Abstract
Objectives
To investigate tissue health around implants with newly attached superstructures over 12 months of preventive maintenance appointments and instrumentation when necessary.
Material and methods
In a randomized, split-mouth study 32 implants (8 participants with 4 implants each) received followed-up care every 3 months after superstructure attachment. Implants and superstructures were randomly assigned to four treatment groups and treated if necessary: (1) titanium curettes (TC), (2) stainless steel ultrasonic tip (PS), (3) erythritol air-polishing powder (EP), or (4) rubber cup polishing (CON). Probing depths (PDs), bleeding on probing (BOP), modified gingival (mucosal) bleeding index (GBI) around implants, and full-mouth Plaque Control Record (PCR) were measured every 3 months. Clinical attachment levels (CALs) and height of keratinized mucosa (KM)/gingival margins (GMs) for implants/teeth and PD, BOP, and GBI for teeth were documented at baseline, 6 months, and 12 months. Matrix metalloproteinase 8 (MMP-8) and periopathogens were measured at baseline and 12 months.
Results
Participants exhibited minimal signs of periodontal inflammation with statistically significant PD improvement (3.0 ± 0.2 to 2.8 ± 0.3 mm; p = 0.022) and overall CAL (4.3 ± 0.8 to 4.0 ± 0.7 mm; p = 0.048) after 1 year. Implants showed no statistically significant differences (p > 0.05) between or within groups at baseline or 12 months for any parameter, except MMP-8 decreased significantly for PS (14.50 ± 17.58 to 4.63 ± 7.56 ng; p = 0.044), and after 12 months, PCR showed a significant difference between TC and PS (p = 0.018).
Conclusions
Treatment was necessary as inflammation was observed around newly placed superstructures within the first year of maintenance care. All tested treatment modalities yielded comparable clinical improvements.
Clinical relevance
Early assessment and diagnosis of mucositis and regular maintenance can promote long-lasting implant health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental implants, like natural teeth, can become diseased and lost. Similar to gingivitis and periodontitis, bacterial biofilm can cause peri-implant diseases. This has been shown in early experimental gingivitis and mucositis models [1, 2] with the peri-implant mucosa resulting in a greater inflammatory reaction in response to plaque accumulation [2].
Two clinical phenotypes exist, namely peri-implant mucositis and peri-implantitis, where the former is characterized by inflammation of the soft tissues around the implant and the latter results in inflammation spreading to the supporting bone [3]. Within 5 to 10 years of implant placement, around 10% of implants and 20% of patients require therapy to treat peri-implantitis [4]. Thus, it is especially important to perform preventive implant care since non-surgical therapy of peri-implantitis has limited or no effect [5, 6] as compared to periodontitis [5].
However, a well-defined therapeutic model for optimal prevention and treatment of peri-implant mucositis does not exist [7, 8]. Still, some kind of prophylactic treatment must be delivered during regular maintenance care, and practitioners must motivate and instruct patients on the importance of optimal oral hygiene [7, 9, 10]. In turn, patients must be compliant and perform daily patient self-care in addition to regularly attending these appointments [5, 6, 11,12,13] that take individual risk factors into consideration [14] such as periodontitis, smoking, and patients’ own biofilm management, all of which can influence the long-term functionality of dental implants [7, 15]. Studies show that patients with periodontitis have greater implant failure compared to patients receiving systematic periodontal therapy [16, 17].
The aim of this randomized clinically controlled study was to follow-up on peri-implant tissue health of new implants at the time new superstructures were attached over the course of 12 months of preventive maintenance appointments every 3 months. Thus, the primary null hypothesis was that any individualized therapy will cause no difference (within or between groups) 12 months after treatment in implant bleeding on probing (BOP) percentages.
Materials and methods
The present follow-up took place at the Department of Periodontology at Philipps-University, Marburg, Germany, and was approved by the Medical Ethics Committee, Philipps-University in Marburg, Germany (no. 159/12). It was conducted in accordance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) and Good Clinical Practice guidelines as well as the Declaration of Helsinki.
Patient recruitment and study population
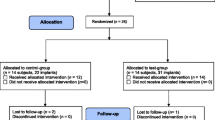
All 18 study participants from a former study [18] were contacted to attempt recruitment for the present maintenance study. Each patient had a history of periodontitis and was already involved in a supportive therapy program with high frequency (every 3 to 6 months). Patients were explained the importance of regular care on implants and teeth and given the option to partake in regular dental hygiene preventive maintenance appointments after they received their prosthodontic superstructures (either in the form of removable partial dentures (RPDs), fixed partial dentures (FPDs), or crowns as presented in Table 3). Recruited participants were consequently renumbered in the order of attendance of their first maintenance appointment (baseline) for the present study. The inclusion and exclusion criteria remained the same as in Schmidt et al. [18]. One year of data (five datasets for every 3 months the patients attended) were collected for this study with baseline representing the time following superstructure attachment (Fig. 1).
Clinical parameters
Assessments were subdivided into parameters on implants and on the remaining dentition.
Assessments of study implants
-
PDs (probing depths) and BOP (bleeding on probing) were measured at baseline and 3, 6, 9, and 12 months at 6 sites (mesiobuccal, buccal and distobuccal, mesiolingual, lingual and distolingual) using a 0.2 N pressure-sensitive probe (DB764R UNC 15, Aesculap, Tuttlingen, Germany). BOP was recorded as being positive if 30 s after probing bleeding was evident and negative if bleeding was absent within 30 s of probing.
-
CAL (clinical attachment level) and the height of KM (keratinized mucosa; similar to the gingival margin or GM around teeth) were also measured in millimeters at 6 sites at baseline and after 6 and 12 months. CAL and GM of implants were measured relative to a fixed reference point on the implant or superstructure [19, 20].
-
PCR (plaque control record; according to O’Leary et al. [21]) and a modified GBI (gingival, i.e., mucosal bleeding index; following the GBI by Ainamo and Bay [22] as the peri-implant mucosa can be monitored with similar methods used for assessing periodontal tissues [19]) were conducted at baseline and 3, 6, 9, and 12 months. Both oral hygiene parameters were measured at four sites (mesiobuccal, distobuccal, mesiolingual, and distolingual). Plaque was documented as present or absent using a disclosing agent (Mira-2-Ton®, Hager Werken, Duisburg, Germany) for the PCR, and mucosal bleeding was documented as being positive when bleeding was present within 10 to 15 s for the modified GBI.
Assessments of the remaining dentition
-
The parameters PD, BOP, GM, CAL, PCR, and GBI were measured for the natural dentition using the same methods as the implant assessments at baseline, 6 months, and 12 months (except PCR was recorded at baseline and 3, 6, 9, and 12 months).
Collection of laboratory parameters
-
MMP-8 (matrix metalloproteinase 8) levels around the study implants were assessed by a commercial immunological test (Bioscientia, Berlin, Germany) at baseline and 12 months. The gingival crevicular fluid (GCF) in each implant sulcus was tested for MMP-8 levels at one site per implant using special collection strips (dentognostics GmbH, Jena, Germany). These strips were inserted into the sulcus for 30 s, placed separately into plastic tubes and then sent to an external laboratory (Bioscientia, Berlin, Germany) for site-specific analysis.
-
A microbial analysis was also conducted at baseline and after 12 months using a commercial bacteria test (qPCR; Bioscientia) to detect 11 periodontal pathogens (Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Treponema denticola, Parvimonas micra, Fusobacterium sp., Campylobacter rectus, Eubacterium nodatum, Eikenella corrodens, and Capnocytophaga gingivalis). This was achieved by inserting 1 paper point (0.02 ISO 40, Dentsply) for each implant to the depth of the sulcus for 20 s. Each paper point was placed in separate plastic tubes and sent to the same external laboratory (Bioscientia).
The GCF strips and paper points were inserted into approximal areas on opposite sides of the implant so that no site was sampled twice. The same sampling sites were used for each test at both time points.
Following assessment of the parameters, the preventive maintenance appointments continued and, again, were differentiated into therapy of study implants and of the remaining dentition (Fig. 2).
Non-surgical therapy on study implants
All implants were polished “supramucosally” if PDs were no more than 3 mm. This included polishing with low relative dentin abrasion (RDA) level prophylaxis paste (Proxyt RDA 7, Ivoclar Vivadent, Schaan, Liechtenstein) using a rubber cup (Prophy Cup, KerrHawe SA, Bioggio, Switzerland).
If clinical assessments demonstrated first signs of inflammation (i.e., higher PDs and bleeding), implants received therapy (according to Mombelli & Lang [23] and Roccuzzo et al. [9]), meaning that they were instrumented “submucosally” following the same randomization scheme (i.e., the same instrument modalities) as in the previous study with the healing caps [18]. Each instrument was used according to the manufacturer’s instructions:
-
1.
Titanium curettes (Ergoplant universal curettes, Aesculap, Tuttlingen, Germany); TC
-
2.
Stainless steel ultrasonic tip (Instrument PS, EMS, Munich, Germany) using an ultrasonic device (Air Flow® Master Piezon, EMS); PS
-
3.
Air-Flow® Plus (EMS) erythritol powder using an air-polishing device (Air Flow® Master Piezon, EMS); EP
-
4.
The control (CON) was only instrumented if required (PD > 5 mm, BOP+) using titanium curettes (Ergoplant universal curettes, Aesculap, Tuttlingen, Germany).
CON was subsequently rinsed with 5 ml 0.9% sodium chloride (NaCl) solution, while the other three instrument groups were rinsed with 5 ml 0.2% chlorhexidine (CHX) (Chlorhexamed forte, GSK, Bühl, Germany).
Non-surgical periodontal therapy on remaining dentition
Treatment involved mechanical debridement of the natural dentition supra- and subgingivally, if indicated, using a combination of different instruments to remove soft and hard deposits from tooth and root surfaces (ultrasonic tip Instrument PS using an ultrasonic device Air Flow® Master Piezon, EMS, Munich, Germany, and stainless steel scalers and curettes, Hu-Friedy, Frankfurt am Main, Germany). Polishing was performed using a rubber cup (Prophy Cup, KerrHawe SA, Bioggio, Switzerland) with low or higher RDA level prophylaxis paste (Proxyt RDA 7, RDA 36 or 83, Ivoclar Vivadent) or an air-polishing device (Air Flow® Master Piezon, EMS) with Air-Flow® Plus erythritol powder (EMS), depending on the individual situation.
Each study participant was shown and demonstrated the appropriate home care techniques to help achieve optimal oral hygiene and long-term health and function of teeth and implants. This normally included proper brushing techniques (manual or electric) and individual interdental cleaning devices. Finally, patients were given a fluoride treatment (Elmex Fluid, GABA, Switzerland).
Statistical analysis
All data were collected in pseudonymous form and statistical analyses were performed blindly using IBM SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY, USA) following similar procedures as in Schmidt et al. [18].
After subsequently decoding the data and excluding the two drop-outs from the data according to the (modified) intention-to-treat concept [24], the mean values and standard deviations (SDs) of all parameters were calculated. This included PD (mm), KM/GM (mm), CAL (mm), BOP (%) (primary outcome), (modified) GBI (%), and PCR (%) for study implants in each treatment group, all study implants combined, and the natural dentition. MMP-8 (ng) and bacterial load were measured for implants in each treatment group and for all implants combined. The laboratory results of the bacteria test were graded from < 104, = 104, < 105, < 106, > 106, to > 107 and converted to a ranking system from 0, 1, 2, 3, 4, to 5, respectively, for all of the bacteria except for A. actinomycetemcomitans. This bacterium had a lower reference area but was also ranked 0, 1, 2, 3, 4, or 5, which corresponds to < 103, < 104, < 105, < 106, > 106, and > 107, respectively.
A within-group analysis between the two time points (i.e., before and 12 months after instrumentation) was completed for all parameters using a paired t test while a between-group analysis (i.e., comparison between the different instrument types) was completed for each parameter using linear mixed models with groups as fixed effects and subject-specific random effects to adjust for possible within-patient correlations.
For all analyses, adjustment for pairwise comparisons among the means was completed using the Bonferroni correction method and a difference was considered significant at a confidence interval of 95% (α = 0.05).
Results
The number of participants that could be included in the analysis in the present study stems from the patients included in a former clinical study [18], which started with 20 subjects and decreased to 18 subjects due to 2 drop-outs. Ten of the original participants could be recruited because the other 8 subjects had received their superstructures at external dental clinics, and thus, baseline data were not able to be collected. Of the 10 participants initially recruited, two were excluded from the analysis; one patient opted to discontinue after the second appointment due to travel distance while the other patient attended appointments irregularly (i.e., not in the scheduled intervals) and did not attend the required number of appointments. Thus, 8 patients (6 female and 2 male) with a mean age of 62.63 ± 7.84 (range 53 to 75) could be included in the analysis, which corresponds to 32 implants. Six of the participants had only fixed (non-removable) superstructures in the form of FPDs or crowns attached to the study implants. One study participant presented with a combination of crowns and a RPD, while another participant only had a RPD attached to the study implants. None of the participants showed any side effects during or following the study.
The baseline characteristics (Table 1) showed moderate periodontally involved patients (GM = −1.3 ± 0.8 mm, CAL = 4.3 ± 0.8 mm) exhibiting minimal signs of inflammation (BOP<22%; GBI < 19%). The periodontal assessment (Table 2) showed a significant improvement in the PDs (3.0 ± 0.2 to 2.8 ± 0.3 mm; p = 0.022) and the overall CAL (4.3 ± 0.8 to 4.0 ± 0.7 mm; p = 0.048) following 1 year of continuous preventive maintenance appointments. GM remained constant between baseline and 12 months (p > 0.05).
During the course of the 12-month study period, 19 out of the 32 study implants (corresponding to 7 of 8 patients) required instrumentation at least one time due to first signs of inflammation. Specifically, 9 out of 18 implants (50.00%) were part of a FPD (8 at 3 months, 1 at 9 months), 5 (of 7; 71.43%) were crowned (4 at 3 months, 1 at 6 months), and 5 (of 7; 71.43%) were used to anchor an RPD (2 at 6 months, 1 at 9 months, 2 at 12 months) and required instrumentation at least once during the 12-month study period. Details regarding implant group, patients, visits, and the type of treatment modality (TC, PS, EP, or CON) used to instrument the specific superstructure (FPD, crown or RPD) for the first time are given in Table 3.
A majority of the instrumentation occurred in the first 6 months of maintenance care (6 implants in the TC and CON groups; 7 implants in the PS and EP groups).
The clinical parameters PD, KM, and CAL at study implants (Table 2) remained stable after 1 year of regular preventive therapy, and therefore, the findings showed no significant differences within the groups at the two time points (p > 0.05). Although not significant, the CON group resulted in a greater increase in PD over the course of the year (2.8 ± 0.6 to 3.3 ± 0.5 mm, p = 0.074). Between the different treatment groups, no significant differences were found at baseline or 12 months for PD, KM, or CAL (p > 0.05).
Results of the oral hygiene parameters BOP, (modified) GBI, and PCR for the study implants and the dentition are shown in Table 4. Comparing both time points (baseline and 12 months after regular therapy), no significant differences were evident within the groups after instrumentation for any of the oral hygiene parameters (p > 0.05). There were also no significant differences (p > 0.05) between the treatment groups at either baseline or 12 months after preventive therapy for BOP, (modified) GBI, or PCR, except for PCR when comparing the two time points (p = 0.025). Specifically, a significant difference was found between TC and PS at 12 months (p = 0.018).
The laboratory parameters of the study implants are shown in Table 5. No significant differences (p < 0.05) were evident for MMP-8 within any treatment group upon comparison of the two time points, except for MMP-8 in the PS group, which significantly decreased from 14.50 ± 17.58 ng at baseline to 4.63 ± 7.56 ng after 12 months (p = 0.044). Bacterial grading showed low levels of bacteria with no significant differences (p < 0.05) within any of the groups at the two time points. Also, no significant differences were evident between the groups at either baseline or 12 months after regular preventive therapy (p > 0.05).
The primary null hypothesis that any treatment modality will cause no difference in implant BOP within and between groups 12 months after instrumentation can be confirmed (p > 0.05).
Discussion
While the main focus of the present study was the instrumentation during maintenance care appointments, the parameters assessed also give valuable information regarding the development and resolution of inflammation surrounding implants through early therapeutic intervention. Baseline data of the implants were collected after attachment of implant superstructures, which is also considered essential in daily routine practice to classify changes such as in PD [3]. In general, data demonstrated largely stable PDs, relatively low BOP, and no suppuration, which characterize clinically healthy peri-implant tissues over the course of 1 year of preventive and minimally invasive therapy.
Similar treatment modalities as well as patient-performed home care for periodontal diseases [13] have been adopted in patients with implants [8, 25]. However, an implant’s more complex threaded design [8, 26] requires safe and effective therapy options since treatment may cause implant surface changes [27] and initiate issues in biocompatibility and long-term success [28]. While several studies propose various treatments for successful mucositis therapy [26, 29, 30], the instrumentation modalities used in the present study were recently evaluated as safe without causing greater biofilm formation or differences in biocompatibility [18, 31]. The fact that the amount of therapy of implants in the present study decreased in the last half of the year demonstrates the benefits of preventive treatment.
Although not statistically significant, the CON group had the greatest increase in PD, CAL, and BOP compared to the other groups. This may be due to only polishing and rinsing with NaCl and the lack of instrumentation and CHX rinsing. Mechanical instrumentation together with chemical agents may reduce adhering biofilm on an implant’s surface [32] though it has been concluded inconsistently in reviews that mechanical therapy with or without adjunctive measures (e.g., antiseptics, local and systemic antibiotics, air abrasive devices) could be effective in treating peri-implant mucositis [5, 11, 14, 33].
When a patient attends maintenance appointments, it is in the practitioner’s interest to ensure peri-implant health is obtained and maintained. Throughout the study, four (PS group) or five implants (TC, EP and CON) in all groups needed extra treatment (other than polishing). In case of signs of peri-implant disease in the CON group, the pre-set randomization scheme required deviation since—following the (modified) intention-to-treat approach to reflect the actual clinical situation [24]—these implants had to be instrumented and not just polished. Since instrumentation can be impeded around an infected implant with increased PDs, by threads that can be felt, or through an inconvenient design or curvature of the superstructure [5], it may be beneficial to combine various mechanical techniques (e.g., curettes, ultrasonic, air-polishing powders, lasers) to achieve more optimal removal of deposits to provide individualized treatment dependent on the patient’s needs [6]. This was done in very few cases (9 out of 160 implant treatment cases) where erythritol powder was used when a titanium curette was unable to reach into a narrow and deep pocket.
The differences in the types of prosthodontic superstructures fixed or anchored to the study implants in the form of crowns/FPDs or RPDs, respectively, were minimal when the implants were instrumented for the first time within the 12-month study period (50.00% for implants with FPDs attached, 71.43% for crowned implants, and 71.43% for implants used for RPDs). When observing the first 6 months of maintenance care where the majority of the instrumentation occurred, the proportions changed to 44.44% for the implants with FPDs attached, 71.43% for the crowned implants, and 28.57% for the RPDs anchored to implants. The variations may lie in the fact that crowns and FPDs hinder access for biofilm removal for both the patient and clinician, thus requiring clinical intervention sooner when compared to RPDs, which increases the accessibility to the implant for cleaning by the patient. However, over time, implants used for the attachment of an RPD may also require treatment due to inflammation as seen in two of these implants requiring treatment at the 12-month time point. This could be due to reasons such as not removing the RPD regularly or poor cleaning of the implant and RPD by the patient.
Even though no specific primary prevention of peri-implant mucositis is available [7, 10, 11, 15], practitioners must be attentive to early signs of inflammation rather than allowing an infection and bone destruction to develop [7].
Regardless of the professional treatment provided, home care plaque control is not only key for patient self-care of periodontal pocket infections [13], but also key in treating mucositis [5]. Clinicians are unable to monitor patients’ daily oral hygiene practices, but they can evaluate and encourage oral hygiene during appointments using bleeding and plaque indices [7]. In the present study, thorough oral hygiene instructions were given at each appointment and based on methods used for the natural dentition due to the lack of evidence [25]. Although the modified GBI around implants showed no significant differences in any of the groups, other studies have shown reductions in bleeding from improvements in oral hygiene and professional treatment, though effective therapies did not always resolve tissue inflammation completely [34]. Plaque decreased in all treatments (except for PS), though the results were not significant. Significant differences in PCR were seen between groups TC and PS after 12 months, which may be more of a case of suboptimal plaque removal by the patients in the study than possible roughening by the instruments. Higher PCR levels could also be due to the dichotomous character of this parameter, which registers plaque as present or absent and documents even small amounts as positive [35]. In two studies [18, 31] testing similar treatments, the amount of biofilm attaching to instrumented implants showed no significant differences, suggesting that surface roughness between the differently treated implants is not significant.
The immunological biomarker matrix metalloproteinase 8 (MMP-8) was measured to further assess inflammation. It has been found at increased levels in affected patients in the gingival crevicular and peri-implant sulcus fluids and is involved in irreversible destruction of collagen in soft and hard tissues of the periodontium and around implants [36, 37]. High MMP-8 levels around implants can be reversed (though healing is slower than the gingiva) upon restoration of normal oral hygiene practices as shown in an experimental gingivitis/mucositis model [2]. Specifically, MMP-8 and PD have shown to be positively correlated in addition to CAL, gingival index, and plaque index [36, 38]. A similar relationship was observed in the present study where one subject generally had both higher PDs and MMP-8 levels compared to other participants. Comparing between groups, PS significantly decreased MMP-8 to healthy levels (<8 ng), CON signified health, and TC and EP represented slight inflammation with no elevated risk of progression of tissue breakdown (8–20 ng). These results are comparable to the former study [18], where MMP-8 levels in the PS group decreased significantly to between 8 and 20 ng after 3 months. The same occurred to the TC group while CON decreased significantly to a healthy level. EP in both studies had similar levels over their respective time points. Treatments generally maintained healthy to slightly inflamed conditions over a 1-year period (< 20 ng). This corresponds to the overall minimal bacterial levels at implants (i.e., ≤ 1 or only slightly above the laboratory detection level), which did not reveal any significant changes. This is also in line with the previous study [18] where none of the groups showed a significant difference in bacterial levels between each other after 3 months except for erythritol air-polishing powder and the control.
Since it was found that in the presence of an increased bacterial load peri-implant mucosa showed a greater inflammatory response than the gingiva [2], the implants in the present study yielded neither increased inflammatory markers nor an increased bacterial load.
With respect to the periodontal status of the patients, the baseline characteristics confirmed stable periodontal conditions (PD = 3 mm; BOP < 22%), which surely helped decrease the risk of periopathogens in periodontal pockets from infecting new dental implants [39, 40]. Furthermore, over the course of the 12 months, PD and CAL decreased significantly, confirming that regular professional maintenance care appointments can improve and maintain clinical signs of periodontal disease [41].
In summary, considering the low number of subjects (that were left for inclusion in this follow-up study), the data have to be interpreted carefully. However, the data show a trend toward peri-implant complications (such as inflammation) in periodontitis patients in an early phase of prosthodontic restoration. It is important to demonstrate such negative events even in the first year of service, which supports the essential value of maintenance care on implant necks and their superstructures. In any case, the study findings provide important implications for future research, which is very rare at present.
Thus, the findings also show that periodontally stable patients with implants and concomitantly facing a higher risk of disease occurrence who attend regular maintenance programs can have long-lasting positive results compared to those not receiving regular preventive care [9, 15, 42].
With respect to the parameters measured, the four treatment modalities in the present study are comparable and generally resulted in positive outcomes over the course of a year following attachment of superstructures to implants. Repeated instrumentation in some of the cases seemed not to have resulted in decreased biocompatibility or increased plaque accumulation, though longer-term studies need to be conducted to determine if these treatments can sustain implant health. Optimal time intervals between peri-implant maintenance appointments should be tailored toward each patient’s individual risk profile (such as smoking, periodontal disease, general disease) as well as poor oral hygiene [15].
References
Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP (1994) Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res 5:254–259
Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP (2012) Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res 23:182–190
Lindhe J, Meyle J, Group D of European Workshop on Periodontology (2008) Peri-implant diseases: consensus report of the sixth european workshop on periodontology. J Clin Periodontol 35(8 Suppl):282–285
Mombelli A, Müller N, Cionca N (2012) The epidemiology of peri-implantitis. Clin Oral Implants Res 23(Suppl 6):67–76
Renvert S, Polyzois I (2015) Clinical approaches to treat peri-implant mucositis and peri-implantitis. Periodontol 2000 68:369–404
Figuero E, Graziani F, Sanz I, Herrera D, Sanz M (2014) Management of peri-implant mucositis and peri-implantitis. Periodontol 2000 66:255–273
Armitage G, Xenoudi P (2016) Post-treatment supportive care for the natural dentition and dental implants. Periodontol 2000 71:164–184
Renvert S, Roos-Jansåker A-M, Claffey N (2008) Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol 35:305–315
Roccuzzo M, Bonino L, Dalmasso P, Aglietta M (2014) Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. Clin Oral Implants Res 25:1105–1112
Ramanauskaite A, Tervonen T (2016) The efficacy of supportive peri-implant therapies in preventing peri-implantitis and implant loss: a systematic review of the literature. J Oral Maxillofac Res 7:e12
Jepsen S, Berglundh T, Genco R, Aass A, Demirel K, Derks J, Figuero E, Giovannoli JL, Goldstein M, Lambert F, Ortiz-Vigon A, Polyzois I, Salvi GE, Schwarz F, Serino G, Tomasi C, Zitzmann NU (2015) Primary prevention of periimplantitis: managing peri-implant mucositis. J Clin Periodontol 42(Suppl 16):S152–S157
Salvi GE, Ramseier CA (2015) Efficacy of patient-administered mechanical and/or chemical plaque control protocols in the management of peri-implant mucositis. A systematic review. J Clin Periodontol 42(Suppl 16):S187–S201
Arweiler NB, Auschill TM, Sculean A (2018) Patient self-care of periodontal pocket infections. Periodontol 2000 76:164–179
Renvert S, Polyzois I (2015) Risk indicators for peri-implant mucositis: a systematic literature review. J Clin Periodontol 42(Suppl 16):S172–S186
Monje A, Aranda L, Diaz KT, Alarcón MA, Bagramian RA, Wang HL, Catena A (2016) Impact of maintenance therapy for the prevention of peri-implant diseases: a systematic review and meta-analysis. J Dent Res 9:372–379
Karoussis IK, Kotsovilis S, Fourmousis I (2007) A comprehensive and critical review of dental implant prognosis in periodontally compromised partially edentulous patients. Clin Oral Implants Res 18:669–679
Wennström JL, Ekestubbe A, Gröndahl K, Karlsson S, Lindhe J (2004) Oral rehabilitation with implant-supported fixed partial dentures in periodontitis-susceptible subjects. J Clin Periodontol 31:713–724
Schmidt KE, Auschill TM, Heumann C, Frankenberger R, Eick S, Sculean A, Arweiler NB (2018) Clinical and laboratory evaluation of the effects of different treatment modalities on titanium healing caps: a randomized, controlled clinical trial. Clin Oral Investig 22:2149–2160
Mombelli A, Lang NP (1994) Clinical parameters for the evaluation of dental implants. Periodontol 2000 4:81–86
Heitz-Mayfield LJ (2008) Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol 35(8 Suppl):292–304
O'Leary TJ, Drake RB, Naylor JE (1972) The plaque control record. J Periodontol 43:38
Ainamo J, Bay I (1975) Problems and proposals for recording gingivitis and plaque. Int Dent J 25:229–235
Mombelli A, Lang NP (1998) The diagnosis and treatment of peri-implantitis. Periodontol 2000 17:63–76
Gupta S (2011) Intention-to-treat concept: a review. Perspect Clin Res 2:109–112
Louropoulou A, Van der Weijden F (2014) Mechanical self-performed oral hygiene of implant supported restorations: a systematic review. J Evid Based Dent Pract 14(Suppl):60–69
Máximo MB, de Mendonça AC, Renata Santos V, Figueiredo LC, Feres M, Duarte PM (2009) Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res 20:99–108
Louropoulou A, Slot DE, Van der Weijden FA (2012) Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res 23:643–658
Louropoulou A, Slot DE, Van der Weijden FA (2015) Influence of mechanical instruments on the biocompatibility of titanium dental implants surfaces: a systematic review. Clin Oral Implants Res 26:841–850
Porras R, Anderson GB, Caffesse R, Narendran S, Trejo PM (2002) Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Periodontol 73:1118–1125
Trejo PM, Bonaventura G, Weng D, Caffesse RG, Bragger U, Lang NP (2006) Effect of mechanical and antiseptic therapy on peri-implant mucositis: an experimental study in monkeys. Clin Oral Implants Res 17:294–304
Schmidt KE, Auschill TM, Heumann C, Frankenberger R, Eick S, Sculean A, Arweiler NB (2017) Influence of different instrumentation modalities on the surface characteristics and biofilm formation on dental implant neck, in vitro. Clin Oral Implants Res 28:483–490
Louropoulou A, Slot DE, Van der Weijden F (2014) The effects of mechanical instruments on contaminated titanium dental implant surfaces: a systematic review. Clin Oral Implants Res 25:1149–1160
Schwarz F, Becker K, Sager M (2015) Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J Clin Periodontol 42(Suppl. 16):S202–S213
Heitz-Mayfield L, Salvi G, Botticelli D, Mombelli A, Faddy M, Lang N (2011) Implant complication research group. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res 22:237–241
Leonhardt A, Gröndahl K, Bergström C, Lekholm U (2002) Long-term follow-up of osseointegrated titanium implants using clinical, radiographic and microbiological parameters. Clin Oral Implants Res 13:127–132
Gupta N, Gupta ND, Gupta A, Khan S, Bansal N (2015) Role of salivary matrix metalloproteinase-8 (MMP-8) in chronic periodontitis diagnosis. Front Med 9:72–76
Sorsa T, Gursoy UK, Nwhator S, Hernandez M, Tervahartiala T, Leppilahti J, Gursoy M, Könönen E, Emingil G, Pussinen PJ, Mäntylä P (2016) Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000 70:142–163
Gonçalves PF, Huang H, McAninley S, Alfant B, Harrison P, Aukhil I, Walker C, Shaddox LM (2013) Periodontal treatment reduces matrix metalloproteinase levels in localized aggressive periodontitis. J Periodontol 84:1801–1808
Papaioannou W, Quirynen M, Van Steenberghe D (1996) The influence of periodontitis on the subgingival flora around implants in partially edentulous patients. Clin Oral Implants Res 7:405–409
Quirynen M, Vogels R, Peeters W, van Steenberghe D, Naert I, Haffajee A (2006) Dynamics of initial subgingival colonization of ‘pristine’ peri-implant pockets. Clin Oral Implants Res 17:25–37
Axelsson P, Lindhe J (1981) The significance of maintenance care in the treatment of periodontal disease. J Clin Periodontol 8:281–294
Pjetursson BE, Helbling C, Weber HP, Matuliene G, Salvi GE, Brägger U, Schmidlin K, Zwahlen M, Lang NP (2012) Peri-implantitis susceptibility as it relates to periodontal therapy and supportive care. Clin Oral Implants Res 23:888–894
Funding
This is a continuation of a study [18] that was financially supported by an unrestricted grant from the Oral Reconstruction Foundation (previously Camlog Foundation; Grant CF41203), Basel, Switzerland. The design, documentation, and analyses of this study were carried out independently. No financial conflict of interest exists between the authors and any previous funding received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Medical Ethics Committee, Philipps-University Marburg in Germany (no. 159/12) and conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and Good Clinical Practice guidelines as well as the Declaration of Helsinki.
Informed consent
All participants gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Schmidt, K.E., Auschill, T.M., Sculean, A. et al. Clinical evaluation of non-surgical cleaning modalities on titanium dental implants during maintenance care: a 1-year follow-up on prosthodontic superstructures. Clin Oral Invest 23, 1921–1930 (2019). https://doi.org/10.1007/s00784-018-2640-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2640-6