Abstract
Objectives
The objective of this study was to assess, by histomorphometric analysis, the degree of bone apposition on two types of dental implant’s surfaces: a novel implant that combines Al2O3 abrasive particle blasting with thermochemical treatment (ContacTi), compared to a standard surface treatment obtained by sandblasting and acid etching (shot blasting).
Materials and methods
Twelve minipigs were used, placing the studied implants in the maxillae, and divided into three groups according to the time of sacrifice: 2, 4, and 8 weeks after implant placement. Histological and histomorphometric analyses were performed following standardized tissue polymerization, cutting, and staining and examined under optical and high-resolution electron microscope.
Results
For all measurements, the novel surface presented higher levels of osseointegration as compared to the shot blasting surface. Bone to implant contact (BIC) in the maxillae for ContacTi presented values of 49.02, 83.20, and 85.58% at 2, 4, and 8 weeks, respectively, significantly higher compared to the shot blasting surface values of 39.32, 46.53, and 46.20% for the same time points. Bone area density (BAD) presented values of 26.52, 61.21, and 59.50% for ContacTi surface implants and 22.95, 36.26, and 49.50% for the shot blasted surface implants. Signs of osteoconductivity were observed in the ContacTi surfaces at 2 weeks.
Conclusions
The ContacTi surface achieved a faster growth of hard tissues around the implants, when compared to the shot blasting surface, and for all evaluated histomorphometric parameters, the values were higher at all measured time points.
Clinical relevance
ContacTi could be a new surface improving the osseointegration in oral implantology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the field of dental implants, researchers are currently endeavoring their efforts in achieving surfaces that will guide tissue to a greater and faster osseointegration. Surface morphology and characteristics have been shown to have a great impact in the different stages of osseointegration [1, 2]. Therefore, over the last 25 years, attempts have been made to improve bone apposition through increasing the surface microroughness or by improving its chemical interaction with the early stages of bone healing. For this purpose, various techniques have been applied, such as acid etching, sandblasting, oxidization, or coatings, among others, as well as their combinations, achieving an improvement in osseointegration when compared to non-treated smooth implants [3, 4].
The surface of dental implants can be modified by tailoring several parameters, such as wettability, chemical composition, zeta potential, texture, or topography, which have a direct impact on cellular interaction with the implant and the site of implantation, which is mediated by the cellular adhesion, proliferation, and differentiation. Actually, these cellular processes are of crucial importance, where surface characteristics are essential in order to obtain a determined cellular response in the bone-implant interface, directly affecting the growth and quality of the formed bone [5,6,7,8].
Recently, a surface treatment for dental implants based on gritblasting or sandblasting with alumina particles and subsequent acid etching, which is known as the Shot Blasting® surface (Klockner Implant System, SOADCO SL, Andorra), allows obtaining a microroughened surface. This surface has demonstrated excellent clinical results by significantly increasing the bone to implant contact (BIC) area as compared to an untreated surface [9, 10].
The seek for improving the bone formation at the implant interface resulted in the development of new generations of implant surfaces by the implementation of different physical and biochemical treatments, which are able to modify the surface at a nanoscale level, which has the ability to induce cellular differentiation towards osteogenesis [11]. Therefore, different techniques have been used in order to achieve bioactive surfaces such as anodizing or coating with CaP (apatite) by different methods such as sol-gel deposition, evaporation, or electrophoresis among others; these methods are capable of speeding up the osseointegration process when compared to traditional smooth surfaces [12,13,14,15]. Bioactive surfaces are those around which new bone formation is produced, characterized by presenting on its surface, besides the different degrees of roughness, bioactive molecules, or growth factors that induce bone formation. In order to achieve this bioactivity, the coating of an implant surface with a CaP layer has received much attention due to its resemblance with natural bone and has demonstrated improved implant osseoconductivity, accelerating early bone healing, due to its fast protein adsorption.
In order to achieve this coating, several techniques have been tested, although the most well-known and widely used is the one proposed by Kokubo [16], consisting in an alkaline immersion process, thermal treatment, and subsequent biomimetic treatment through immersion in a simulated body fluid. The result is the formation of an apatite layer that allows the interaction with the underlying bone and hence accelerating the integration process [17, 18].
Following this process performed by Kokubo, a surface treatment designated as ContacTi® (Klockner Implant System, SOADCO, Andorra) was designed in a two stage treatment of the titanium, followed by an alumina particle sandblasting. This process allowed obtaining an optimal microroughness for the adhesion, proliferation and differentiation of human osteoblastic cells, and a subsequent treatment (alkaline immersion and thermal treatment) based on the previously cited technique [16], which conferred the bioactivity [19]. This surface has demonstrated, in vitro, the formation of a hydroxyapatite layer when immersed in a simulated body fluid [20, 21].
The present work has the objective of assessing by histomorphometric analysis the degree of bone apposition on implant external surface, comparing two different types of surfaces, one obtained by sandblasting and acid etching (shot blasting) and the novel treatment that obtains the ContacTi surface, tested in different time intervals.
Materials and methods
Surface treatment
In this study, two different surface treatments were evaluated. The first surface was a titanium surface treated by sandblasting and acid etching, coded as shot blasting, while the second treatment to obtain the new bioactive surface, coded as ContacTi, was obtained by a two-stage technique. Initially, the process began with sandblasting with abrasive alumina particles over the titanium surface, followed by chemical and thermal treatment similar to the one described by Kokubo et al. [16]. The process consists on the immersion of the metal in a 10-ml 5 M NaOH solution at 60 °C for 24 h, then rinsed with distilled water and dried at 40 °C for 24 h and finally submitted to a thermal treatment in a tubular furnace at 600 °C for 1 h.
Dental implants
All implants used in this study were Essential Cone (Klockner Implant System, Soadco, Andorra) with 4-mm diameter and 8 mm of length, 1.5-mm machined polished collar, and surface treatment according to its experimental group (ContacTi or shot blasting).
Implant roughness was evaluated according to the recommendations described by Wennerberg and Albrektsson on topographic evaluation for dental implants [3, 4]. A white light interferometer microscopy (Wyko NT1100, Veeco) was used to quantify the surface roughness of each implant. The surface analysis area was 189.2 × 248.7 μm2 for the smooth control surfaces and 459.9 × 604.4 μm2 for all the micro-rough surfaces. Data analysis was performed with Wyko Vision 232TM software (Veeco, USA). A Gaussian filter was used to separate waviness and form from the roughness of the surface. Cut-off values, λc = 0.8 mm, for ShotBlasting, and ContacTi surfaces and λc = 0.25 mm for control surfaces were applied, according to previous tests [9]. The measurements were made in three different surfaces of each type of surface treatment to characterize the amplitude and spacing roughness parameters Ra and Pc, respectively.. Ra (the average roughness) is the arithmetic average of the absolute values of the distance of all points of the profile to the mean line. Pc is the number of peaks in the profile per length of analysis. Ra and Pc were calculated by averaging the values of all individual profiles that were evenly distributed along the surface analyzed. A scanning electron microscope (SEM, JSM 6400, Jeol, Japan) was used to qualitatively analyze the surface topography of the implants before being implanted.
Animal model
The present study was carried out in maxillae of 12 six-year-old female minipigs in the Córdoba University’s Servicio Centralizado de Animales de Experimentación located in the Campus de Rabanales and approved by the University of Seville Ethics Experimentation Committee (MED2016-01-324). All requirements and regulations for animal experimentation, according to the Spanish and European Union, were fulfilled.
Teeth extractions of each animal were performed 4 months before the surgical implantation. After teeth extraction, hemorrhages were spontaneously resolved.
Radiographic pictures of the maxilla of each animal were taken 1 day before the surgeries were performed to assess appropriate bone regeneration, to discard the presence of root residues, and to plan the location of the dental implants during surgery.
The animals were fed with a liquid and powder-based food diet throughout the study and submitted to oral prophylaxis, by means of an aseptic technique, 3 weeks before the experimental surgeries.
In vivo implantation
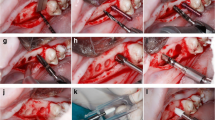
A total number of 48 implants were placed by a surgeon with more than 10 years of experience in oral implantology and highly experienced in the described implants. Manufacture’s guidelines for implant placement were followed. This animal model and procedures have been validated in previous studies [22,23,24]. Figure 1 shows relevant images of the surgical procedure, showing the teeth extraction (Fig. 1a), the removed teeth (Fig. 1b), the root canal cleaning (Fig. 1c), and the dental implant placement (Fig. 1d).
Animal feeding was suspended the night before the surgery. The animals were pre-anesthetized with xylazine hydrochloride and ketamine and maintained with gaseous anesthetic (5% isoflurane–oxygen). Hydration was maintained by infusion of a Ringer lactate solution. They were also monitored by measuring heart and breathing frequencies. The study consisted of two surgeries as described below.
During the first intervention, posterior teeth of the 12 pigs were carefully extracted from both sides of the maxilla—bicuspids and molars. For this purpose, a wide incision was performed with the elevation of a full thickness flap, followed by a careful osteotomy in order to obtain a sufficient edentulous maxilla for the placement of two implants in the subsequent second surgical stage.
Once the surgery was finished, buprenorphine hydrochloride was administrated for pain management and amoxicillin for infection prevention. Animal follow-ups were performed, controlling edema, dehiscence, and possible infections.
After the 4-month healing period, each animal received four implants with each of the surface finishings. Two implants of each type were alternately placed in each of the two maxillae quadrants. Consequently, 16 dental implants were analyzed for each implantation time (8 ShotBlasting and 8 ContacTi). These implants were placed in the edentulous maxilla of each minipig. The manufacture’s guidelines for implant placement were followed accordingly to a semi-submerged technique, completely covered by skin, in such a manner that the machined polished collar was placed completely supraosseous with a distance between them of at least 4 mm. Healing abutments of 2-mm height were placed, and after irrigation with a sterile saline solution, the surgical incision was closed by primary intention with synthetic polyamide interrupted sutures, Supramid® 4–0.
Animal sacrifice
Each group was sacrificed at different time points as already previously described: 2, 4, and 8 weeks after the second surgery. Euthanasia was performed by overdose of sodium pentothal perfused in the carotid arteries, mixed with 5% glutaraldehyde and 4% formaldehyde at a pH of 7.2.
Sample processing
This study was performed in such a way that the histopathologist entrusted with the histomorphometric analysis was unaware of which type of implant surface was being evaluated in each animal. After euthanasia, 16-mm-thick block sections including implants, alveolar bone, and surrounding mucosa were collected by cutting with an irrigated diamond-saw (Accutom 50, Struers, Germany) and radiographed. The specimens were thoroughly rinsed in sterile saline solution and immersed in buffered 10% formol solution. The tissue blocks were fixed for 5 days in the 10% formol solution, dehydrated in ethanol solutions (70, 80, 96, and 100% alcohol for 3 days each), and embedded into molds (Exact 41440-4150, Exact Apparetabau GmbH, Germany) in a photopolymerizable resin (Technovit 7200 VLC, Sulzer, Germany) using a polymerization unit (Exact 520-530, Exact Apparatebau GmbH, Germany). Activation of the polymerization of the resin was achieved by irradiation with yellow and blue light for 12 h. Sample processing was performed with the sawing and grinding technique described by Donath and Breuner [25], using an EXAKT system (Exact Vertriebs, Norderstedt, Germany) and methylmetacrilate (MMA) as the inclusion medium.
The embedded implants were cut (Accutom 50, Struers, Germany) midaxially in a buccal-lingual plane into sections of approximately 200 μm thick, and further treated using the cutting-grinding technique to obtain a final polished 50-μm-thick section. Sections were then stained with toluidine blue (Toluidine Blue O, Fisher Scientific, USA) for 20 min.
Sample analysis
The histopathologic and histometric analysis were performed with a digital camera system (DP12, Olympus, Japan) attached to a light microscope (BX51, Olympus, Japan) and an image analyzer software (MicroImage 4.0, Olympus, Japan). × 80 images were taken from end to end of the bottom part of the implant collar. More than 500 samples were studied by optical microscope. Polished sections were examined under a high-resolution scanning electron microscope (SEM, JSM-6400, Jeol, Japan) using retro-dispersed electrons to differentiate in greater detail bone from the surface of the implant. Back-scattered electrons allowed determining differences based on atomic numbers, clearly showing the calcified tissue and bone-implant contact. The resolution used in the current study was of 8 nm. This technique is the most sensitive in order to evaluate and quantify the two parameters analyzed:
BIC (bone to implant contact): amount of mineralized bone that is in intimate contact with the implant surface. This is a length value and therefore a two-dimensional static variable.
-
BAD (bone area density): amount of mineralized bone arranged both in trabeculae and osteons in relation to the total area of tissue in the space between two spirals (bone area per total area). This is also a two-dimensional variable.
Data analysis
Statistical analysis was performed by the Department of Statistics of the Faculty of Dentistry, University of Seville, using the IBM SPSS 20.0 statistical package for Windows. Average and standard deviations were analyzed by groups, and multiple comparison tests were carried out to determine statistical significance (established at p < 0.05).
Average and standard deviations were determined overall and by groups. For the comparison of the numerical variables between groups, a one factor ANOVA was applied, or a one factor ANOVA with Welch correction (in variance heterogeneity), or the Kruskal-Wallis non-parametric test (in case of non-normal distributions); when these tests were significant, multiple comparison tests were used (DMS, Tamhane, Bonferroni).
Results
The surfaces of dental implants after shot blasting and ContacTi treatments are shown in Fig. 2. The roughness of dental implants with shot blasting was 3.1 ± 0.4 μm, and the surface roughness with ContacTi was 3.5 ± 0.3 μm, not presenting significant differences among them. This implied that the formation of sodium titanate did not affect the roughness of the implants treated by shot blasting. For all the time points and conditions studied, samples showed a normal macroscopic anatomy. There was normal bone tissue in contact with all implants, not presenting any signs of fibrosis or inflammation. .
Table 1 specifies the values obtained in terms of BIC% and BAD%, in the different measured time points of the study. The percentage of BIC for the ContacTi surface was higher compared to the shot blasting surface in all measurements, presenting statistical significant differences between the two different surfaces at 4 and 8 weeks.
The highest percentage of BIC corresponded to the new surface at 8 weeks, being superior to 85%. Figure 3 graphically collects comparative average BIC values between the two surfaces in the different time points. The percentage of BAD for the ContacTi surface was higher to the shot blasting surface in all measurements, with statistically significant differences between surfaces at 4 and 8 weeks. The highest value corresponded to the ContacTi surface at the 4 weeks, with 61.2%. Figure 4 graphically represents the average BAD values at the different time points.
Histological samples allowed assessing the interaction between the implant and the surrounding bone. At low magnifications, it was possible to observe the drilling grooves and primary contact with the implant threats (Fig. 5). For both treatments, the contact between the bone and the implant increased and the integration between the implant and the bone seemed higher in the ContacTi-treated surfaces compared to the shot blasting surfaces. At higher magnifications, at the 2-week time point, the ContacTi surface already showed immature bone formation, exhibiting contact osteogenesis, with new bone growth from the implant to the surrounding mature bone (Figs. 6 and 7). Figure 7 shows that osteoblastic actions take place at the interface of the implant indicated with red arrows, showing the osteogenic capacity of the bone lining cells, which induce the formation of new bone. In the shot blasting surface, only contact between the implant spirals and surgical drilling grooves was observed. The old bone and new bone were clearly visible with the back-scattered images, showing in darker color the new bone (NB, indicated with arrows) and the old bone (OB) in a lighter gray color. Figure 8 shows the quantification of new bone formed for each implant, showing that the amount of NB in the ContacTi samples was higher at 2 weeks than the shotblasted samples, presenting 37 and 17%, respectively.
After 4 weeks, the shot blasting surface continued to show the drilling grooves and primary contact, with some initial secondary contact. The ContacTi surface showed great osteoconductivity, with the presence of large amounts of immature bone growing from the implant surface, as well as bone remodeling due to the mechanical stimulation (Fig. 6). At this time point, the amount of NB increased for the shotblasted up to 24%, while the amount of ContacTi NB increased up to 59% (Fig. 8).
After 8 weeks, the ContacTi-treated implants presented a large amount of lamellar tissue deposited in the implant interthread gaps. The contact base that forms the bone extension with the implant surface was much greater than the thickness of the bone trabecula from which it originates. There was a large amount of mature lamellar tissue (type II) and little vascular space, therefore indicating a high level of maturity and bone quality, which indicated advanced stage bone remodeling. The amount of NB after 8 weeks was 47 and 55% for the shotblasted and ContacTi, respectively (Fig. 8). The shot blasting surface showed a slower maturation process (Fig. 9). It is worth highlighting that in the ContacTi samples at 8 weeks shown in Fig. 9, there seems to be the presence of osteoclastic actions, resorbing old bone, as shown by the Howship’s lacunae indicated with red arrows, which tend to resorb old bone forming islets of new bone. The control samples presented lower signs of osteoclastic actions.
Discussion
Titanium is inherently bioinert which hinders the stimulation of the surrounding bone in order to achieve greater and faster bone regeneration. In order to overcome this issue, the coating of the implant with some sort of osteoconductive calcium phosphate such as apatite—the bone’s mineral phase—was thought as a possible improvement for this problem, as it could improve the implant’s performance immediately after its insertion, by interacting with the native bone and facilitate a quick bone regeneration around the mineral layer [26, 27]. Over the past years, various attempts have been made to cover the implant with this mineral, applying a thick layer over the implant surface by a plasma spray process or by electrodeposition [28, 29]. However, in-depth characterization of the deposited calcium phosphate demonstrated that these techniques lead to the degradation, fragmentation, and mechanical failure of the coating with medium and long-term implant failure [30]. For this type of surface deposition of HA, a fast primary osseointegration occurs which is then followed by the dissolution of the coating, which in turn results in a significant gap between the implant and the bone, producing mobility and implant failure. It has been previously shown that the presence of calcium ions on the surface which may induce the formation of HA, rather than the deposition of HA nanocrystals or the presence of a hydrophilic rough surface, may enhance bone apposition on the early stages of bone remodeling to a higher extent [31, 32]. Therefore, we consider that the novel ContacTi surface solves the aforementioned problems, by forming a crystalline apatite layer with higher chemical and mechanical stability, forming a chemical bond to the titanium to avoid fractures and debondings. The treatment with a strong alkaline solution results in the formation of sodium titanate, which is negatively charged, that allows, when reacting with calcium cations and phosphate anions, the formation of an apatitic calcium phosphate layer, which densifies with the thermal treatment.
The increased efficiency of the novel ContacTi surface has been demonstrated in the present study by comparing the results with another surface, the shot blasting surface, which has been extensively used and tested with several years of clinical experience. ContacTi surface histomorphometric data were clearly superior to the shot blasting surface in all measurements of this study, showing that the percentage of BIC was 10% higher at 2 weeks (49 vs 39%), 37% higher at 4 weeks (83 vs 46%), and 39% higher at 8 weeks (85 vs 46%). Regarding BAD, the data presented superior values for the new surface, presenting a 3.6% higher value at 2 weeks (26 vs 22%), 25% at 4 weeks (61 vs 36%), and 10% at 8 weeks (59 vs 49%).
The previously stated data shows that the new ContacTi surface presents an improved performance in the osseointegration process when compared to the classical surface, accelerating and achieving better results in the histomorphometric parameters. These results could evidence that the apatite layer formed over the implant surface when in contact with the surrounding bone has an adequate surface roughness achieved by the formation of bone like material on its surface, promoting osteoblastic migration, bonding, proliferation, and differentiation. Several previous studies have shown results that are in agreement with the findings in the present study, in which it was stated that an increased surface roughness stimulates osteogenesis and changes the expression of integrin and cellular growth factor [23, 33,34,35,36].
Several studies have performed histomorphometric analysis in animal models to assess the behavior of new surfaces with respect to peri-implant bone healing. It is worth highlighting that the different sizes and metabolisms of the different animal models make comparison as general guidelines although differences are expected [33]. Gahlert et al. [34] in 2012 compared the tissue response of acid etched zirconia (ZrO2) implants to a sand blasting and acid etching surface (SLA) that served as experimental control. BAD values were 60.4% at 4 weeks, 65.4% at 8 weeks, and 63.3% at 12 weeks, while BIC values were 70, 67.1, and 68.3% for the same time points for the experimental group. The values of our study for the bioactive surface were similar regarding the BAD values, but presented much higher BIC values at 4 and 8 weeks (83.2 and 85.5%).
One of the most studied surfaces in the last years has been the SLActive (Institut Straumann AG, Basel, Switzerland), which is a modification of the classically obtained sand blasted and acid etch surface (SLA), submitted to a nitrogen atmosphere to avoid the contact of passive air elements to come in contact with implant surface and stored in a isotonic saline solution, achieving the hydroxylation of the titanium oxides without changes in the surface topography with increased wettability. Buser et al. [23] implanted the mentioned implants in a minipig animal model, showing BIC values significantly higher at 2- and 4-week time points than the control SLA surface with values of 49.3, 81.9, and 78.4% at 2, 4, and 8 weeks, respectively. These values match ours at 2 and 4 weeks, with the result for ContacTi of 85.5% at 8 weeks versus 78.4% achieved in the cited study. It is noteworthy that in this study, at 8 weeks, BIC values were equal for both surfaces. Since the topography was the same, the authors concluded that difference in favor of the SLActive rested in the chemical changes of the surface. A similar study was undertaken by Schwarz et al. [35], obtaining a BIC value of 74% for the modified SLActive surface while the standard surface achieved a value of 56%. However, at 12 weeks, this difference was reduced without any statistical significance (84 vs 76%). The results therefore indicated that the modified bioactive surface improved bone apposition only in the initial integration stages. The difference surface properties between the SLA and SLactive were clearly indicating the effect of the surface roughness and surface hydrophobicity in the initial periods of bone recovery, despite at longer time points (8 weeks), the differences are not significant [36, 37]. This was also related with a higher ability of the implant to induce the formation of new bone, showing that ContacTi allows an enhanced new bone formation and these values are slightly higher than those showed in the literature at short times, although the values tend to be similar at longer time points [38].
The ContacTi surface achieved a faster growth of hard tissues around the implants, when compared to the shot blasting surface, and for all evaluated histomorphometric and histological parameters, the values were higher after 4 and 8 weeks. Similarly, this surface showed osteoconductive behavior with bone growth from the implant surface in the first 2 weeks, although other studies showed direct bone growth at 3 days after placement [39, 40,41,42,43]. All this could be consequence of the stimulating effect that the surface treatment on the bone growth, which can be attributed to the formation of the hydroxyapatite layer on its surface after placement in the bone tissue with contact with body fluids, as demonstrated by Aparicio et al. [19].
These results are very encouraging, but before being clinically tested, new animal model studies should be carried out evaluating shorter time points and relating the histomorphometric results with different clinical variables, such as implant stability. After adequate clinical trials in order to validate these results, shorter loading times can be proposed as well as its use in situations of maximum demand, such as immediate loading in poor quality bones.
Conclusions
The new blasted, alkaline-etched, and thermally treated surfaces produced micro-rough and bioactive implants that accelerated bone tissue regeneration in the bone bed at short time periods of implantation in comparison with control implant tested. This new surface can rapidly stimulate (a) bone nucleation directly on the implant surface and (b) bone growing from the implant surface. We consider that ContacTi implants are good candidates to be used in short-term loading clinical scenarios.
References
Steigenga JT, al-Shammari KF, Nociti FH et al (2003) Dental implant design and its relationship to long-term implant success. Implant Dent 12:306–317. https://doi.org/10.1097/01.ID.0000091140.76130.A1
Aljateeli M, Wang HL (2013) Implant microdesigns and their impact on osseointegration. Implant Dent 22:127–132. https://doi.org/10.1097/ID.0b013e318278a90b
Albrektsson T, Wennerberg A Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont 17:536–43
Albrektsson T, Wennerberg A (2004) Oral implant surfaces: Part 2--review focusing on clinical knowledge of different surfaces. Int J Prosthodont 17:544–564. https://doi.org/10.1098/rsta.2009.0062
Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF (2000) Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 22:87–96. https://doi.org/10.1016/S0142-9612(00)00174-5
Lamers E, Frank Walboomers X, Domanski M et al (2010) The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition. Biomaterials 31:3307–3316. https://doi.org/10.1016/j.biomaterials.2010.01.034
Lincks J, Boyan BD, Blanchard CR et al (2006) Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomater Silver Jubil Compend 19:147–160. https://doi.org/10.1016/B978-008045154-1.50019-8
Von Der Mark K, Park J, Bauer S, Schmuki P (2010) Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res 339:131–153. https://doi.org/10.1007/s00441-009-0896-5
Aparicio C, Gil FJ, Fonseca C et al (2003) Corrosion behaviour of commercially pure titanium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 24:263–273
Gil FJ, Planell JA, Padrós A (2002) Fracture and fatigue behavior of shot-blasted titanium dental implants. Implant Dent 11:28–32
Stanford CM (2008) Surface modifications of dental implants. Aust Dent J. https://doi.org/10.1111/j.1834-7819.2008.00038.x
Bressan E, Sbricoli L, Guazzo R et al (2013) Nanostructured surfaces of dental implants. Int J Mol Sci 14:1918–1931. https://doi.org/10.3390/ijms14011918
Novaes AB, de Souza SLS, de Barros RRM et al (2010) Influence of implant surfaces on osseointegration. Braz Dent J 21:471–481. https://doi.org/10.1590/S0103-64402010000600001
Mendes VC, Moineddin R, Davies JE (2009) Discrete calcium phosphate nanocrystalline deposition enhances osteoconduction on titanium-based implant surfaces. J Biomed Mater Res - Part A 90:577–585. https://doi.org/10.1002/jbm.a.32126
Tanaka Y, Matin K, Gyo M et al (2010) Effects of electrodeposited poly(ethylene glycol) on biofilm adherence to titanium. J Biomed Mater Res - Part A 95:1105–1113. https://doi.org/10.1002/jbm.a.32932
Kokubo T, Miyaji F, Kim H-M, Nakamura T (1996) Spontaneous Formation of Bonelike Apatite Layer on Chemically Treated Titanium Metals. J Am Ceram Soc 79:1127–1129. https://doi.org/10.1111/j.1151-2916.1996.tb08561.x
Yan WQ, Nakamura T, Kawanabe K et al (1997) Apatite layer-coated titanium for use as bone bonding implants. Biomaterials 18:1185–1190. https://doi.org/10.1016/S0142-9612(97)00057-4
Yan WQ, Nakamura T, Kobayashi M et al (1997) Bonding of chemically treated titanium implants to bone. J Biomed Mater Res 37:267–275. https://doi.org/10.1002/(SICI)1097-4636(199711)37:2<267::AID-JBM17>3.0.CO;2-B
Aparicio C, Padrós A, Gil F-J (2011) In vivo evaluation of micro-rough and bioactive titanium dental implants using histometry and pull-out tests. J Mech Behav Biomed Mater 4:1672–1682. https://doi.org/10.1016/j.jmbbm.2011.05.005
Aparicio C, Manero JM, Conde F et al (2007) Acceleration of apatite nucleation on microrough bioactive titanium for bone-replacing implants. J Biomed Mater Res - Part A 82:521–529. https://doi.org/10.1002/jbm.a.31164
Gil F, Padrós A, Manero J et al (2002) Growth of bioactive surfaces on titanium and its alloys for orthopaedic and dental implants. Mater Sci Eng C 22:53–60. https://doi.org/10.1016/S0928-4931(01)00389-7
Buser D, Nydegger T, Oxland T et al (1999) Interface shear strength of titanium implants with a sandblasted and acid-etched surface: a biomechanical study in the maxilla of miniature pigs. J Biomed Mater Res 45:75–83
Buser D, Broggini N, Wieland M et al (2004) Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res 83:529–533. https://doi.org/10.1177/154405910408300704
Germanier Y, Tosatti S, Broggini N et al (2006) Enhanced bone apposition around biofunctionalized sandblasted and acid-etched titanium implant surfaces: A histomorphometric study in miniature pigs. Clin Oral Implants Res 17:251–257. https://doi.org/10.1111/j.1600-0501.2005.01222.x
Donath K, Breuner G (1982) A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol 11:318–326
Puleo D, Nanci A (1999) Understanding and controlling the bone–implant interface. Biomaterials 20:2311–2321. https://doi.org/10.1016/S0142-9612(99)00160-X
Rønold HJ, Lyngstadaas SP, Ellingsen JE (2003) Analysing the optimal value for titanium implant roughness in bone attachment using a tensile test. Biomaterials 24:4559–4564. https://doi.org/10.1016/S0142-9612(03)00256-4
Geesink RGT, De Groot K, Klein CPAT (1987) Chemical Implant Fixation Using Hydroxyl-Apatite Coatings. Clin Orthop Relat Res:147–170
Shirkhanzadeh M (1991) Bioactive calcium phosphate coatings prepared by electrodeposition. J Mater Sci Lett 10:1415–1417. https://doi.org/10.1007/BF00735695
Hulshoff JEG, Hayakawa T, Van Dijk K et al (1997) Mechanical and histologic evaluation of Ca-P plasma-spray and magnetron sputter-coated implants in trabecular bone of the goat. J Biomed Mater Res 36:75–83. https://doi.org/10.1002/(SICI)1097-4636(199707)36:1<75::AID-JBM9>3.0.CO;2-I
Favero R, Botticelli D, Antunes AA et al (2016) Sequential Healing at Calcium- versus Calcium Phosphate-Modified Titanium Implant Surfaces: An Experimental Study in Dogs. Clin Implant Dent Relat Res 18:369–378. https://doi.org/10.1111/cid.12311
Favero V, Lang NP, Favero R et al (2016) Sequential morphometric evaluation at UnicCa(®) and DCD(®) implant surfaces. An experimental study in the dog. Clin Oral Implants Res. https://doi.org/10.1111/clr.12888
Botticelli D, Lang NP (2016) Dynamics of osseointegration in various human and animal models - a comparative analysis. Clin Oral Implants Res. https://doi.org/10.1111/clr.12872
Gahlert M, Roehling S, Sprecher CM et al (2012) In vivo performance of zirconia and titanium implants: A histomorphometric study in mini pig maxillae. Clin Oral Implants Res 23:281–286. https://doi.org/10.1111/j.1600-0501.2011.02157.x
Schwarz F, Herten M, Sager M et al (2007) Bone regeneration in dehiscence-type defects at chemically modified (SLActive®) and conventional SLA titanium implants: A pilot study in dogs. J Clin Periodontol 34:78–86. https://doi.org/10.1111/j.1600-051X.2006.01008.x
Lang NP, Salvi GE, Huynh-Ba G et al (2011) Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res 22:349–356. https://doi.org/10.1111/j.1600-0501.2011.02172.x
Bosshardt DD, Salvi GE, Huynh-Ba G et al (2011) The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin Oral Implants Res 22:357–364. https://doi.org/10.1111/j.1600-0501.2010.02107.x
Favero R, Lang NP, Salata LA et al (2016) Sequential healing events of osseointegration at UnicCa(®) and SLActive(®) implant surfaces: an experimental study in the dog. Clin Oral Implants Res 27:203–210. https://doi.org/10.1111/clr.12591
Rossi F, Lang NP, De Santis E et al (2014) Bone-healing pattern at the surface of titanium implants: An experimental study in the dog. Clin Oral Implants Res 25:124–131. https://doi.org/10.1111/clr.12097
Gil FJ, Manzanares N, Badet A et al (2014) Biomimetic treatment on dental implants for short-term bone regeneration. Clin Oral Investig 18:59–66. https://doi.org/10.1007/s00784-013-0953-z
Groessner-Schreiber B, Tuan RS (1992) Enhanced extracellular matrix production and mineralization by osteoblasts cultured on titanium surfaces in vitro. J Cell Sci 101 ( Pt 1:209–217
Fischer K, Stenberg T (2004) Early loading of ITI implants supporting a maxillary full-arch prosthesis: 1-year data of a prospective, randomized study. Int J Oral Maxillofac Implants 19:374–381
Gottlow J, Dard M, Kjellson F et al (2012) Evaluation of a New Titanium-Zirconium Dental Implant: A Biomechanical and Histological Comparative Study in the Mini Pig. Clin Implant Dent Relat Res 14:538–545. https://doi.org/10.1111/j.1708-8208.2010.00289.x
Acknowledgments
The authors would like to acknowledge the Ministry of Science of Spain for funding to this project (MAT2012-30706) as well as Klockner, S.L. for donating the implants used.
Funding
The work was supported by the Spanish government. Ministerio Economía y Competitividad by the research project number MAT2012-30706.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The present study was carried out in maxillae of 12 six-year-old female minipigs in the Córdoba University’s Servicio Centralizado de Animales de Experimentación located in the Campus de Rabanales and approved by the University of Seville Ethics Experimentation Committee (MED2016-01-324). All requirements and regulations for animal experimentation, according to the Spanish and European Union, were fulfilled.
Conflict of interest
Mariano Herrero-Climent declares that he has no conflict of interest. Manuel María Romero declares that he has no conflict of interest. Pedro Lázaro declares that he has no conflict of interest. José Vicente Rios declares that he has no conflict of interest, and F. Javier Gil Mur declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants. For the animal study, the study was approved by the University of Seville Ethics Experimentation Committee (MED2016-01-324).
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Herrero-Climent, M., Romero Ruizª, M.M., Calvo, P.L. et al. Effectiveness of a new dental implant bioactive surface: histological and histomorphometric comparative study in minipigs. Clin Oral Invest 22, 1423–1432 (2018). https://doi.org/10.1007/s00784-017-2223-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2223-y