Abstract
Objectives
This study aimed at identifying and quantifying Actinomyces naeslundii, Bifidobacterium spp., Streptococcus mitis group, Lactobacillus acidophilus, Lactobacillus casei group, Streptococcus gordonii, and Streptococcus mutans in active and inactive carious dentine lesions of children with early childhood caries by using quantitative polymerase chain reaction.
Material and methods
Fifty-six dentin lesion samples, classified as active (n = 39) or inactive (n = 17), were collected from children aged from 2 to 5 years old. Dentinal-cavitated lesions were evaluated by Nyvad criteria for the assessment of caries lesion activity.
Results
Relative quantification revealed that Bifidobacterium spp. and the L. casei group were significantly more abundant in active dentin lesions (p < 0.05). Concentrations of A. naeslundii, S. mitis group, and S. gordonii were not significantly different when comparing dentin lesion activity. The relative proportion of S. mutans was significantly greater in inactive than in active lesions (p < 0.05). Bifidobacterium spp. and L. casei group demonstrated a positive correlation (p = 0.001) in active lesions. The positive detection of L. acidophilus (odds ratio = 15.1) and S. gordonii (odds ratio = 7.7) was significantly associated to the active lesions.
Conclusions
The data indicate that higher detection levels of Bifidobacterium spp. and the L. casei group may be linked to dentin lesion activity. Additionally, the presence of L. acidophilus and S. gordonii was associated with lesion activity.
Clinical relevance
Considering that information about the oral microbiota related to dentin caries activity status is relevant, this study provides insights to better understand the differences in the microbiotas between active and arrested dentin cavities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental caries is one of the most prevalent diseases of childhood worldwide, especially in socially disadvantaged populations [1]. Several factors, including microbial, genetic, immunological, behavioral, and environmental, are involved and contribute to its development [2]. In preschool children, in particular, this condition defined as early childhood caries (ECC), can devastate the primary teeth, affect the child’s self-esteem, impact general health, and lead to nutritional deficiency [3]. Due to the aggressive pattern of ECC, areas of demineralization can rapidly progress, develop cavitation, and involve dental pulp tissues, causing serious consequences such as pain and pulp infection [4, 5].
Cavitated dentin carious lesions are considered as the last stage of dental caries and also diverse ecosystems with high variability in microbiota [6]. Dentin provides a different environment for bacteria involved in caries progression, where only specialized bacteria are able to colonize and exploit [7]. The bacterial profile in enamel and dentinal caries are significantly different [5, 7], since the microbiota in dentin is constantly submitted to changes, such as nutrient availability, oxygen concentration, and pH [8, 9]. Moreover, this tissue contains a higher proportion of organic matrix and a lower inorganic component than enamel. Thus, the critical pH for dentin dissolution is higher when compared to enamel, which allows colonization of bacteria that may not be as acidogenic and aciduric as those required for initial enamel demineralization [10].
The advent of molecular research that characterizes the oral microbiota in health and disease revealed the diversity of oral biofilms and dentinal caries, introducing new candidates for disease-associated bacterial species [11–14]. Considering that the microbiota involved in dental caries are known to be highly diverse and variable [9, 15], understanding the microbial etiology of caries and how environmental conditions in the oral cavity impact the disease process continues to change as technology advances [16]. Even though the strong association of mutans streptococci and ECC is established in the literature [1, 17–19], it seems that these bacteria are not present in all children with caries [12, 20]. Beyond S. mutans, molecular approaches have revealed a greater variability of the community of dentine caries-associated microbiota including Streptococcus spp. and bacteria of the genera Actinomyces, Bifidobacterium, Lactobacillus, Propionibacterium, Veillonella, Selenomonas, and Atopobium [6, 11, 12, 15, 21–23].
On the other hand, it is possible that other microorganisms contribute to the inactivation of caries lesions, since several streptococci, such as members of the S. mitis group and S. gordonii are able to produce alkalis by the arginine deiminase system (ADS) [24]. These bacteria generate ammonia that neutralizes acids in cariogenic biofilms and favors pH increase, which could explain the pH of arrested lesions as previously demonstrated [25, 26]. However, the role of these bacteria in caries progression remains inconclusive.
Research into the microbial communities present in dentinal caries lesions is not only important in understanding the pathogenesis of dentinal caries, but also in developing novel approaches to dental caries treatment [16]. Early in the caries process, the pulp reflects changes within lesion activity [27]. However, little is known about the dynamic characteristics of oral microbiota in caries progression [28] and how the oral bacterial community changes within carious lesion activity [9, 16].
Molecular studies dealing with the microbiota in dentinal caries have mainly focused on comparing plaque samples within the same patient [11–14, 29], rarely defining dentinal lesion activity, which may reveal different microbial patterns [9]. Therefore, the aim of the present study was to quantify Actinomyces naeslundii, Bifidobacterium spp., Lactobacillus acidophilus, Lactobacillus casei group, Streptococcus mitis group, Streptococcus gordonii, and Streptococcus mutans in active and inactive dentine carious lesions from severe ECC children and also to evaluate whether caries lesion activity could be linked to a certain microbial composition.
Methods
Ethics statement
The study protocol was approved by the ethics committee of the Federal University of Ceará, Brazil (COMEPE/UFC) (Protocol Number 158/2011). Verbal and written consents were obtained from the parents of all subjects. Samples were taken only after obtaining the approval from the children and their parents.
Study population
Thirty-nine subjects aged from 2 to 5 years old with severe ECC were recruited for this study from public preschools in Fortaleza, Ceará, Brazil. The schools were selected based on convenience and located in a suburban area of the city. A total of 420 children was examined in the first phase of the study in order to select the study population. Patients were excluded from the study if history of significant medical disease or antimicrobial therapy was reported by their parents within the last 3 months prior to the study. None of the subjects had salivary gland disorders or systemic diseases, and none showed spontaneous symptoms associated with the carious lesions. Informed consent of the respective children and their families, their willingness to participate, and the presence of at least one cavitated dentinal carious lesion with no pulp exposure in primary teeth were used as inclusion conditions.
The presence of dental caries was assessed using the International Caries Detection and Assessment System (ICDAS) II [30, 31]. Children were examined under standardized conditions by two calibrated examiners (BGN and DSB). Professional oral cleaning was performed prior to clinical examination. A WHO periodontal probe, a mirror, an air syringe, and adequate illumination were used by the examiners during the clinical evaluation.
Dentinal cavitated lesions were evaluated by the Nyvad criteria [32] for the assessment of caries lesion activity, based on visual and tactile diagnoses. The teeth were examined after air-drying for 5 s. The examiners reviewed the clinical appearance of the selected sites based on color, opacity, and the presence of surface discontinuities or cavities. Gentle probing was used to assess the lesion surface integrity or texture (rough or smooth) and to remove dental plaque, if needed or not removed by professional oral cleaning. Dentine cavities classified as active were those easily visible with the naked eye, soft on gentle probing, and with a whitish/yellowish appearance. Inactive lesions were characterized by a whitish/brown/black shiny appearance and were smooth/hard on gentle probing. Each site was ranked according to the scores proposed for caries lesion activity [32].
All children involved in this study were enrolled in a dental care program that included preventive counseling and dental treatment at the dental clinic of the Faculty of Dentistry at the Federal University of Ceará in Fortaleza, Ceará, Brazil.
Sampling and clinical data collection
All sample collections were performed by two calibrated dentists at the dental clinic of the Dental School at the Federal University of Ceará located at Fortaleza, Ceará, Brazil. Samples were collected from 39 children over a period of 15 months.
The dentinal caries lesions selected were opened cavities scored with codes 5 (distinct cavity with visible dentin) and 6 (extensive distinct cavity with visible dentin), according to the ICDAS II criteria [30]. Selected teeth had no clinical signs or symptoms of irreversible pulpitis. Absence of pulp exposure and radiolucent areas were evaluated radiographically.
After being classified as active or inactive, as previously reported, one to three dentin carious samples were collected from each child. Samples were not pooled across dentin lesions or patients. Carious samples were collected from cavitated dentine lesions from primary teeth with local anesthesia under rubber dam isolation with a sterile spoon excavator in order to reduce the risk of saliva contamination. Prior to sampling procedure, dental plaque on the surfaces of cavitated dentin lesions was swiped. Subsequently, all cavities were restored with resin-modified glass ionomer cement (Vitro Fil LC, DFL, Rio de Janeiro, Brazil). When necessary, an indirect pulp capping with calcium hydroxide liner was placed. Dentine carious samples were placed in a sterile 1.5-mL microcentrifuge tube containing 150 μL of TE (10 mM Tris-HCl, 1 mM EDTA pH 7.6) and immediately transported on ice to the laboratory, where they were frozen at a temperature of −20 °C until analysis.
Laboratory methods
Bacterial strains and culture conditions
Quantitative PCR was performed to detect the presence/absence and to quantify targeted bacterial DNA in carious dentin samples. The presence of A. naeslundii, Bifidobacterium spp., L. acidophilus, L. casei group, S. mitis group, S. gordonii, and S. mutans was investigated using specific forward and reverse primers as listed on Table 1.
The bacteria strains used as positive controls to test the specificities of the primers included A. naeslundii (ATCC 12104), Bifidobacterium animalis subsp. lactis BB-12 ® (Chr. Hansen), L. acidophilus (ATCC 4356), Lactobacillus paracasei subsp. paracasei (ATCC 335), S. gordonii (ATCC 35105), Streptococcus mitis (ATCC 49456—NTCC 12261), and S. mutans (UA 159).
Isolated bacteria were cultured in broth for 24 h as recommended by the Bergey’s Manual of Determinative Bacteriology [39]. The cells were centrifuged and washed in sterile saline solution (sodium chloride 0.9%). The quality and purity of bacterial cultures were checked by Gram staining.
Extraction and purification of DNA from dentinal samples and bacterial cultures
All samples were transferred into a fresh 2-mL screw cap tube. Mechanical disruption of cells was carried out with 0.16 g of 0.1-mm diameter zirconia beads (Biospec Products, Bartlesville, OK, USA) on a Mini-beadbeater (Biospec Products) at maximum power for 60 s. DNA was recovered from all samples using an organic extraction protocol based on phenol/chloroform purification and alcohol precipitation [40]. The DNA concentration (A260) and purity (A260/A280) of the samples were evaluated using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Electrophoresis of the extracted DNA was performed on a 1.2% agarose gel in Tris/borate/EDTA buffer and stained with 0.1 μg/mL ethidium bromide.
Quantification of target bacterial DNA in dentinal samples by qPCR
Serial dilutions starting from 300 to 0.0003 ng (10-fold) of reference bacterial DNA concentrations were used as standards and positive controls for relative quantification of the targeted bacteria. A standard amplification curve and a melting-point product curve were obtained for each primer set. Amplifications of qPCR were performed using a MicroAmpFast Optical 48-Well Reaction Plate (Applied Biosystems, Foster City, CA, USA) covered with Optical Adhesive Film (Applied Biosystems) in a StepOne Real-Time PCR System (Applied Biosystems). Each reaction mixture (10 μL) contained 5 μL of 2x Power SYBR Green Mastermix (Applied Biosystems), 0.3 μL of each forward/reverse primer 10 μM, 2 μL of DNA sample, and 2.4 μL nuclease-free water. Assays were carried out in duplicate, and the final analyses were based on the mean of the two reactions. Negative control included reactions without template. The standard curves were used to transform the cycle threshold (Ct) values to the mass of DNA, and the results of the concentrations of bacteria in carious dentin samples were normalized relative to the total bacterial load estimated by the primer Bacteria 16S rDNA [33].
Statistical analysis
Data were tabulated in Microsoft Excel and exported to a statistical software Statistical Package for Social Sciences (SPSS) version 17.0, on which all analyses were performed considering a confidence level of 95%.
After examining the pattern of sample distribution (normality test Kolmogorov-Smirnov test), data were expressed as the mean and standard deviation and compared between groups using the Kruskal-Wallis test followed by post-test Mann-Whitney with Bonferroni correction (nonparametric data). For bacterial prevalence data, the values of the concentrations of the bacterial species were calculated as a percent of the total bacterial load.
Data were dichotomized according to the presence or absence of bacteria in active and inactive dentin carious lesions and stratified according to higher or lower values than the median proportion for bivariate analysis (chi-square test). Presence and absence of the bacteria in dentine lesions were described and also relative median of all groups was considered for this analysis. A Spearman correlation (nonparametric data) was performed for evaluation of the interaction between different bacteria and lesions activity (active or inactive).
Results
A total of 56 carious dentin samples were collected from cavitated dentin lesions (ICDAS 5, 6). Dentin samples were divided into two groups: active (n = 39) and inactive (n = 17) lesions, according to Nyvad criteria [32]. From one to three samples were collected from each patient, and five children had lesions of both types.
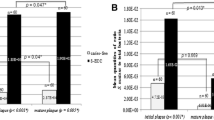
The concentrations of each strain were obtained by normalization to total bacteria present in the same dentine sample as determined using specific primers. Table 2 details the mean and median values of the prevalence of oral bacteria in active and inactive dentin carious lesions. Active lesions presented significantly higher concentrations of Bifidobacterium spp. and the L. casei group (p < 0.05) compared to inactive lesions. Concentrations of A. naeslundii, S. gordonii, and the S. mitis group were not significantly different when comparing active and inactive dentin lesions. The relative proportion of S. mutans was significantly greater in inactive than in active lesions (p < 0.05).
The presence and absence of the bacteria in dentine lesions and also relative median of all groups are summarized in Table 3 . L. acidophilus was completely absent in all inactive carious dentin samples. S. mutans was identified in all samples of the current study. The L. casei group was absent in about 40% of inactive lesions. Considering presence in a carious dentin lesion in a bivariate analysis, L. acidophilus showed a statistically significant association with active dentin lesions (OR = 15.1) and also S. gordonii presented a greater chance to be present in active lesions (OR = 7.7). Only 29.4% of the inactive dentin samples presented levels of the L. casei group higher than the median value of both groups (p < 0.05; OR = 3.5) (Table 3).
According to the results of Spearman’s rank correlations, there was a significant inverse correlation between L. casei group and S. mutans in active dentine lesions (p = 0.039); when analyzing the bacterial relationships in the different groups, Bifidobacterium spp. and the L. casei group demonstrated a positive correlation (p = 0.001). A significant direct correlation was observed between the S. mitis group and S. gordonii in both active and inactive dentin lesions (Supplemental Tables).
Discussion
The microbiota in dental caries is highly complex and varies between individual lesions [15, 41]. Consequently, etiological studies must focus on site-specific analyses [7]. It has been suggested that the proportions and numbers of acid-base-producing bacteria are the core of dental caries activity [42]. Different proportions of some bacterial populations in active and inactive dentin lesions were observed in the current study.
The current results showed a higher concentration of the L. casei group in active dentin lesions when compared to the arrested lesions, which was previously demonstrated by a study that verified these bacteria as dominant in active dentine lesions in adults [26]. Although present in low quantity, the presence of L. acidophilus was related to dentin lesion activity and it showed a statistical significant association to active dentin lesions (OR = 15.1), since it was completely absent in all inactive carious dentin samples. These findings demonstrated that dentin lesions, where these bacteria are present, were 15.1 times more likely to be active. Lactobacillus spp. have the ability to produce organic acids, promoting low levels of pH and being responsible for the decalcification of the dentinal matrix [5, 43], which is a common situation in active lesions. The link between Lactobacillus count and carious decay is unquestionable. However, the relationship between the Lactobacillus count and the carious activity remains to be established [44]. L. casei and L. paracasei were frequently isolated from dentine sites in ECC [45]. Moreover, Lactobacilli have shown robust association with more advanced stages of caries in many studies [5, 7, 8, 12, 21, 23, 28, 43] and have also been implicated in the initial stages of pulp infection [46], indicating that they present a pathogenic potential and play a crucial role in caries progression.
Low proportions of Bifidobacterium spp. were detected in dentine lesions in the current study, which is in accordance with a previous study on adult dentine lesions [7]. Interestingly, a significantly higher proportion was verified in active ECC dentine lesions compared to inactive lesions, in agreement with an earlier study that isolated Bifidobacterium spp. from soft and active dentine lesions in primary teeth [22]. Bifidobacteria have been detected in dentine carious lesions [7, 11, 12, 21], suggesting that these bacteria may be implicated in dental caries progression [22], since these species are acidogenic and aciduric and also known to produce lactate [22, 47]. Additionally, another study has observed spatial distribution of bacterial taxa in vivo with confocal microscopy, showing a bacterial invasion into the dentinal tubules of Bifidobacterium inside cavitated caries lesions [48].
Several studies have associated S. mutans with progressive stages of caries and have detected these bacteria in cavitated lesions in dentin [12, 22, 45], and this was confirmed by the present results, since all dentine samples examined harbored S. mutans. Mutans streptococci comprise about 30% of the total microbiota according to microbial culture approaches [49, 50]. These species may form biofilm on dentine, and incorporate and collaborate with various bacteria for induction of dentine acidulation [51]. Surprisingly, S. mutans was identified with higher concentration in arrested lesions, which is not in agreement with Kuribayashi et al. [26], who demonstrated a high prevalence of S. mutans in carious dentine lesions regardless of the caries activity. These discrepant results may be due to the studied population, as their study evaluated adults. It has been suggested that S. mutans have a more dominating role in dentine and deep dentine caries of primary teeth that in permanent teeth [12].
Other studies have demonstrated a low prevalence or even an absence of S. mutans in dentinal caries lesions [6, 7], contradicting the findings of this study. However, it must be emphasized that the earlier findings were based on smaller samples. Possible reasons for different detection levels of S. mutans in different studies are probably related to different approaches used for species detection, including the DNA extraction and the bacterial lysis method, as well as PCR and primer bias [18, 23]. In addition, it was observed that the oral microbiota is diverse in different ethnicities and races, which may explain discrepancies in the composition of the microbiota in dentinal caries in different studies [5, 14].
One particularly interesting finding of this study was the positive correlation between bacteria from the L. casei group and Bifidobacterium spp. in active dentine lesions. Both bacterial genera are commonly detected in ECC lesions [11, 12], where they play important roles in lowering the pH in active lesion environments and proliferating in acidic carious lesions [22]. A similar result was found in a study with active dentine lesions in deciduous teeth that verified a correlation between the proportion of Bifidobacterium spp. and Lactobacillus spp. [22]. S. mutans and L. casei group presented a negative correlation in active dentine lesions, which is supported by the idea that mutans streptococci and lactobacilli are more competitive under severely acidic conditions [9]. This finding corroborates with the results of a previous in vitro study in which the lactobacilli were capable of inhibiting the growth of mutans streptococci, being L. paracasei, Lactobacillus plantarum, and Lactobacillus rhamnosus the species with maximum interference capacity against mutans streptococci [52].
In this study, the presence of S. gordonii was significantly associated with active dentine lesions. The data is consistent with those reported by Peterson et al. [2] in a dental plaque microbiome study in which S. gordonii was associated with caries activity and also with a metagenomic study that detected abundance of this species in caries-active individuals [6]. The role of S. gordonii in dental caries is still undefined [53]. Despite being considered as a pioneer for dental plaque formation and associated with health [29], an in vitro study showed that these bacteria were able to increase their acid tolerance and acidogenicity when exposed to an acidic environment [54]. However, the contribution of S. gordonii on the acidification of dentin remains unclear and deserves further investigation.
With regard to A. naeslundii, no statistically significant difference was found between active and inactive lesions, which is not surprising as Actinomyces spp. are prevalent in oral cavity and frequently found in association with both carious and sound surfaces [45]. In addition, these species have been associated with dentin and root caries in adults [16, 55] and does not seem to play a relevant role in childhood caries [11]. Likewise, although members of the mitis group were previously detected in active carious dentine lesions in adults and children [7, 45], they have been frequently associated with health [13, 29], making the contribution of these bacteria to caries inactivation inconclusive.
Other microorganisms, like Candida spp., have been proposed to produce acid and contribute to dental caries. However, the role of Candida species in this disorder has not been clearly established. Studies have indicated a relationship between the presence of Candida species and dental caries progression [7], reflecting the polymicrobial etiology of dental caries although the invasion of these species into carious dentin has been questioned [56]. Since our goal was to mainly identify bacterial differences in active and inactive dentine caries lesions in primary teeth, fungal analysis was not accomplished in this study.
Real-time PCR was the chosen method to this study, since the use of qPCR is an accepted technology for the quantitative analysis of bacteria from mixed samples. Furthermore, this methodology allows the microorganisms to be assessed more accurately than they can be by cultural analysis. Quantitative PCR has the potential to account for the uncultivable portion of the oral microbial community, as well as, species which are more difficult to culture [57]. According to Chhour et al. [21], real-time PCR analysis of the total bacterial load in advanced carious lesions has shown that the total load exceeds the number of cultivable bacteria. A limitation of this methodology is that qPCR cannot distinguish between viable and non-viable bacterial cells, even though a recent technique has been proposed to assess the viability of live and dead cells with qPCR [58]. However, it seems that this study presents important findings, since differences in the microbiotas between active and arrested cavities have not yet been elucidated, according to Takahashi & Nyvad [59].
Moreover, the current study did not evaluate the bacterial composition among different depths in the same lesion. Nevertheless, no significant difference in the microbial composition in the different layers of dentinal caries lesions has been reported [8, 23]. In addition, stratified analysis of dentinal caries might be possible only with in vitro studies evaluating extracted teeth where sampling could be precisely taken from different zones, which may provide information about the dynamics of the disease process even in a cross-sectional study. In the present study, the dentine caries was well established and in an advanced stage of progression or arrest, and therefore was not representative of the initial stages of carious lesions.
Contamination during sampling technique must not be considered, since sampling of carious dentine was carried out carefully by rubber dam isolation and after removal of dental plaque and debris. Additionally, our study analyzed an in vivo situation with children with severe ECC, differently from numerous studies with dentine caries that commonly evaluated extracted carious teeth [10, 16, 21, 45]. It is possible to infer that if these teeth were already necrotic, their microbial composition might have been altered. It seems important to highlight that unlike plaque studies, there is no biologic control available for an established lesion where the bacterial DNA is extracted directly from the caries mass [16]. Furthermore, dentine lesions present a different profile compared to other caries samples affecting different tissues [7].
The higher concentration of Bifidobacterium spp. and the L. casei group as well as the presence of L. acidophilus and S. gordonii in active ECC-dentine lesions show that these bacteria may be implicated in the activity status of dentin lesions.
References
Berkowitz RJ (2003) Acquisition and transmission of mutans streptococci. J Calif Dent Assoc 31:135–138
Peterson SN, Snesrud E, Liu J, Ong AC, Kilian M, Schork NJ, Bretz W (2013) The dental plaque microbiome in health and disease. PLoS One 8:e58487
Ramos-Gomez FJ, Weintraub JA, Gansky SA, Hoover CI, Featherstone JD (2002) Bacterial, behavioral and environmental factors associated with early childhood caries. J Clin Pediatr Dent 26:165–173
American Academy of Pediatric Dentistry (2014) Policy on early childhood caries (ECC): classifications, consequences and preventive strategies. Reference manual. Pediatr Dent 36:50–52
Obata J, Takeshita T, Shibata Y, Yamanaka W, Unemori M, Akamine A, Yamashita Y (2014) Identification of the microbiota in carious dentin lesions using 16S rRNA gene sequencing. PLoS One 9:e103712
Belda-Ferre P, Alcaraz LD, Cabrera-Rubio R, Romero H, Simón-Soro A, Pignatelli M, Mira A (2012) The oral metagenome in health and disease. ISME J 6:46–56
Simón-Soro A, Belda-Ferre P, Cabrera-Rubio R, Alcaraz LD, Mira A (2013) A tissue-dependent hypothesis of dental caries. Caries Res 47:591–600
Lima KC, Coelho LT, Pinheiro IVA, Rocas IN, Siqueira JF Jr (2011) Microbiota of dentinal caries as assessed by reverse-capture checkerboard analysis. Caries Res 45:21–30
Takahashi N, Nyvad B (2011) The role of bacteria in the caries process: ecological perspectives. J Dent Res 90:294–303
Kianoush N, Nguyen K-AT, Browne GV, Simonian M, Hunter N (2014) pH gradient and distribution of streptococci, lactobacilli, prevotellae, and fusobacteria in carious dentine. Clin Oral Investig 18:659–669
Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL (2002) Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40:1001–1009
Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ (2008) Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol 46:1407–1417
Corby PM, Lyons-Weiler J, Bretz WA, Hart TC, Aas JA, Boumenna T, Goss J, Corby AL, Junior HM, Weyant RJ, Paster BJ (2005) Microbial risk indicators of early childhood caries. J Clin Microbiol 43:5753–5759
Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL (2012) Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One 7:e47722
Martin FE, Nadkarni MA, Nicholas A, Hunter N, Jacques NA (2002) Quantitative microbiological study of human carious dentine by culture and real-time PCR: association of anaerobes with histopathological changes in chronic pulpitis. J Clin Microbiol 40:1698–1704
Kianoush N, Adler CJ, Nguyen K-AT, Browne GV, Simonian M, Hunter N (2014) Bacterial profile of dentine caries and the impact of pH on bacterial population diversity. PLoS One 9:e92940
Koehler B, Andreen I, Jonsson B (1988) The earlier the colonization by mutans streptococci, the higher the caries prevalence at 4 years of age. Oral Microbiol Immunol 3:14–17
Kanasi E, Dewhirst FE, Chalmers NI, Kent R, Moore A (2010) Clonal analysis of the microbiota of severe early childhood caries. Caries Res 44:485–497
Parisotto TM, Steiner-Oliveira C, Silva CM, Rodrigues LK, Nobre-dos-Santos M (2010) Early childhood caries and mutans streptococci: a systematic review. Oral Health Prev Dent 8:59–70
Mattos-Graner RO, Corrêa MS, Latorre MR, Peres RC, Mayer MP (2001) Mutans streptococci oral colonization in 12-30-month-old Brazilian children over a one-year follow-up period. J Public Health Dent 61:161–167
Chhour K, Nadkarni MA, Byun R, Martin FE, Jacques NA, Hunter N (2005) Molecular analysis of microbial diversity in advanced caries. J Clin Microbiol 43:843–849
Mantzourani M, Fenlon M, Beighton D (2009) Association between Bifidobacteriaceae and the clinical severity of root caries lesions. Oral Microbiol Immunol 24:32–37
Munson MA, Banerjee A, Watson TF, Wade WG (2004) Molecular analysis of the microflora associated with dental caries. J Clin Microbiol 42:3023–3029
Nascimento MM, Burne RA (2014) Caries prevention by arginine metabolism in oral biofilms: translating science into clinical success. Curr Oral Heal Reports 1:79–85
Hojo S, Komatsu M, Okuda R, Takahashi N, Yamada Y (1994) Acid profiles and pH of carious dentin in active and arrested lesions. J Dent Res 73:1853–1857
Kuribayashi M, Kitasako Y, Matin K, Sadr A, Shida K, Tagami J (2012) Intraoral pH measurement of carious lesions with qPCR of cariogenic bacteria to differentiate caries activity. J Dent 40:222–228
Bjørndal L, Demant S, Dabelsteen S (2014) Depth and activity of carious lesions as indicators for the regenerative potential of dental pulp after intervention. J Endod 40:S76–S81
Jiang W, Ling Z, Lin X, Chen Y, Zhang J, Yu J, Xiang C, Chen H (2014) Pyrosequencing analysis of oral microbiota shifting in various caries states in childhood. Microb Ecol 67:962–969
Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, Asnani K, Griffen AL (2010) Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol 48:4121–4128
Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, Pitts NB (2007) The international caries detection and assessment system (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol 35:170–178
Pitts NB (2004) Modern concepts of caries measurement. J Dent Res 83:43–47
Nyvad B, Machiulskiene V, Baelum V (1999) Reliability of a new caries diagnostic system differentiating between active and inactive caries lesions. Caries Res 33:252–260
Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257–266
Park S, Kim YK, Kook J (2013) Development of quantitative real-time PCR primers for detecting 42 bacterial species. Arch Microbiol 195:473–482
Rintillä T, Kassinen A, Malinen E, Krogius L, Palva A (2004) Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol 97:1166–1177
Furet J, Quéneé P, Tailliez P (2004) Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int J Food Microbiol 97:197–207
Wolff D, Freese C, Maier-Kraus T, Krueger T, Wolff B (2013) Bacterial biofilm composition in caries and caries-free subjects. Caries Res 47:69–77
Yano A, Kaneko N, Ida H, Yamaguchi T, Hanada N (2002) Real-time PCR for quantification of Streptococcus mutans. FEMS Microbiol Lett 217:23–30
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology. Williams & Wilkins, Baltimore
Wilson K (2001) Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol Chapter 2:Unit 2.4. doi: 10.1002/0471142727.mb0204s56
Hahn CL, Liewehr FR (2007) Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. J Endod 33:213–219
Kleinberg I (2002) A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med 13:108–125
Byun R, Nadkarni MA, Chhour KL, Martin FE, Jacques NA, Hunter N (2004) Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol 42:3128–3136
Badet C, Thebaud NB (2008) Ecology of lactobacilli in the oral cavity: a review of literature. Open Microbiol 2:38–48
Marchant S, Braislsford SR, Twomey AC, Roberts GJ, Beighton D (2001) The predominant microflora of nursing caries lesions. Caries Res 35:397–406
Nadkarni MA, Simonian MR, Harty DW, Zoellner H, Jacques NA, Hunter N (2010) Lactobacilli are prominent in the initial stages of polymicrobial infection of dental pulp. J Clin Microbiol 48:1732–1740
van Houte J, Lopman J, Kent R (1996) The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J Dent Res 75:1008–1014
Dige I, Grønkjær L, Nyvad B (2014) Molecular studies of the structural ecology of natural occlusal caries. Caries Res 48:451–460
Loesche WJ, Eklund S, Earnest R, Burt B (1984) Longitudinal investigation of bacteriology of human fissure decay: epidemiological studies on molars shortly after eruption. Infect Immun 46:765–772
Milnes AR, Bowden GH (1985) The microflora associated with developing lesions of nursing caries. Caries Res 19:289–297
Maeda T, Kitasako Y, Senpuku H, Burrow MF, Tagami J (2006) Role of oral streptococci in the pH-dependent carious dentin. J Med Dent Sci 53:159–166
Simark-Mattsson C, Emilson CG, Håkansson EG, Jacobsson C, Roos K, Holm S (2007) Lactobacillus-mediated interference of mutans streptococci in caries-free vs. caries-active subjects. Eur J Oral Sci 115:308–314
Tanzer JM, Livingston J, Thompson AM (2001) The microbiology of primary dental caries in humans. J Dent Educ 65:1028–1037
Takahashi N, Yamada T (1999) Acid-induced acidogenicity and acid tolerance of non-mutans streptococci. Oral Microbiol Immunol 14:43–48
Brasilsford SR, Tregaskis RB, Leftwich HS, Beighton D (1999) The predominant Actinomyces spp. isolated from infected dentin of active root caries lesions. J Dent Res 78:1525–1534
Maijala M, Rautemaa R, Järvensivu A, Richard- son M, Salo T, Tjäderhane L (2007) Candida albicans does not invade carious human dentine. Oral Dis 13:279–284
Dalwai F, Spratt DA, Pratten J (2007) Use of quantitative PCR and culture methods to characterize ecological flux in bacterial biofilms. J Clin Microbiol 45(9):3072–3076
Yasunaga A, Yoshida A, Morikawa K, Maki K, Nakamura S, Soh I, Awano S, Ansai T (2013) Monitoring the prevalence of viable and dead cariogenic bacteria in oral specimens and in vitro biofilms by qPCR combined with propidium monoazide. BMC Microbiol 13:157. doi:10.1186/1471-2180-13-157
Takahashi N, Nyvad B (2016) Ecological hypothesis of dentin and root caries. Caries Res 50:422–431
Acknowledgements
This study was supported by the CNPq—the National Counsel of Technological and Scientific Development (Process # 475346/2011-4 MCT/CNPq 14/2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the ethics committee of the Federal University of Ceará, Brazil (COMEPE/UFC) (Protocol Number 158/2011). Verbal and written consents were obtained from the parents of all subjects. Samples were taken only after obtaining the approval from the children and their parents.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by CNPq—National Counsel of Technological and Scientific Development (Process # 475346/2011-4 MCT/CNPq 14/2011).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 485 kb)
Rights and permissions
About this article
Cite this article
Neves, B.G., Stipp, R.N., da Silva Bezerra, D. et al. Molecular detection of bacteria associated to caries activity in dentinal lesions. Clin Oral Invest 21, 2053–2061 (2017). https://doi.org/10.1007/s00784-016-1995-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1995-9