Abstract
Aims
To quantitatively identify Bifidobacterium, S. wiggsiae and S. mutans in plaque samples obtained from children with severe-ECC and caries-free groups and to analyze their association with caries-related factors retrieved from the questionnaire in each group.
Study design
To establish the 2 study groups, clinical examination in 122 Thai children, aged 2–5 years, recorded decayed, missing and filled teeth scores (dmft), in addition to plaque and gingival indices. Sixty one children in the caries-free group and 61 in the S-ECC group were identified. A questionnaire was used to assess the parent’s attitudes and behavior regarding the child’s oral hygiene care and diet.
Methods
Pooled overnight supra gingival plaque was collected from each child using a sterile toothpick, released in 1 ml of TE buffer, transported on ice to the Laboratory and stored at – 20 °C. DNA was extracted from the plaque based on enzymatic lysis and quantitative real-time PCR using fluorescent dye (SYBR green) in addition to Agarose gel electrophoresis were performed. All laboratory and retrieved from the questionnaire data per child were recorded and statistically analysed.
Results
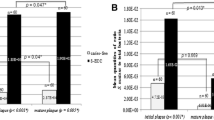
S. wiggsiae (p < 0.005) and S. mutans (p < 0.001) were higher in the S-ECC group. Bifidobacterium, S. mutans, and S. wiggsiae were associated with the dmft score and gingival index (p < 0.001). The dmft scores of children who detected only S. mutans were significantly lower than the dmft scores of children who detected two bacteria; S. mutans + S. wiggsiae (p = 0.028), S. mutans + Bifidobacterium (p = 0.026), and three bacteria; S. mutans + Bifidobacterium + S. wiggsiae (p = 0.007). Children who found all three bacteria (Bi + Sm + Sw) had the highest dmft scores, followed by children who had two bacteria (Bi + Sw, or Bi + Sm, or Sw + Sm). The guardians’ education levels, occupations, household income, prolonged bottle feeding, taking of water after bottle or breast feeding, eating sugar-coated crackers or bread with sweetened cream, and premature birth were the factors that related to S-ECC.
Conclusion
Levels of S. wiggsiae and S. mutans, guardian’s education, family economics, prolonged bottle feeding, eating high sugar-containing snacks and premature birth were associated with S-ECC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe early childhood caries (S-ECC) in children is one of major health problems worldwide (Academy on Pediatric Dentistry 2008). In the USA, the prevalence of caries in primary dentition in children aged 2–5 years old was 23% (Academy on Pediatric Dentistry 2008). Thailand is a developing country experiencing high levels of ECC. A recent national dental health survey showed that dental caries affects 43% and 76% of 3- and 5-year-old children, respectively (National survey).
ECC is an advanced progressive demineralization of the tooth due to interaction among cariogenic diets, a susceptible host and oral microbiota in children younger than 6 years old (Palmer et al. 2010). The ecological plaque hypothesis envisages that a cariogenic oral environment that is reduced in pH will select for increased proportions and numbers of acidogenic and aciduric microbiota. Streptococcus mutans is commonly isolated microorganism from dental plaque (Tanzer et al. 2001; Tanner et al. 2011; Mitrakul et al. 2017; Mitrakul et al. 2019). Not only it is aciduric and acidogenic, but also has the capability to adhere and deposit on the tooth surfaces. In the presence glucosyltransferases (Gtfs) (an enzyme of S. mutans), sucrose molecules are cleaved and the glucose component is polymerised into adherent glucans (Tanzer et al. 2001). S. mutans is also able to generate the acid from carbohydrate and tolerate low pH environment (Tanzer et al. 2001; Tanner et al. 2011). Previous studies have shown an association between S. mutans and ECC and are used as one of the microbial parameters for assessing children’s caries risk (Tanzer et al. 2001; Tanner et al. 2011). Recent studies in Thai children found that S. mutans in plaque was higher in ECC. Contributing factors, which include the guardian’s demographic data, the habit of milk bottle and breast feedings, oral hygiene care and the consumption of cariogenic snacks were all associated with ECC (Mitrakul et al. 2017; Mitrakul et al. 2019). Other species have been recognized that are significantly associated with S-ECC when S. mutan is not detected, including Bifidobacteria and Scardovia wiggsiae (Henne et al. 2015; Valdez et al. 2016; Mitrakul et al. 2017).
Bifidobacterium is anaerobic, gram-positive, rod-shaped, and generally colonizes the gastrointestinal tract. It has been isolated from saliva, dental plaque and dentinal caries (Valdez et al. 2016; Modesto et al. 2006; Brighton et al. 2008; Mantzourani et al. 2009; Nair et al. 2017). Bifidobacterium were shown to have similar acidogenicity and aciduricity to S. mutans and the ability to produce an acidic environment, to resist low pH and to promote biofilm formation when co-adhered with primary colonizers (Modesto et al. 2006). Recent studies have demonstrated an association between Bifidobacterium and ECC (Mantzourani et al. 2009; Nair et al. 2017). A previous study in Thai children also reported that Bifidobacterium levels were significantly higher in the supra gingival plaque of ECC children when compared with caries-free children (Mitrakul et al. 2017).
Scardovia wiggiae is an anaerobic Gram-positive bacillus. In vitro studies show that S. wiggsiae growth and acid tolerance are similar to S. mutans (Henne et al. 2015). Also, it is a strong acid producer, equal to or greater than S. mutans (Henne et al. 2015). Recent studies found a strong association between S. wiggsiae and S-ECC, and have suggested that S. wiggsiae might be a considerable primary pathogen of dental caries (Henne et al. 2015). A few studies have found that, in S. mutans-negative samples, S. wiggsiae was detected, which suggests that S. wiggsiae might be a secondary aggressor and implicated with caries progression at a later stage of disease when S. mutans is not the main pathogenic specie (Henne et al. 2015). Furthermore, some studies found an association with the combination of Bifidobacteria, S. wiggsiae and S. mutans with caries, and this might be valuable in caries risk assessment (Henne et al. 2015; Valdez et al. 2016; Mitrakul et al. 2017). S. wiggsiae was found to be associated with advanced dentinal caries on occlusal surfaces in young children, initial white spot lesions in older children and dentinal caries in adults (Henne et al. 2015; Tanner et al. 2016).
This study aims to quantitatively detect Bifidobacterium, S. wiggsiae and S. mutans in two groups of Thai children which were S-ECC and caries-free, and to analyse the association between these bacteria and caries-associated factors. The hypothesis is that the levels of Bifidobacterium, S. wiggsiae and S. mutans in plaque samples from S-ECC and caries-free children should be different.
Materials and methods
This is a cross-sectional study. The study protocol was approved by the Human Institutional Review Board of the Faculty of Dentistry and the Faculty of Pharmacy, Mahidol University (MU-DT/PY-IRB 2019/ 021.2404). A statistician consultation was done based on previous studies, performed with α = 0.05 and power of 80%, using the software package Primer of Biostatistics (McGraw-Hill, NY, USA) (Mitrakul et al. 2017). A minimum of 61 children in each group was enough to achieve statistical difference.
Subject selection: Thai children aged 2–5 years old recruited from four primary schools in Prachuap Khiri Khan Province which is located in the Southern part of Thailand. Consent forms were signed. Total subjects were 122 (n = 61 in each group). A clinical examination was performed following the diagnosis protocol of AAPD 2018–2019, which defines S-ECC. In children younger than 3 years of age, any sign of smooth-surface caries is indicative of S-ECC. From ages 3 through 5, 1 or more cavitated, missing (due to caries), or filled smooth surfaces in primary maxillary anterior teeth, or a decayed, missing, or filled score of ≥ 4 (age 3), ≥ 5 (age 4), or ≥ 6 (age 5) surfaces also constitutes S-ECC (Academy on Pediatric Dentistry, 2018). For the caries-free group, subjects had no caries nor existing restorations (dmft = 0). Bitewing radiograph was obtained once interproximal caries was suspected. Subjects who had any systemic disease(s), taking any kind of antibiotics, had professional fluoride application or any dental treatment within 2 months prior to the sample collection period were excluded.
Clinical examination, plaque and modified gingival indices: Two examiners who are in a residency training program in pediatric dentistry performed a clinical examination at public schools using World Health Organization criteria (Ismail et al. 2009). Recorded dmft scores, plaque index using a modified debris index of the simplified oral hygiene index for primarydentition and gingiva inflammation index as mentioned in previous study (Greene et al. 1964; Lobene et al. 1986; Ismail et al. 2009; Mitrakul et al. 2016).
The questionnaire: All participants’ parents or caretakers were asked to complete the questionnaire by face-to-face interview. All questions were close ended. Besides the parents’ general information, three categories were examined: (1) child’s general information; (2) parental attitude towards child’s diet: (a) Is your child still bottle feeding?; (b) Did your child ever have breast and/or bottle feeding ad lib?; (c) Did your child breast and/or bottle feed ad lib and fall asleep?; (d) Did you always give your child water after breast or bottle feeding?; (e) What type of snacks does your child have per day?; (f) type and frequency of snacks; 3. parent’s attitude and behaviour in child’s oral hygiene care: (a) How many times per day do you brush your child’s teeth?; (b) When did you last take your child to the dentist.
Plaque sample collection: All parents/guardians were instructed to brush their child’s teeth at 8.00 PM the night before plaque collection day. No food or drink before sample collection. Pooled overnight dental plaque was collected using a sterile toothpick and released in 1 ml of TE buffer. All samples were immediately transported on ice to the Oral Biology Laboratory and stored at − 20 °C until the DNA extraction process.
DNA extraction: DNA was extracted based on enzymatic lysis using a commercial kit (Flavogen, Taiwan) as previously described (Mitrakul et al. 2017). In brief, 20 µl of Proteinase K was added, 400 µl of FABG buffer and 20 µl of a lysozyme mixture (lysozyme 20 mg/ml and mutanolysin (Sigma Aldrich, USA) in 1:10 proteinase K) and vortex. This was incubated at 60 °C for 1 h.; 200 µl ethanol was added and centrifuged at 11,000 rpm for 30 s. The solution was transferred into a spin column and centrifuged for 1 min. The supernatant was discarded, 500 µl of W1 buffer was added and centrifuged for 1 min. The supernatant was discarded. Then 750 µl of wash buffer was added and centrifuged for 1 min. The next step was adding 50 µl of elution buffer, left at room temperature for 3 min, before a final centrifuge for 2 min. The extracted DNA concentration and purity was measured using a spectrophotometer at 260 nm/280 nm (Nanodrop 2000C Thermo Scientific, Delaware, USA).
Culture condition and standard strains: Three bacterial strains (Bifidobacterium longum (subspecies 51,139), S. mutans ATCC 25,175, S. sobrinus ATCC 6715) were used as standard strains. Bifidobacterium longum (subspecies 51,139) was inoculated in Brain Heart Infusion broth and incubated at 37 °C for 24 h. S. mutans and S. sobrinus strains were grown anaerobically (5% CO2) in Brain Heart Infusion broth at 37 °C for 24–48 h. Genomic DNA was extracted from the overnight culture as described above. A ten-fold serial dilution, starting from 108 to 102 CFU/ml, was performed.
Quantitative real-time PCR: Using specific primers (Table 1), the reaction mixture (total volume of 20 μl) contained (varied from 2 to 9.1 μl) of water, 10 μl of 2X KAPA SYBR® FAST qPCR Master Mix, 0.4 μl of 10 μM forward and reverse primer, and (varied from 0.1 to 7.2) μl of bacteria DNA. The thermocycler (C1000™ Thermal cycler and CFX 96 Real-time System) was set for 40 cycles. Each cycle consisted of enzyme activation at 95 °C for 3 min, denaturing at 95 °C for 3 s, annealing at 52 °C, 53 °C and 53 °C for 20 s for universal BAC16S, Bifidobacterium and S. wiggsiae, respectively. Melting curves were generated from 60 °C to 95 °C and read every 0.5 °C for 5 s (Mitrakul et al. 2017).
Agarose gel electrophoresis: Amplified PCR products were checked with 2% agarose gel (UltraPure Agarose, ThermoFisher Scientific, USA) which was stained with ethidium bromide and the gel images were captured with a digital imaging system (Molecular Imager ®Gel docTM Systems, Bio-Rad Laboratories Inc., CA, USA).
Statistical analysis
All data were recorded and analyzed by SPSS 18.0 software (Microsoft Corporation. CA, USA). Normality of the data was tested using a Kolmogorov–Smirnov and Shapiro–wilk test (p <). The different levels of bacteria between the S-ECC and caries free groups were analyzed using a Mann–Whitney U test (p < 0.05). Association between Bifidobacterium and S. wiggsiae with S. mutans were analyzed by McNemar’s test (p < 0.05). Pearson chi-square was used for analysis of the detection of each bacteria and association of factors in the questionnaire for caries status.
Results
General informational data of the subjects are shown in Table 2. Age and gender of the children and guardians were not statistically different between the two groups and were not associated with the caries status of the children. The plaque index was not statistically different between the two groups, but the gingival index was significant higher in the S-ECC group (Table 3).
The specificity test for all primers was done. For S. wiggsiae, the standard strain was not obtained due to the difficulty in laboratory culture. Standard curves were plotted from the quantities and threshold cycle of universal primers BAC16S, Bifidobacterium primers and S. mutans primers. The detection limit of BAC16S, Bifidobacterium primers and S. mutans primers were 103, 101 and 102, respectively. The prevalence of S. mutans (p < 0.001) and S. wiggsiae (p = 0.005) were significantly higher in the S-ECC group when compared to the caries-free group. The prevalence of Bifidobacterium was not different between the two groups (Table 4). When analysing association between the three bacteria, results showed that all of them were associated with the dmft scores and gingiva index (Table 5). The dmft scores of children who detected only S. mutans were significantly lower than the dmft scores of children who detected two bacteria; S. mutans + S. wiggsiae (p = 0.028), S. mutans + Bifidobacterium (p = 0.026), and three bacteria; S. mutans + Bifidobacterium + S. wiggsiae (p = 0.007). Children who had all three bacteria (Bi + Sm + Sw) detected had the highest dmft scores, followed by children who had two bacteria detected (Bi + Sw, or Bi + Sm, or Sw + Sm) (Fig. 1), when analysing the correlation between bacteria and dmft scores, and plaque and gingiva indices. Results showed that level of Bifidobacterium, ratio of Bifidobacterium to total bacteria, S. mutans and ratio of S. mutans to total bacteria were correlated with dmft scores in the S-ECC group but only the level of S. mutans correlated with the plaque index. The level of S. mutans and ratio of S. mutans to total bacteria were correlated with the gingival index (Table 6).
The relationships between caries status and related factors in the questionnaires are shown in Table 7. The variables that were significantly associated with dental caries were the guardians’ education levels, guardian’s occupation, household income, prolonged bottle feeding (> 18 months of age), taking water after bottle or breast feeding, eating snacks while watching TV, premature birth and eating of sugar-coated crackers or bread with sweetened cream (p < 0.05).
Discussion
It has been supported by numerous studies that S. mutans is strongly associated with dental caries and S-ECC (Tanzer wt al. 2001; Anil et al. 2017; Kanasi et al. 2010; Hughes et al. 2012; Choi et al. 2009; Mitrakul et al. 2017; Mitrakul et al. 2019). Results from this study also supports this association. In this study, the prevalence of S. mutans in S-ECC was 73.77%, which was similar to previous studies (Carmona et al. 2011; Singla et al. 2016; Vacharaksa et al. 2015). Two previous studies showed that the level of S. mutans and the ratio of S. mutans to total bacteria in dental plaque were strongly correlated with dmft scores (Mitrakul et al. 2017; Choi et al. 2009). Results from this study found similar correlation. In addition, this study found a positive correlation between levels of S. mutans and the ratio of S. mutans to total bacteria and the gingival index, which is consistent with previous studies (Mitrakul et al. 2017). This can be explained by the fact that mature plaque is a high level and climax community, consistent with many microorganisms associated with gingival inflammation, and bacterial infection is the main cause of gingivitis (Beighton et al. 1996).
In this study, results showed that the detection and quantities of Bifidobacterium from the S-ECC group and caries-free group were not significantly different, which was inconsistent with our previous study that showed a significantly higher level of Bifidobacterium in the S-ECC group than in the caries-free group (Mitrakul et al. 2017). This could be from the different demographic and level of caries severity. Although both studies were done in Thai children, the subjects were recruited from different regions. Not only was the dmft scores from our previous study higher, but also the mean quantities of S. mutans and Bifidobacterium were higher. The mean quantity of S. mutans (in CFU) in the caries-free and S-ECC groups in a previous study were 2.87 × 104 and 1.9 × 105, while in this study they were 1.28 × 103 and 1.13 × 104, respectively (Mitrakul et al. 2017). The mean quantity of Bifidobacterium in the caries-free and S-ECC groups in the previous study were 1.67 × 102 and 3.4 × 104, while in this study they were 1.78 × 102 and 1.68 × 103, respectively (Mitrakul et al. 2017). It is possible that the severity of dental caries might have been related to the amount of S. mutans and Bifidobacterium and resulted in a different outcome. However, this study also found that levels of Bifidobacterium and the ratio of Bifidobacterium to total bacteria were positively correlated with the dmft scores, which corresponded with the previous study (Mitrakul et al. 2017). Several previous studies found Bifidobacterium mostly in deep dentinal caries but rarely found it in intact or white spot lesions (Mantzourani et al. 2009; Becker et al. 2002; Torlakovic et al. 2012; Haukioja et al. 2006). Haukioja and colleagues reported that Bifidobacterium did not bind to saliva-coated hydroxyapatite, but bound well to F. nucleatum-coated surfaces, indicating the importance of other oral bacteria in modulating the colonization potential of the strains, and consistent with our result that did not detect Bifidobacterium in S-ECC children, whom both S. mutans and S. wiggsiae were absent (Haukioja et al. 2006). It is possible that Bifidobacterium might be associated with caries progression after tooth surface destruction has already begun by other bacteria. The current literature mentions the special properties of Bifidobacterium that increases the violence of caries progression because it can store intracellular polysaccharides and degrade them into acids under carbohydrate-limited conditions, such as between meals, and it has tolerance to fluoride due to its unique metabolic pathway (Manome et al. 2019). Taken together, Bifidobacterium seems to play an important role in caries progression. This study also found that Bifidobacterium was associated with the gingiva index in the same way as our previous study (Mitrakul et al. 2017). Results from both studies are similar to the study by Palmer and colleagues, which showed that gingival inflammation and gingival bleeding were associated with Bifidobacterium (Palmer et al. 2010).
S. wiggsiae had belonged to the Bifidobacteriaceae family (Valdez et al. 2016). It was separated from the genus Bifidobacterium and detected in S-ECC as an unclassified Bifidobacterium in 2002 (Haukioja et al. 2006; Jian et al. 2002). Tanner and colleagues found that S. wiggsiae was significantly associated with S-ECC by anaerobic culture using enriched blood agar and acid agar from dental plaque samples which were collected from the interproximal surfaces of molars and plaque from carious lesions from S-ECC children (Tanner et al. 2011). Later on, they developed a PCR assay for S. wiggsiae and their results showed that S. wiggsiae was associated with S-ECC and its prevalence was higher than S. mutans (Tanner et al. 2011). In contrast, this study showed that the prevalence of S. mutans (74%) was higher than S. wiggsiae (49%) in the S-ECC group. However, in this study found that the prevalence of S. wiggsiae from supra gingival plaque was significantly higher in the S-ECC group than in the caries-free group, which is consistent with previous studies (Topcuoglu et al. 2017; Vacharaksa et al. 2015). In previous studies, S. wiggsiae was significantly higher in the saliva of S-ECC than caries-free children, but in dental plaque it was detected higher in white spot and carious lesions (Colombo et al. 2017; Chandna et al. 2018; Carr et al. 2020; Kameda et al. 2020). This study showed that dmft scores and the gingival index of children who presented with S. wiggsiae were significantly higher than in children who were absent of S. wiggsiae. Previous studies showed a positive correlation between the numbers of S. wiggsiae in saliva and dmft scores, which corresponded to our study (Chandna et al. 2018; Colombo et al. 2017). This is the first study to report the association between S. wiggsiae and gingival index in Thai children. However, Carr and colleagues reported that the gingival crevice was not the specific oral location that harbors S. wiggsiae (Carr et al. 2020). This study also found S. wiggsiae in S-ECC children who did not show the detection of S. mutans (Vacharaksa et al. 2015).
Taken together, this finding suggests that both S. mutans and S. wiggsiae are associated with dental caries, either these two species living together or living alone. The mean dmft scores of children who were detected with S. mutans, S. wiggsiae or Bifidobacterium were significantly higher than children who detected only S. mutans. It is possible that when the two or three species are living together, it might enhance their ability and severity of dental caries when compared with having S. mutans alone. Based on previous study, Bifidobacterium and S. wiggsiae have interesting properties that encourage dental caries formation and progression. These interesting properties of Bifidobacterium have already been aforementioned. For S. wiggsiae, it is an anaerobic bacterium, while for S. mutans it is a facultative anaerobic bacterium, so S. wiggsiae can continue to grow and produce acid in a low oxygen environment like in mature plaque (Vacharaksa et al. 2015). This species exhibits high acid productivity and tolerance to acidic conditions (Kameda et al. 2020). They produce acid from glucose and can reduce the environmental pH to 3.5 (Kameda et al. 2020). They produce acetic acid as the end product through a fructose-6-phosphate pathway (F6PPK shunt) similar to Bifidobacterium but different from S. mutans that produces lactic acid from the glycolytic pathway (Chandna et al. 2018; Carr et al. 2020; Kameda et al. 2020). Moreover, S. wiggsiae is arginine deaminase negative, so it is incapable of increasing intraoral pH. All of these properties provide S. wiggsiae with high ecological competitiveness in acidic environments and intensify the cariogenic effect of this bacteria. Although mean dmft scores of children who detected only S. wiggsiae (without S. mutans) were not significantly different from children who detected only S. mutans (without S. wiggsiae), they were also not significantly different from the mean dmft scores of children who detected both S. mutans and S. wiggsiae. While the mean dmft scores of children who detected only S. mutans were lower than in children who detected both S. mutans and S. wiggsiae, these results suggest that S. wiggsiae might play an important role in the caries process. However, further studies are needed.
From our study, the plaque index was not different between the caries-free and the S-ECC groups. The result was inconsistent with previous reports that the presence of plaque was associated with ECC (Chanpum et al. 2020; Pierce et al. 2019; Rugg-Gunn et al. 2017). The reason for this result might be from the study design, which instructed parents to brush their child’s teeth at the same period before dental plaque examination and collection so the amount of plaque accumulation was not different between the two groups, unlike other previous studies that examined the plaque index without disturbing.
Data from the questionnaire showed factors that are significantly related to S-ECC are the guardians’ education levels, guardian’s occupation, household income, prolonged bottle feeding, taking water after bottle or breast feeding, eating snacks while watching TV, and eating of sugar-coated crackers or bread with sweetened cream. The parent’s educational level, employment status, and low socio-economic status were found to be associated with ECC, similar to previous studies (Chanpum et al. 2020; Pierce et al. 2019; Rugg-Gunn et al. 2017). Dietary practices also play a significant role in the development of ECC (Rugg-Gunn et al. 2017). Prolonged duration of bottle feeding was identified as one of the causes of dental caries, but the result from our study surprisingly found that caries-free children had significantly longer durations of bottle feeding than S-ECC children (Ghimire et al. 2013). However, it was found that in the caries-free group, the percentage taking water after bottle feeding was higher than in the S-ECC group. This implies that dental caries is a multifactorial disease, if an unfavorable diet is accompanied by appropriate oral cleaning it might lower the risk of having ECC (Ghimire et al. 2013; Zeng et al. 2014). Among a variety of foods, data showed that only sugar-coated crackers and bread with sweetened cream were significantly associated with S-ECC, as reported in our previous study (Mitrakul et al. 2017). As a mixture of starch and sucrose, cooked starch is more cariogenic than raw starch. Fermentable sugar that is retained in the mouth for a long period and frequency of consuming are considered factors that contribute to tooth demineralization (Pierce et al. 2019; Rugg-Gunn et al. 2017). Eating while watching TV is a possible risk factor for developing dental caries, as children are more likely to consume more sweetened beverages and dwell on snacks while watching TV (Zeng et al. 2014). Previous studies have shown a correlation between duration and frequency of television viewing and dental caries (Anil et al. 2011). Our study also found a relationship between eating while watching TV with dental caries. The meta-analysis indicates that preterm birth is associated with ECC, while low birth weight is not, and our study also confirmed these results (Shi et al. 2020). Limitations of this study is that the cross-sectional design was able to show a snap-shot in time only and did not allow us to determine the true dynamic of the oral biofilm community and find the predicting factors in the questionnaire. Secondly, this small sample size in one area of Thailand might not be representative of the whole population.
Conclusions
Considering the limitations of the present study the following conclusions can be made:
-
The detection of S. wiggsiae and S. mutans in plaque from the S-ECC group were significantly higher than the caries-free group, while the detection of Bifidobacterium was nt significantly different between the two groups.
-
The number of S. mutans and ratio of S. mutans to total bacteria from the S-ECC group were significantly higher than in the caries-free group, while the number of Bifidobacterium and ratio of Bifidobacterium to total bacteria were not significantly different between the two groups.
-
The prevalence of Bifidobacterium, S. wiggsiae and S. mutans were associated with dmft scores and the gingival index.
-
Factors that were significantly related to S-ECC were the guardians’ education levels, guardian’s occupation, household income, prolonged bottle feeding, taking water after bottle or breast feeding, eating snacks while watching TV, and eating of sugar-coated crackers or bread with sweetened cream and premature birth.
References
American Academy of Pediatric Dentistry. Policy on early childhood caries (ECC): classifications, consequences, and preventive strategies. Pediatr Dent. 2016;38(6):52–4.
Anil S, Anand PS. Early childhood caries: prevalence, risk factors, and prevention. Front Pediatr. 2017;5:157. https://doi.org/10.3389/fped.2017.00157.
Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, et al. Molecular analysis of bacterial species associated with childhood caries. J Clinicalmicrobiol. 2002;40(3):1001–9. https://doi.org/10.1128/JCM.40.3.1001-1009.2002.
Beighton D, Adamson A, Rugg-Gunn A. Associations between dietary intake, dental cariesexperience and salivary bacterial levels in 12-year-old English schoolchildren. Arch Oral Biol. 1996;41(3):271–80. https://doi.org/10.1016/0003-9969(96)84555-9.
Beighton D, Gilbert SC, Clark D, et al. Isolation and Identification of Bifidobacteriaceae from Human Saliva. Appl Environ Microbiol. 2008;74(20):6457–60. https://doi.org/10.1128/AEM.00895-08.
Carmona LE, Reyes N, González F Polymerase chain reaction for detection of Streptococcus mutans and Streptococcus sobrinus in dental plaque of children from Cartagena, Colombia. Colombia Médica. 2011;42:430–7. https://doi.org/10.25100/cm.v42i4.943
Carr G, Alexander A, Nguyen L, Kingsley K. Oral site specific sampling reveals differential location for scardovia wiggsiae. Microbiol Res J Int. 2020. https://doi.org/10.9734/mrji/2020/v30i130189.
Chandna P, Srivastava N, Sharma A, Sharma V, Gupta N, Adlakha V. Isolation of Scardovia wiggsiae using real-time polymerase chain reaction from the saliva of children with early childhood caries. J Indian Soc Pedodont Prevent Dentistry. 2018;36:290. https://doi.org/10.4103/JISPPD.JISPPD_225_17.
Chanpum P, Duangthip D, Trairatvorakul C, Songsiripradubboon S. Early childhood caries and its associated factors among 9- to 18-month old exclusively breastfed childrenin Thailand: a cross-sectional study. Int J Environ Res Public Health. 2020;17:3194. https://doi.org/10.3390/ijerph17093194.
Choi EJ, Lee SH, Kim YJ. Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int J Paediat Dentist. 2009;19(2):141–7. https://doi.org/10.1111/j.1365-263X.2008.00942.x.
Colombo NH, Kreling PF, Ribas LFF, Pereira JA, Kressirer CA, Klein MI, et al. Quantitative assessment of salivary oral bacteria according to the severity of dental caries in childhood. Arch Oral Biol. 2017;83:282–8. https://doi.org/10.1016/j.archoralbio.2017.08.006.
de Matos BM, Brighenti FL, Do T, Beighton D, Koga-Ito CY. Acidogenicity of dual-species biofilms of bifidobacteria and Streptococcus mutans. Clin Oral Invest. 2017;21(5):1769–76. https://doi.org/10.1007/s00784-016-1958-1.
Ghimire N, Rao A. Comparative evaluation of the influence of television advertisements on children and caries prevalence. Glob Health Action. 2013;6:20066. https://doi.org/10.3402/gha.v6i0.20066.
Greene JG, Vermillion JR. The simplified oral hygiene index. J Am Dental Assoc 1964;68(1):7–13. https://doi.org/10.14219/jada.archive.1964.0034
Hallett KB, O’Rourke PK. Caries experience in preschool children referred for specialist dental care in hospital. Aust Dent J. 2006;51(2):124–9. https://doi.org/10.1111/j.1834-7819.2006.tb00415.x.
Haukioja A, Yli-Knuuttila H, Loimaranta V, Kari K, Ouwehand AC, Meurman JH, et al. Oral adhesion and survival of probiotic and other lactobacilli and bifidobacteria in vitro. Oral Microbiol Immunol. 2006;21(5):326–32. https://doi.org/10.1111/j.1399-302X.2006.00299.x.
Henne K, Rheinberg A, Melzer-Krick B, Conrads G. Aciduric microbial taxa including Scardovia wiggsiae and Bifidobacterium spp in caries and caries free subjects. Anaerobe. 2015;35(Pt A):60–5. https://doi.org/10.1016/j.anaerobe.2015.04.011.
Hughes CV, Dahlan M, Papadopolou E, Loo CY, Pradhan NS, Lu SC, et al. Aciduric microbiota and mutans streptococci in severe and recurrent severe early childhood caries. Pediatr Dent. 2012;34(2):e16-23.
Ismail AI, Sohn W, Lim S, Willem JM. Predictors of dental caries progression in primary teeth. J Dent Res. 2009;88(3):270–5. https://doi.org/10.1177/0022034508331011.
Jian W, Dong X. Transfer of Bifidobacterium inopinatum and Bifidobacterium denticolens to Scardovia inopinata gen. nov., comb nov., and Parascardovia denticolens gen. nov., comb nov., respectively. Int J Syst Evolut Microbiol. 2002;52:809–12. https://doi.org/10.1099/00207713-52-3-809.
Kameda M, Abiko Y, Washio J, Tanner ACR, Kressirer CA, Mizoguchi I, et al. Sugar metabolism of Scardovia wiggsiae, a novel caries-associated bacterium. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2020.00479.
Kanasi E, Dewhirst FE, Chalmers NI, Kent R Jr, Moore A, Hughes CV, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010;44(5):485–97. https://doi.org/10.1159/000320158.
Li Y, Tanner A. Effect of antimicrobial interventions on the oral microbiota associated with early childhood caries. Pediatr Dent. 2015;37(3):226–44.
Lobene R. A modified gingival index for use in clinical trials. Clin prevent Dent. 1986;8:3–6.
Manome A, Abiko Y, Kawashima J, Washio J, Fukumoto S, Takahashi N. Acidogenic Potential of Oral Bifidobacterium and Its High Fluoride Tolerance. Front Microbiol. 2019;10:1099. https://doi.org/10.3389/fmicb.2019.01099
Mantzourani M, Gilbert SC, Sulong HN, Sheehy EC, Tank S, Fenlon M, et al. The isolation of bifidobacteria from occlusal carious lesions in children and adults. Caries Res. 2009;43(4):308–13. https://doi.org/10.1159/000222659.
Matsuki T, Watanabe K, Fujimoto J, Kado Y, Takada T, Matsumoto K, et al. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl Environ Microbiol. 2004;70(1):167–73. https://doi.org/10.1128/AEM.70.1.167-173.2004.
Mitrakul K, Akarapipatkul B, Thammachat P. Quantitative Analysis of Streptococcusmutans, Streptococcus sobrinus and Streptococcus sanguinis and their association with Early Childhood Caries. J Clin Diagn Res. 2019;13(10):17–21. https://doi.org/10.7860/JCDR/2020/43086.13513.
Mitrakul K, Chan.vitan S, Jeamset A, Vongsawan K. Quantitative analysis of S. mutans, Lactobacillus and Bifidobacterium found in initial and mature plaques in Thai children with early childhood caries. Eur Arch Paediat Dentist. 2017;18(4):251–61. https://doi.org/10.1007/s40368-017-0295-7.
Modesto M, Biavati B, Mattarelli P. Occurrence of the family bifidobacteriaceae in human dental caries and plaque. Caries Res. 2006;40(3):271–6. https://doi.org/10.1159/000092237.
Nair S, Kumar VS, Krishnan R, Rajan P. A comparative evaluation of bifidobacteria levels in early childhood caries and severe early childhood caries. J Pharm Bioall Sci 2017;9, Suppl S1:82–4 https://doi.org/10.4103/jpbs.JPBS_75_17
Palmer CA, Kent R Jr, Loo CY, Hughes CV, Stutius E, Pradhan N, et al. Diet and caries associated bacteria in severe early childhood caries. J Dent Res. 2010;89(11):1224–9. https://doi.org/10.1177/0022034510376543.
Pierce A, Singh S, Lee J, Grant C, Cruz de Jesus V, Schroth RJ. The Burden of Early Childhood Caries in Canadian Children and Associated Risk Factors. Front Public Health. 2019;7:328. https://doi.org/10.3389/fpubh.2019.00328
Rugg-Gunn AJ, Woodward M, editors. Review of the aetiology of Early Childhood Caries 2017
Rusali R, Hamali N, Razi F, Mustafa N, Harun N, Azwani N. Early Childhood Feeding Practices and Its Association with Early Childhood Caries. 2019:801–4. https://doi.org/10.12691/jfnr-7-11-7
Shi L, Jia J, Li C, Zhao C, Li T, Shi H, et al. Relationship between preterm, low birth weight and early childhood caries: a meta-analysis of the case-control and cross-sectional study. 2020. Biosci Rep. https://doi.org/10.1042/BSR20200870.
Singla D, Sharma A, Sachdev V, Chopra R. Distribution of Streptococcus mutans and Streptococcus sobrinus in Dental Plaque of Indian Pre-School Children Using PCR and SB-20M Agar Medium. J Clin Diagn Res. 2016;10(11):Zc60-zc3. https://doi.org/10.7860/JCDR/2016/19256.8909
Sinsimer D, Leekha S, Park S, Marras SA, Koreen L, Willey B, et al. Use of a multiplex molecular beacon platform for rapid detection of methicillin and vancomycin resistance in Staphylococcus aureus. J Clin Microbiol. 2005;43(9):4585–91. https://doi.org/10.1128/JCM.43.9.4585-4591.2005.
Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, et al. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 2011;49(4):1464–74. https://doi.org/10.1128/JCM.02427-10.
Tanner AC, Kressirer CA, Faller LL. Understanding caries from the oral microbiome perspective. J Calif Dent Assoc. 2016;44(7):437–46.
Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65(10):1028–37.
The 8th national oral health survey in 2017, Thailand. Bangkok: Ministry of Public Health.
Topcuoglu N, Aktoren O. Scardovia wiggsiae and the other microorganisms in severe early childhood caries. J Dentist Oral Care Med. 2017. https://doi.org/10.1128/JCM.02427-10.
Torlakovic L, Klepac-Ceraj V, Ogaard B, Cotton SL, Paster BJ, Olsen I. Microbial community succession on developing lesions on human enamel. J Oral Microbiol. 2012. https://doi.org/10.3402/jom.v4i0.16125.
Vacharaksa A, Suvansopee P, Opaswanich N, Sukarawan W. PCR detection of Scardovia in combination with Streptococcus mutans for early childhood caries-risk prediction. Eur J Oral Sci. 2015. https://doi.org/10.1111/eos.12208.
Valdez RM, Dos Santos VR, Caiaffa KS, Danelon M, Arthur RA, Negrini TC, et al. Comparative in vitro investigation of the cariogenic potential of bifidobacteria. Arch Oral Biol. 2016;71:97–103. https://doi.org/10.1016/j.archoralbio.2016.07.005.
Yano A, Kaneko N, Ida H, Yamaguchi T, Hanada N. Real-time PCR for quantification of Streptococcus mutans. FEMS Microbiol Lett. 2002;217(1):23–30. https://doi.org/10.1111/j.1574-6968.2002.tb11451.x.
Zeng X, Sheiham A, Sabbah W. The association between dental caries and television viewing mong Chinese adolescents in Guangxi. China BMC Oral Health. 2014;14:138. https://doi.org/10.1186/1472-6831-14-138.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that he/she have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tantikalchan, S., Mitrakul, K. Association between Bifidobacterium and Scardovia Wiggsiae and caries-related factors in severe early childhood caries and caries-free Thai children: a quantitative real-time PCR analysis and a questionnaire cross-sectional study. Eur Arch Paediatr Dent 23, 437–447 (2022). https://doi.org/10.1007/s40368-022-00702-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40368-022-00702-0