HCs. None of the participants showed any side effects during or following the study. However, one case demonstrated the need for systemic antibiotic administration due to increased levels of A. actinomycetemcomitans (> 106+ or a grade of 4) discovered during the pooled sample analysis of four periodontally compromised teeth at the outset of the study. The elimination of A. actinomycetemcomitans was confirmed by a further microbiological analysis before implantation.
The baseline characteristics (Table 1) 2 months after implantation and prior to instrumentation show only patients exhibiting no or minimal signs of periodontal inflammation.
Part 1. Clinical evaluation of parameters before and after instrumentation
Results of the clinical evaluation are presented in Table 2. Comparing both time points (baseline and 3 months after instrumentation), no significant differences were evident after instrumentation for any of the clinical parameters PD and BOP (p > 0.05) except for TC, which improved PD slightly but statistically significant from 2.82 ± 0.67 mm to 2.56 ± 0.77 mm (p = 0.049). Between the different treatment groups, no significant differences could be found (p > 0.05).
MMP-8 levels (ng) were significantly reduced in all groups before and after instrumentation except those for EP (p = 0.793), which had already had a very low level at baseline (11.11 ± 16.49 ng). Interestingly, CON significantly reduced the MMP-8 level from 19.83 ± 26.50 ng to 6.00 ± 7.40 ng (p = 0.022). Regarding the groups, no significant differences were found at baseline or after 3 months.
Bacterial load was very low at baseline (0 or far under 103 for A. actinomycetemcomitans and 104 for the other strains) and did not change after instrumentation except for EP, where the ten bacterial strains were significantly reduced (p = 0.001). This is a result of the EP group having a reduction in the number of participants with signs of any of the ten periopathogens from baseline to 3 months after instrumentation (from 17 to 12 participants). The other groups remained similar before and after baseline with respect to the presence of the ten bacteria. For A. actinomycetemcomitans, most cases showed no signs of the bacterium at either baseline or 3 months later (and if so, levels were no higher than a grade of 1, except for one case in the EP group, which had a grade of 4 at 3 months after instrumentation). No significant differences were evident between the treatment groups or the two time points (p > 0.05) except for between groups when comparing the baseline means of A. actinomycetemcomitans (p = 0.025) and when comparing the difference between the means for the ten bacterial strains (p = 0.023). Specifically, a significant difference was found between the difference in the means of the two time points for EP and CON (p = 0.015).
Part 2. Laboratory evaluation of instrumented healing caps
After bacterial colonization on the 72 HCs, no significant differences in the mean log10CFUs were evident between any of the four groups for neither the single bacterium, S. gordonii (p = 0.362), nor the mixed anaerobic culture (p = 0.357) (Table 3).
Concerning attachment of fibroblasts, the four groups revealed no significant difference after 5 days of incubation (p = 0.417) (Table 3).
Scoring of the SEM photographs (two scoring periods and overall mean of the two scoring periods) for surface roughness is presented in Table 4. Kappa test was conducted for repeatability of the two time points and concerning intra-rater agreement. It revealed very little variation between the examiners and the two time points (0.42–0.88 and 0.84–0.95). Between the two scoring periods, no significant difference was evident (p > 0.05). The overall mean of the two scoring periods showed that PS was ranked the roughest (3.51 ± 0.33), TC was slightly rougher (2.49 ± 0.53), and EP (1.87 ± 0.40) was similar to that of CON (2.0). PS and TC were significantly different from CON (p < 0.001, p = 0.001), while EP was not (p = 0.188). Comparing the instrumentation groups (TC, PS, EP) with one another, it has been shown that all differed significantly from each other (p < 0.001).
It should be noted that all examiners reported difficulties with classifying some photographs into the grades by Bain [17]. The SEM images of the four treatment modalities each began with a standardized R a = 0.2 μm and is portrayed in the CON image (Fig. 3). The machined (smoothed) surface of the non-instrumented HCs is generally characterized by parallel grooves. On the one hand, distinctly altered surfaces that for the most part “erased” the original grooves of the machined surface were seen in PS and TC images, but instead created other scratches and streaks compared to the already existing parallel grooves in the CON. On the other hand, EP showed a deposit on the machined surface in some photographs and was scored as a smoother surface compared to CON. Furthermore, examiners saw small, isolated scratches and artifacts, but could not consider these as a general change in surface.
The primary null hypothesis that any treatment modality will cause no difference in PD within and between groups 3 months after instrumentation can be confirmed (p > 0.05) with TC being the only exception (baseline to 3 months, p = 0.049).
Concerning the secondary null hypotheses, BOP, biofilm formation, and fibroblast attachment showed no differences within and/or between groups (p > 0.05) and can be confirmed. The other secondary null hypotheses (MMP-8, bacterial load, surface roughness) have to be rejected.
Discussion
To the authors’ knowledge, this is the first clinical study that evaluated different preventive cleaning modalities on implants in vivo and their surface characteristics including bacterial and fibroblast adhesion.
Since previous studies have revealed similarities between experimental peri-implant mucositis and gingivitis in terms of microbiological and clinical parameters [18, 19], proposed therapies for implant diseases are currently based on the treatment of periodontal disease [2, 13, 20]. However, the surface anatomy of an implant is more complex with its threaded design compared to natural teeth [2, 20], suggesting that instrumentation options may not be easily transferred from teeth to implants. Any type of prophylactic instrumentation can alter physical and chemical properties of the implant surface [21] and influence an implant’s biocompatibility [5]. Therefore, clinicians need to have options to safely and effectively remove biofilm from implants to prevent the onset and progression of peri-implant diseases, especially when applied repeatedly (e.g., in maintenance therapy).
A healthy and stable periodontal condition is key to minimizing the risk of colonizing newly inserted dental implants with bacterial pathogens [20, 22] as some periodontal pockets could potentially harbor periopathogens and increase the potential for cross-infection to peri-implant sites [23,24,25]. This strengthens the importance of regular maintenance care appointments to support periodontal and peri-implant health [22].
Part 1. Clinical evaluation of parameters before and after instrumentation
Participants in the present study were periodontally stable prior to implant insertion as well as before instrumentation of the titanium HCs. This criterion may have helped avoid increased colonization of pathogens around the implants [20, 26].
Specially constructed HCs (made of pure titanium and with mean R a = 0.2 μm) were aimed to mimic the clinical situation as closely as possible, with the advantage that after removal from the oral cavity, they could be subjected to additional laboratory procedures allowing subsequent measurement of bacterial colonization, attachment of fibroblasts, and changes in surface roughness.
Moreover, the clinical part of the study determined the effects of instrumentation on the surrounding tissues. As seen from the results of the clinical parameters, PD and BOP did not show significant changes following the various treatment modalities except for TC, which showed a slight, but statistically significant reduction in PD. Although clinical attachment and gingival margin levels were not measured in this early healing phase, the decrease in PD may be the result of mucosal recession (possibly due to a more thorough instrumentation by TC) rather than a gain in clinical attachment. Nevertheless, all data correspond to relatively healthy findings (PD < 3 mm and BOP < 28%) and is noteworthy for the CON group, which showed similar results. There are only a few studies [20, 26, 27] that investigated these clinical parameters in conjunction with single mechanical therapy. These studies found improvements in clinical parameters and reduction of periodontal pathogens, which support positive outcomes of mechanical instrumentation modalities for the treatment of mucositis. The present data derived from this prospective study where inflamed sites were generally non-existent only allow the findings to be conditionally comparable with the other studies.
To follow up the presence of inflammation after implant and HC insertion, MMP-8 was assessed. Here, too, no studies were found with respect to the effects of specific instrument types on MMP-8 levels surrounding titanium implants. However, positive correlations have been observed between PD and MMP-8 [28, 29] and MMP-8 was found to correlate significantly with bleeding in periodontal studies [30]. Nevertheless, testing for MMP-8, a key biomarker for the presence of inflammation that is released in response to the presence of periopathogens, can demonstrate the state of peri-implant health and possibly predict disease progression [29,30,31]. In the present study, TC, PS, and CON resulted in a significant decrease in MMP-8. While EP showed no significant change after 3 months, a mean baseline value of 11 ng was already very low and nearly only half the value of the other groups. It should be kept in mind that values of < 8 ng around the teeth are defined as healthy and 8 to 20 ng indicate the presence of slight inflammation at the sampled site with no elevated risk of progression of periodontal tissue breakdown at the time the sample was taken. When evaluating MMP-8 levels on implants, it was demonstrated to be present in significantly greater amounts compared to teeth in response to a bacterial challenge [19]. Regarding the present results, it can be concluded that further inflammation was prevented in patients originally exhibiting healthy to slightly inflamed conditions (< 20 ng).
With respect to bacterial load, only EP showed a significant decrease in bacterial load of the ten bacteria. While this may suggest that EP more effectively removed biofilm, made the surface less rough, or left behind a residue which reduced biofilm formation, it has to be considered that bacterial grading in all groups ranged between 0 and 1, which corresponds to levels around 104 and only slightly above the detection level. EP consisting of an erythritol-chlorhexidine combination may have possibly contributed to an overall stronger and sustained anti-bacterial effect in vivo. The effect of using an air-polishing powder needs to be investigated further in a longer-term maintenance care study where treatment can be repeated up to every 3 months.
Few studies have investigated the presence of periopathogens in the implant’s sulcus before and after treatment. The use of various instruments (e.g., rubber cup polish and plastic scalers or teflon curettes and sodium carbonate air-polishing powder), with or without CHX, or following open flap surgery, resulted in a beneficial reduction or elimination of pathogenic bacteria [20, 26].
Part 2. Laboratory evaluation of instrumented healing caps
Further laboratory investigations were undertaken following careful removal of the titanium HCs to help explain clinical effects of the different treatment modalities.
Clinical plaque indices (assessment after or before brushing) are generally prone to error, due to the Hawthorne effect of patients being included in a clinical study as well as a plaque disclosing agent disturbing other clinical evaluations. Therefore, biofilm formation on the instrumented titanium surface was simulated using the same model as in a former in vitro study [15] since it is an indicator for surface roughness from a clinical perspective. This included a single colonization with S. gordonii, an early colonizer [32, 33], and a separate colonization with a mixed culture known to be associated with periodontal and peri-implant diseases. The results revealed similar log10CFUs among all four treatment modalities for both single and mixed cultures, and none proved to be statistically significant suggesting that all possible surface changes had no influence on biofilm formation. Overall results are comparable to those of a previous in vitro study [15].
Therefore, with respect to the claim that any instrument used for biofilm removal should not increase plaque retention [8] due to increased surface roughness [34], all instrumentation modalities examined in the present study (including mere polishing) can be recommended.
While most studies focused on bacterial adhesion only, the present study also included fibroblast attachment after instrumentation, since any alteration to the implant surface after therapy also has an effect on its biocompatibility [5, 12, 35, 36]. Already in 1996, Bollen et al. [9] pointed out that an optimal surface roughness (R a = 0.2 μm) may be of benefit to slow bacterial growth as well as to promote the soft tissue seal around the implant. It seems a fine balance must be found between the roughness and smoothness of the implant surface to discourage biofilm formation, but at the same time encourage attachment of fibroblasts, which will support the establishment of a tight tissue seal [9, 10, 12]. When fabricating the HCs, care was taken that they possessed an initial surface roughness of R a = 0.2 μm. Microgrooved surfaces have shown to promote early activation of fibroblasts, thus leading to a quicker formation of cells and a biological seal around the implant [12]. In the present study, regarding the number of fibroblasts that attached to the treated titanium surface, no significant differences were found between any of the groups, suggesting that the surface characteristics may not have been altered to an extent that make fibroblasts incompatible for attachment. Other studies have found that not only polishing powders but also some instruments may deposit themselves to the surface of the implant, which may impair adhesion of fibroblasts and osteoblasts [8, 35, 36].
Finally, SEM images of the titanium surfaces should detect smoothening or roughening following treatment. The images were scored using the method proposed by Bain [17] for the same reasons described in Schmidt et al. [15] where the method contained a score for surfaces appearing smoother than the control [17]. Since the primary focus was not only on evaluating titanium surfaces but on the clinical impact of changes, other more objective measures of surface roughness such as laser profilometry and atomic force microscopy were omitted. Although profilometry can measure profile height, depth, and deviations [4], our analysis of surface roughness using only a SEM scoring method is in line with a recent review of 34 studies [4], where two thirds used SEM only to evaluate surface roughness while the remaining one third of the studies quantified surface roughness using both profilometry and SEM.
When compared to CON images, PS proved to be most rough, followed by TC and lastly EP, which had a score showing a tendency towards being smoother than CON, but not reaching the level of significance. These results differ slightly from a similar in vitro study [15], which compared eight treatment groups to a control. EP and TC scored similar to the untreated control while PS scored smoother than the control. It should be noted that in the present study, CON was polished with a rubber cup and fine polishing paste, while in the other study, the control was not treated at all, which could result in differences.
This short-term study of 3 months provides an insight into various aspects including the effects of different instrumentation modalities on titanium surfaces. Taking into consideration the clinical and laboratory findings, the present study suggests that all four treatment modalities yielded comparable results with no negative outcomes. Therefore, any of the treatment modalities may be recommended to prevent or treat mild peri-implant mucositis.
The results are especially interesting for the control group, which, although it did not receive instrumentational treatment like the other three groups, did receive thorough polishing and saline rinsing. Thus, when dental health professionals are unsure of which prophylactic measures they can implement on an implant, they can or should at least polish and rinse the implant. This is advantageous over completely ignoring the implant in maintenance therapy; a common practice in fear of damaging the implant surface. Naturally, the choice of the instrument should consider the presence of plaque only or strong staining and calculus. Most importantly, the clinical study suggests that all instrumentation types are safe and non-damaging and can be implemented as needed. However, from the point of view of surface roughness, erythritol air-polishing powder did achieve a surface appearing smoother than the titanium curettes and ultrasonic, but as smooth as the control. Further investigation is needed regarding this finding.
While the present study evaluated single instrumentation, a succeeding study (follow-up appointments) evaluating the repeated application of the modalities on supraconstructions of the implants using the same clinical parameters is being conducted.
As recently discussed in detail [3], periodontal maintenance therapy and supporting implant therapy are necessary for long-term success. However, it should not be forgotten that alongside professional measurements, a patient’s daily home-care regimen also significantly influences the success [37].
References
Derks J, Tomasi C (2015) Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol 42(Suppl 16):S158–S171. https://doi.org/10.1111/jcpe.12334
Renvert S, Roos-Jansåker A-M, Claffey N (2008) Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol 35(8 Suppl):305–315. https://doi.org/10.1111/j.1600-051X.2008.01276.x
Armitage GC, Xenoudi P (2016) Post-treatment supportive care for the natural dentition and dental implants. Periodontol 71(1):164–184. https://doi.org/10.1111/prd.12122
Louropoulou A, Slot DE, Van der Weijden FA (2012) Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res 23(6):643–658. https://doi.org/10.1111/j.1600-0501.2011.02208.x
Louropoulou A, Slot DE, Van der Weijden FA (2015) Influence of mechanical instruments on the biocompatibility of titanium dental implants surfaces: a systematic review. Clin Oral Implants Res 26(7):841–850. https://doi.org/10.1111/clr.12365
Quirynen M, Bollen CM (1995) The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol 22(1):1–14
Teughels W, Van Assche N, Sliepen I, Quirynen M (2006) Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res 17(S2):68–81. https://doi.org/10.1111/j.1600-0501.2006.01353.x
Duarte PM, Reis AF, de Freitas PM, Ota-Tsuzuki C (2009) Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J Periodontol 80(11):1824–1832. https://doi.org/10.1902/jop.2009.090273
Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D (1996) The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res 7(3):201–211. https://doi.org/10.1034/j.1600-0501.1996.070302.x
Marín-Pareja N, Salvagni E, Guillem-Marti J, Aparicio C, Ginebra MP (2014) Collagen-functionalised titanium surfaces for biological sealing of dental implants: effect of immobilisation process on fibroblasts response. Colloids Surf B Biointerface 122:601–610. https://doi.org/10.1016/j.colsurfb.2014.07.038
Zhao B, van der Mei HC, Subbiahdoss G, de Vries J, Rustema-Abbing M, Kuijer R, Busscher HJ, Ren Y (2014) Soft tissue integration versus early biofilm formation on different dental implant materials. Dent Mater 30(7):716–727. https://doi.org/10.1016/j.dental.2014.04.001
Guillem-Marti J, Delgado L, Godoy-Gallardo M, Pegueroles M, Herrero M, Gil FJ (2013) Fibroblast adhesion and activation onto micro-machined titanium surfaces. Clin Oral Implants Res 24(7):770–780. https://doi.org/10.1111/j.1600-0501.2012.02451.x
Figuero E, Graziani F, Sanz I, Herrera D, Sanz M (2014) Management of peri-implant mucositis and peri-implantitis. Periodontol 66(1):255–273. https://doi.org/10.1111/prd.12049
Schwarz F, Schmucker A, Becker J (2015) Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: a systematic review and meta-analysis. Int J Implant Dent 1(1):22. https://doi.org/10.1186/s40729-015-0023-1
Schmidt KE, Auschill TM, Heumann C, Frankenberger R, Eick S, Sculean A, Arweiler NB (2017) Influence of different instrumentation modalities on the surface characteristics and biofilm formation on dental implant neck, in vitro. Clin Oral Implants Res 28(4):483–490. https://doi.org/10.1111/clr.12823
Eick S, Bender P, Flury S, Lussi A, Sculean A (2013) In vitro evaluation of surface roughness, adhesion of periodontal ligament fibroblasts, and Streptococcus gordonii following root instrumentation with Gracey curettes and subsequent polishing with diamond-coated curettes. Clin Oral Investig 17(2):397–404. https://doi.org/10.1007/s00784-012-0719-z
Bain CA (1998) An in vitro and in vivo evaluation of various implant-cleaning instruments. Quintessence Int 29(7):423–427
Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP (1994) Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res 5(4):254–259. https://doi.org/10.1034/j.1600-0501.1994.050409.x
Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP (2012) Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res 23(2):182–190. https://doi.org/10.1111/j.1600-0501.2011.02220.x
Máximo MB, de Mendonça AC, Renata Santos V, Figueiredo LC, Feres M, Duarte PM (2009) Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res 20(1):99–108. https://doi.org/10.1111/j.1600-0501.2008.01618.x
Cochis A, Fini M, Carrassi A, Migliario M, Visai L, Rimondini L (2013) Effect of air polishing with glycine powder on titanium abutment surfaces. Clin Oral Implants Res 24(8):904–909. https://doi.org/10.1111/j.1600-0501.2012.02490.x
Brägger U, Bürgin WB, Hämmerle CH, Lang NP (1997) Associations between clinical parameters assessed around implants and teeth. Clin Oral Implants Res 8(5):412–421. https://doi.org/10.1034/j.1600-0501.1997.080508.x
Papaioannou W, Quirynen M, Van Steenberghe D (1996) The influence of periodontitis on the subgingival flora around implants in partially edentulous patients. Clin Oral Implants Res 7(4):405–409. https://doi.org/10.1034/j.1600-0501.1996.070415.x
Quirynen M, Papaioannou W, van Steenberghe D (1996) Intraoral transmission and the colonization of oral hard surfaces. J Periodontol 67(10):986–993. https://doi.org/10.1902/jop.1996.67.10.986
Quirynen M, Vogels R, Peeters W, van Steenberghe D, Naert I, Haffajee A (2006) Dynamics of initial subgingival colonization of ‘pristine’ peri-implant pockets. Clin Oral Implants Res 17(1):25–37. https://doi.org/10.1111/j.1600-0501.2005.01194.x
Porras R, Anderson GB, Caffesse R, Narendran S, Trejo PM (2002) Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Periodontol 73(10):1118–1125. https://doi.org/10.1902/jop.2002.73.10.1118
Trejo PM, Bonaventura G, Weng D, Caffesse RG, Bragger U, Lang NP (2006) Effect of mechanical and antiseptic therapy on peri-implant mucositis: an experimental study in monkeys. Clin Oral Implants Res 17(3):294–304. https://doi.org/10.1111/j.1600-0501.2005.01226.x
Gonçalves PF, Huang H, McAninley S, Alfant B, Harrison P, Aukhil I, Walker C, Shaddox LM (2013) Periodontal treatment reduces matrix metalloproteinase levels in localized aggressive periodontitis. J Periodontol 84(12):1801–1808. https://doi.org/10.1902/jop.2013.130002
Gupta N, Gupta ND, Gupta A, Khan S, Bansal N (2015) Role of salivary matrix metalloproteinase-8 (MMP-8) in chronic periodontitis diagnosis. Front Med 9(1):72–76. https://doi.org/10.1007/s11684-014-0347-x
Chen HY, Cox SW, Eley BM, Mäntylä P, Rönkä H, Sorsa T (2000) Matrix metalloproteinase-8 levels and elastase activities in gingival crevicular fluid from chronic adult periodontitis patients. J Clin Periodontol 27(5):366–369. https://doi.org/10.1034/j.1600-051x.2000.027005366.x
Sorsa T, Gursoy UK, Nwhator S, Hernandez M, Tervahartiala T, Leppilahti J, Gursoy M, Könönen E, Emingil G, Pussinen PJ, Mäntylä P (2016) Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 70(1):142–163. https://doi.org/10.1111/prd.12101
Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS (2003) Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol 11(2):94–100. https://doi.org/10.1016/S0966-842X(02)00034-3
Subramani K, Jung RE, Molenberg A, Hammerle CH (2009) Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants 24(4):616–626
Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Darius PL, van Steenberghe D (1990) The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J Clin Periodontol 17(3):138–144. https://doi.org/10.1111/j.1600-051X.1990.tb01077.x
Dmytryk JJ, Fox SC, Moriarty JD (1990) The effects of scaling titanium implant surfaces with metal and plastic instruments on cell attachment. J Periodontol 61(8):491–496. https://doi.org/10.1902/jop.1990.61.8.491
Schwarz F, Rothamel D, Sculean A, Georg T, Scherbaum W, Becker J (2003) Effects of an Er:YAG laser and the Vector ultrasonic system on the biocompatibility of titanium implants in cultures of human osteoblast-like cells. Clin Oral Implants Res 14(6):784–792. https://doi.org/10.1046/j.0905-7161.2003.00954.x
Arweiler NB, Auschill TM, Sculean A (2017) Patient self-care of periodontal pocket infections. Periodontol 2000 76(1):164–179. https://doi.org/10.1111/prd.12152
Acknowledgements
Special thanks to Dr. Ralf Rössler for inserting the dental implants.
Funding
This study was partially supported by an unrestricted grant from the Oral Reconstruction Foundation (previously Camlog Foundation), Basel, Switzerland (Grant CF41203), who also provided the dental implants and the specially constructed titanium healing caps for the experimental investigation. The design, documentation, and analyses of this study were carried out independently.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol of the study was reviewed and approved by the Medical Ethics Committee, Philipps-University Marburg in Germany (no. 159/12). The study was conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and Good Clinical Practice guidelines as well as the Declaration of Helsinki.
Informed consent
All participants gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Schmidt, K.E., Auschill, T.M., Heumann, C. et al. Clinical and laboratory evaluation of the effects of different treatment modalities on titanium healing caps: a randomized, controlled clinical trial. Clin Oral Invest 22, 2149–2160 (2018). https://doi.org/10.1007/s00784-017-2287-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2287-8