Abstract
Objectives
This study evaluated the color alteration, cytotoxicity, and hydrogen peroxide (HP) diffusion associated with different in-office bleaching protocols.
Materials and methods
Bovine enamel/dentin disks were subjected to three bleaching sessions with 35 % HP (three 15-min applications), 35 % HP (one 45-min application), or 20 % HP (one 45-min application). The control group was not bleached. Before bleaching, the disks were adapted to artificial pulp chambers positioned in compartments containing 1 ml of acetate buffer or medium, so that the dentin remained in contact with these substances. Immediately after bleaching, the HP that diffused through the disks was stabilized by acetate buffer and was quantified (two-way repeated measures ANOVA/Fisher’s protected least significant difference (PLSD) test; α = 5 %). Cells of mouse dental papilla cell-23 (MDPC-23) were incubated in this culture media for 1 h, followed by analysis of cellular metabolism (methyl tetrazolium assay) (one-way ANOVA/Tukey test; α = 5 %) and morphology (scanning electron microscopy). The specimen color alteration (ΔE) was analyzed by reflection spectrophotometry (two-way repeated measures ANOVA/Fisher’s PLSD test; α = 5 %).
Results
All protocols showed equal effectiveness at the end of the treatment. HP diffusion was significantly higher in the groups bleached with 35 % HP. Reapplication of 35 % HP resulted in increased diffusion only in the first session; however, the decrease in cell metabolism was similar for all studied protocols.
Conclusion
Despite greater peroxide diffusion in the groups treated with 35 % HP, all protocols showed the same effectiveness and were cytotoxic to MDPC-23 cells.
Clinical relevance
Bleaching protocols using high HP concentrations should be avoided because they exert aggressive actions on odontoblast-like cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tooth whitening is currently one of the most popular cosmetic procedures for patients in dental clinics since it is an effective and noninvasive treatment [1]. However, the current challenge of bleaching treatments is to define a technique that provides good cosmetic results without damaging the dental tissues, promotes high levels of satisfaction for patients who want an affordable and fast treatment, and produces minimal side effects.
The bleaching process is believed to occur because of the low molecular weight of hydrogen peroxide (HP), which diffuses through the enamel and dentin [2], releasing reactive oxygen species (ROS) that react with other free or weakly bound substances, and there after regains molecular stability. This oxidant phenomenon may explain the complex mechanism of dental bleaching [2].
Despite the esthetic improvement obtained from most of the bleaching procedures currently available, the penetration of HP and its toxic by products in the pulp-dentin complex [3–6] is responsible for pulpal damage ranging from a transient inflammatory response to the occurrence of local necrosis [3, 5, 7]. The intensity of these negative effects is thought to be closely related to the amount of ROS that come into contact with the pulp cells; thus, the resulting damage increases as the concentration and exposure time to the bleaching product increase [3, 7–11]. Most of the in-office bleaching products are 35 to 38 % HP based; however, new products based on 20 % HP were placed on the market, but there are few studies comparing the efficacy and the effects on pulp of this new concentration to traditional products.
Within this context, although the literature questions whether the use of bleaching agents with a high concentration of HP is necessary or even safe, these products are being indicated, applied, and reapplied multiple times in the same clinical session in order to increase the speed of changing the color of the teeth. Although the whitening effect is known to be related to the diffusion of peroxide through the dental tissues, studies suggest that this diffusion is not related to the constant reapplication of the gel because good results have been obtained with the technique of a single clinical application [12, 13]. The continuous exchanges have been justified by the rapid degradation of the peroxide after its application in trays in the at home technique [14, 15]. However, recent studies show that the rate of decomposition is relatively small for the products used by the “in-office” technique [16, 17], and this finding might support the adoption of a new dosage that is based on a single application of the bleaching product. Thus, given that high concentrations of peroxide are potentially harmful to the pulp cells [3, 7, 9–11], the study of posologies that are guided by the adoption of milder protocols is both appealing and justifiable in an effort to find safe alternatives to bleaching.
Thus, the aim of this study was to compare the bleaching effect, the HP diffusion, and the cytotoxicity associated with three in-office dental bleaching protocols. Specifically, the traditional posology based on three applications of a 35 % HP-based product was compared with one application of the same product and with a product with a lower concentration of peroxide (20 % HP).
Materials and methods
Obtaining specimens
Sound permanent bovine incisors from 24 and 30-month-old bullocks were used. Teeth with stains, excessive wear of the incisal third of the crown, morphological changes in enamel, or cracks were excluded.
Enamel/dentin disks with a diameter of 5.2 mm were removed from the middle third of the labial surface of the bovine incisor. Each bovine tooth gave rise to 1 disk. The dentin surface was reduced with 400 and 600 grit silicon carbide paper (T469-SF, Norton Abrasives, Saint-Gobam Abrasivos Ltda., Jundiai, SP, Brazil) until the disk had a thickness of 3.5 mm: approximately 1.3 mm (±0.2 mm) enamel and 2.2 mm (±0.2 mm) of dentin. The dentin surface of the disks was treated with 0.5 M ethylenediaminetetraacetic acid (EDTA), pH 7.4, for 30 s, followed by a rinse with deionized water to remove the smear layer [18].
Preparation of the artificial pulp chamber
The artificial pulp chambers (APCs) [10, 11, 19] were made of stainless steel and had two compartments. The upper compartment contained an opening with a diameter of 8 mm, while the lower portion had a diameter of 6 mm, which allowed appropriate positioning of the enamel/dentin disk. Thus, the specimens were adapted in the upper compartment between two silicon rings, which maintained fixation of the disks. Additional sealing was performed between the disk and the sidewall of the APC by using wax. The lower compartment side had holes to allow circulation of the solutions that remained in contact with the dentin surface (the acetate buffer solution used in the peroxide quantification or the culture medium for evaluation of cytotoxicity).
Bleaching procedure
Group 1 (G1), the control group, received no bleaching treatment. For group 2 (G2), the specimens were treated with a product containing 35 % HP (Whiteness HP Maxx, FGM, Joinvile, SC, Brazil). The product was handled according to the manufacturer’s instructions, and 0.04 ml of the product was applied to each specimen and was kept in contact with the enamel for 15 min. Subsequently, the gel was aspirated from the enamel surface and the product was reapplied twice, resulting in a total exposure period of 45 min to the bleaching material, as indicated by the manufacturer. For group 3 (G3), the specimens were treated with the same product described above; however, the material was applied only once and was left on the enamel for 45 min. For group 4 (G4), the specimens were treated with a product containing 20 % HP (Whiteness HP Blue, FGM, Joinvile, SC, Brazil). After the product was prepared according to the manufacturer’s instructions, 0.04 ml of the product was applied on the tooth surface for 45 min.
The bleaching procedures were performed three times with an interval of 1 week between treatments (three bleaching sessions, one session per week). The enamel and the dentin remained moistened with artificial saliva and distilled water, respectively, during the periods between the bleaching procedures. The APCs with the disks were left in 24-well plates at 37 °C that worked like a humidified chamber.
Color evaluation
Color assessment of the specimens (n = 15) was performed by using an ultraviolet visible spectrophotometer, Model UV-2450 (Shimadzu, Kyoto, Japan), which uses the color model CIE L* a* b* established by the Commission Internacionale I’Eclairage—CIE (International Commission on Illumination). For this purpose, we made a black silicone mold containing a central hole, which allowed standard positioning of the specimen during the readings. Three measurements were obtained for each specimen, and the average between them was calculated. The analyses were performed before the initiation of treatment, 24 h after each bleaching session, and 7 days after the end of treatment. The CIE L* a* b* color calculates the distance between two points by using the following formula: ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2. After calculating the ΔE values, the data were analyzed by two-way repeated measures ANOVA/Fisher’s protected least significant difference (PLSD) test, with an accepted significance level of 5 %.
Quantification of HP diffusion
To quantify the diffusion of HP (n = 15), the disks of enamel/dentin were positioned on the APCs and were placed in 24-well plates (Costar Corp., Cambridge, MA, USA) containing 1 ml of acetate buffer solution (2 mol/M, pH 4.5). The dentin surface remained in contact with this solution, and the enamel was exposed to the bleaching gel. Immediately after bleaching, 20 μl of the buffer solution was transferred to tubes containing 100 μl of 0.5 mg/ml leucocrystal violet (Sigma Chemical Corp., St. Louis, MO, USA), 50 μl of 1 mg/ml horseradish peroxidase enzyme solution (Sigma Chemical Co.), and 2,750 ml of distilled water. This method is based on the reaction of HP with the leucocrystal violet, which is catalyzed by the peroxidase enzyme [20]. The color of the mixture varies in intensity according to the amount of peroxide, making it possible to evaluate the amount of diffused peroxide. The optical density of the resulting blue solution was measured in a reflection ultraviolet visible spectrophotometer (UV-2450, Shimadzu, Kyoto, Japan) at a wavelength of 596 nm. A standard curve with absorbance values of known HP concentrations was used to convert the obtained optical density values into micrograms of peroxide per milliliter of acetate buffer solution. The HP quantification was performed after each bleaching session, and the data were analyzed statistically by two-way repeated measures ANOVA/Fisher’s PLSD test, with significance accepted at 5 %. The color alteration analyses and quantification of HP diffusion were done concomitantly on the same disks, while the other specimens were used for cytotoxicity tests.
Cytotoxicity
Other disks were done for these tests, and different specimens were used for each period analyzed; thus, for cytotoxicity, we had ten groups (Table 3). Each bleaching period was carefully programmed so that all experimental groups finished the treatment on the same day with the purpose of applying the extract collected on the same cell culture seeded at the same time for all groups. Initially, cells of mouse dental papilla cell-23 (MDPC-23) [21] were seeded (30,000 cells/cm2) into the wells of 24-well acrylic plates using plain Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (Gibco, Grand Island, NY, USA). The plates were maintained in an incubator (Isotemp, Fisher Scientific, Pittsburgh, PA, USA) at 37 °C with 5 % CO2 for 72 h. The disks/APC sets were sterilized by ethylene oxide and then placed in 24-well plates containing 1 ml DMEM without fetal bovine serum. Bleaching was performed on the enamel surface, according to the experimental groups described above, for one, two, or three sessions. The culture medium in contact with the dentin was replaced in each session. Immediately after each session, 500 μl of the extract (culture medium containing the components of the degraded bleaching gel that diffused through the dentin/enamel specimens) was applied on previously cultured MDPC-23 cells for 1 h. Cell metabolism was assessed by using the methyl tetrazolium (MTT) assay, and cell morphology was examined by scanning electron microscopy (SEM). Three independent experiments were performed in the present investigation in order to verify the reproducibility of data.
MTT assay
Cell metabolism analysis (n = 11) was performed by using the MTT assay [22] by cytochemical demonstration of succinic dehydrogenase enzyme (SDH), which represents the rate of mitochondrial respiration. The cells were incubated with the extract, which was subsequently replaced with MTT solution (5 mg/ml, Sigma Chemical Co.) for an incubation period of 4 h at 37 °C with 5 % CO2. Then, the formazan crystals formed by the viable cells were diluted with acidified isopropanol solution (0.04 N HCl), and the absorbance was measured in an ELISA reader (570 nm; Tp Reader). The mean absorbance values of the control group were considered as 100 % cell viability. Then, the absorbance values of each sample were transformed into percentages according to this parameter, and the percentages values were used for statistical analysis. The data were submitted to ANOVA and Tukey test, with significance accepted at 5 %.
Cell morphology (SEM)
Cell morphology (n = 2) was examined with a scanning electron microscope (Philips FEG XL 30; Oxford Instruments, Inc., Concord, MA, USA). Previously sterilized glass cover slips (12 mm in diameter) were placed in the bottom of the compartments immediately before cultivating the cells. After 60 min of incubating the cells with the extracts, the extract was aspirated, the cells were rinsed with phosphate buffer saline solution, and the cells were fixed with 2.5 % buffered glutaraldehyde at room temperature. Subsequently, the cells were postfixed with 1 % osmium tetroxide, dehydrated in a series of increasing ethanol concentrations (30, 50, 70, 95, and 100 %), and immersed in 1,1,1,3,3,3-hexamethyldisilazane. The cover glasses containing the cells were mounted on stubs, stored in a vacuum desiccator for 72 h at room temperature, and sputter-coated with a gold layer.
Results
All bleaching protocols evaluated promoted a significant ΔE alteration when compared to the control group. Color alteration was continuous from the first bleaching session, but partial color rebound was observed 1 week after the end of bleaching (Table 1). Although the group in which 20 % HP was used (G4) showed less color change compared to the group in which three applications of 35 % HP were used (G2) after the last session, all treatments showed similar performance at the other evaluated periods, including at 28 days.
The lowest HP penetration value was obtained in the 20 % HP group (G4) in all sessions. The groups in which 35 % HP for one application of 45 min (G3) and three applications of 15 min (G2) were used differed from each other only in the first session, with the protocol that used the reapplication causing the highest diffusion (Table 2).
Further, we observed a significant reduction in cell viability in all bleached groups when compared to the negative control. No differences were observed between sessions, as well as when the bleaching protocols were compared (Table 3).
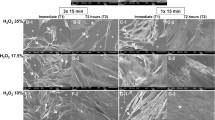
Representative images of the effects of each bleaching protocol on MDPC-23 cells are shown in Fig. 1. A large number of cells adhering to the glass substrate were observed in the control group, and these cells exhibited a wide cytoplasm covering practically the entire substrate. On the other hand, significant changes in cell morphology were observed when the bleaching gels were applied to the enamel/dentin disks, regardless of the bleaching protocol used. Large areas of the glass substrate were exposed in the bleached groups, indicating that cell death and detachment from the glass had occurred, and the few cells that remained attached showed profound alterations in morphology.
SEM × 1,000. Representative micrographs of the control and experimental groups. a Control: MDPC-23 cells exhibited a wide cytoplasm and covered the glass of the substrate on which they had been cultured, with visible cell proliferation (mitosis) (asterisks). b 35 % HP 3 × 15 min: Numerous cells that had been attached to the substrate died and detached from the glass substrate (GS). The few cells that remained on the substrate exhibited remarkable shrinkage of the cytoplasm. This cellular reaction caused the rounded morphology of the cells, and some thin cytoplasmic processes originating from the cell membranes appeared to adhere these cells to the glass substrate (arrow). c 35 % HP 1 × 45 min: Similar to the observations in image b, a decreased number of cells adhered to the glass, and these cells exhibited a more rounded morphology. Large areas of the cover glass were exposed (GS). d 20 % HP: Although more MDPC-23 cells remained on the glass substrate compared to the images of the 35 % HP groups, these cells showed significant morphological changes (arrow)
Discussion
The in-office bleaching technique that is performed by a dentist in the dental office employs high concentrations of peroxide on the enamel surface. The clinical sessions intended for this procedure last up to 50 min, and the majority of the manufacturers recommend frequent reapplication of the product during the same clinical session.
Reis et al. [23] showed in vivo that 35 % HP in three 15-min applications caused more color alteration than a single 45-min application. However, in our study, all bleaching techniques provided similar color alterations, indicating that they are equally effective. A similar effectiveness using protocols with 35 % HP with and without the reapplication of the product was reported in other studies [12, 13, 24]. This topic is controversial and there are few studies comparing these posologies showing the need for more research to clarify this finding.
Marson et al. [24] evaluated the decomposition rate of the Whiteness HP Maxx product and noted that after 45 min of contact with the tooth surface the degradation of the product was 11.1 %, suggesting that product replacement is unnecessary. However, other factors must be considered for the maintenance of the product on the tooth surface. The pH on the enamel must be maintained at safe levels during product application in order to prevent significant changes in the surface of this tissue [16, 25]. Trentino et al. [26] evaluated the pH of the Whiteness HP Maxx product based on 35 % HP (with and without reapplication) and showed that maintaining the product for an extended time does not compromise the safety of the procedure because the pH remained above the critical value for the enamel.
Other studies have also shown that products with different concentrations of the active component, including those used in at-home techniques [27–29] and in-office techniques [28–30], have the same bleaching effect at the end of treatment. Matis et al. [29] compared eight HP-based products at different concentrations (15 to 35 %) applied for different periods on the tooth surface. The authors suggested that, for the in-office technique, the duration of contact of the product with the dental tissue has a greater influence on the bleaching effect compared to the concentration. The same authors reported a color rebound rate of 51 % at 1 week after treatment and of 65 % at 6 weeks after treatment [29]. This high color rebound rate is probably due to the timing of the initial readings because they were performed immediately after the end of bleaching. This fact, associated with the use of light sources and the high osmotic power of the products, possibly increased dental dehydration. In this study, to avoid registering a “false whitening effect” caused by dehydration after bleaching [31], the color reading was performed 24 h after each session. Although the analysis was performed 24 h post treatment, color rebound was observed after a week, which is probably because complete rehydration occurs up to 2 weeks after bleaching [32].
Regarding HP diffusion, higher penetration was observed in the group that received three 15-min applications of 35 % HP compared to the group that received a single application of 45 min of 35 % HP only in the first session. Although the statistical analysis reveals this specific difference, a global analysis of our data produced results that were consistent with those obtained by Kwon et al. [17]. These authors found a constant penetration of HP during 1 h application, suggesting the possibility of maintaining the product on the enamel for a period longer than 15 min.
The group that was bleached with 20 % HP (G4) showed lower diffusion compared to the two protocols in which 35 % HP product (G2, G3) was used. The pH of Whiteness HP Blue and Whiteness HP Maxx with or without reapplications remains above the critical value for enamel (pH 5.5). Thus, we believed that the higher penetration of 35 % HP-based product can be explained by the different diffusion coefficients for each product, which depends on the amount of available peroxide, the thickness to be penetrated, and the application time. Thus, the diffusion of the peroxide to the pulp chamber tends to be proportional to the concentration of the bleaching agent [4]. Furthermore, there is calcium in the composition of the Whiteness HP Blue, which tends to minimize possible structural alterations in the enamel resulting from the application of the bleaching product by increasing the saturation of the gel, reducing mineral loss, and increasing enamel resistance to demineralization [33]. This fact may also have contributed to the lower penetration of this product than that seen in other protocols that used the Whiteness HP Maxx. However, this difference in the amount of HP that diffused to the disks did not significantly affect the cytotoxicity in vitro. In part, this discrepancy in the results can be attributed to the fact that the test we used detects the diffusion of HP, but it does not register other types of ROS, which may have also diffused and induced toxic effects in MDPC-23 cells. Similar observations were reported by Sacono et al. [10], who observed no significant difference in cytotoxicity on MDPC-23 cells when using bleaching gels based on 38 and 20 % HP. Our results showed that all of the protocols used in the experimental model were toxic to MDPC-23 cells.
The average decrease in cell viability observed in this study was 35.49 % for G2 (treatment with three 15-min applications of 35 % HP), 37.10 % for G3 (a single application of 35 % HP for 45 min), and approximately 30 % for G4 (an application for 45 min of 20 % HP). The aggressive effect of high concentrations of peroxide has been highlighted by several researchers. Coldebella et al. [11] and Trindade et al. [3] observed decreases of 62.09 and 92.03 % in MDPC-23 cell metabolism after one and three 15-min applications of 35 % HP, respectively. Sacono et al. [10] found a reduction of 97.18 % in the metabolism of the same cells after three 10-min applications of 38 % HP and 96.29 % after a 45-min application of 20 % HP. However, the cells had contact with the extract containing HP for 24 h in these studies, whereas the cells had contact for 1 h in the present study. In a recent study, Soares et al. [19] observed a reduction of approximately 40.4 % in MDPC-23 cell metabolism after contact for 1 h with the extract obtained from three applications of a gel with 35 % HP in enamel/dentin disks adapted in the APCs. Thus, HP may remain active for long periods in culture medium, and the major in vitro toxic effects may increase as the contact time with the cells increases.
The SEM images of the bleached groups revealed that the components of the gels that diffused through the enamel and dentin had deleterious effects on MDPC-23 cells. These effects were characterized by severe morphological alterations in MDPC-23 cells that remained attached to the glass substrate. Only cell membrane remnants were observed in the cell-free areas, showing that local toxicity caused the death of a large number of MDPC-23 cells. These effects can be attributed to the action of the peroxide itself, as well as to hydroxyl radicals (OH−) and other ROS resulting from the degradation of the bleaching gel [2]. The presence of high concentrations of these substances in contact with the cells has been shown to cause oxidative stress [34], which may result in a decrease in cell proliferation [35], lipid peroxidation and protein fragmentation, with consequent injury to the cellular membrane [8], causing cell death by necrosis or apoptosis [36].
In general, it can be speculated that the technique with a single application provides benefits such as reducing the clinical time and treatment costs because a smaller amount of material would be required for the patient. Furthermore, the risks of accidents due to possible contact of the gel with the adjacent soft tissues would be reduced. However, in the experimental model used in this study, it was found that all of the techniques were cytotoxic to odontoblast-like cells. Thus, the negative results observed in this study corroborate with previous studies [3, 7, 9–11, 19, 37] and indicate the necessity of reassessing the safety of the currently used in-office bleaching technique.
The results of this study should be interpreted cautiously because it was an in vitro study. This study had several limitations. First, bovine teeth were used because human teeth are more susceptible to penetration of peroxide than bovine teeth [4]. Further, because the data on peroxide diffusion and cytotoxicity only refer to the last application of the product, it was not possible to assess the cumulative effect of the sessions, in which the bleaching procedure may have been more aggressive. In addition, several factors such as intrapulpal pressure, the presence of cytoplasmic prolongations in dentinal tubules [38, 39], antioxidant enzymes, and the defense mechanisms of the pulp [40] could decrease the diffusion of peroxide as well mitigate the intensity of the aggression.
Although physiological mechanisms exist that make it difficult for HP and its by-products to diffuse through the enamel and dentin and reach the pulp, in an in vivo study, de Souza Costa et al. [7] observed an intense inflammatory reaction associated with areas of coagulation necrosis in the coronal pulp tissue of human mandibular incisors subjected to 38 % HP applied by the 30-min technique, which is similar to the techniques evaluated in this research. In the study by Sato et al. [37], the premolars of young patients subjected to 45 min of treatment with 35 % HP showed induction of oxidative stress in the pulp tissue that was associated with the intense activity of metalloproteinase and cathepsin B, which are proteases closely associated with extracellular matrix degradation. Thus, according to the results of the present study and the results of other laboratory and clinical studies in the literature, the evaluated techniques present risks such as pulp tissue damage, especially when the thickness of the enamel and dentin of the targeted teeth is small.
Conclusion
According to the methodology used in this study, it was concluded that all treatments using bleaching gels with high concentrations of HP (20–35 %) result in the same color change in tooth structure and result in diffusion of HP through the enamel and dentin, which significantly reduces the metabolism of MDPC-23 odontoblast-like cells.
References
Almeida LC, Riehl H, Santos PH, Sundfeld ML, Briso AL (2012) Clinical evaluation of the effectiveness of different bleaching therapies in vital teeth. Int J Periodon Restor Dent 32:303–309
Kawamoto K, Tsujimoto Y (2004) Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J Endod 30:45–50
Trindade FZ, Ribeiro APD, Sacono NT, Oliveira CF, Lessa FC, Hebling J, Costa CA (2009) Trans-enamel and trans-dentinal cytotoxic effects of a 35% H2O2 bleaching gel on cultured odontoblast cell lines after consecutive applications. Int Endod J 42:516–524
Camargo SE, Valera MC, Camargo CH, Gasparoto Mancini MN, Menezes MM (2007) Penetration of 38% hydrogen peroxide into the pulp chamber in bovine and human teeth submitted to office bleach technique. J Endod 33:1074–1077
Fugaro JO, Nordahl I, Fugaro OJ, Matis BA, Mjör IA (2004) Pulp reaction to vital bleaching. Oper Dent 29:363–368
Briso A, Lima A, Gonçalves R, Gallinari M, Santos PD (2014) Transenamel and transdentinal penetration of hydrogen peroxide applied to cracked or microabrasioned enamel. Oper Dent 39:166–173
de Souza Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 10:59–64
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
de Almeida LC, Costa CA, Riehl H, dos Santos PH, Sundfeld RH, Briso AL (2012) Occurrence of sensitivity during at-home and in-office tooth bleaching therapies with or without use of light sources. Acta Odontol Latinoam 25:3–8
Sacono NT, Coldebella CR, Ribeiro APD, Soares DGS, Trindade FZ, Hebling J, Costa CAS (2010) Cytotoxic effect of a 20% and a 38% hydrogen peroxide bleaching agents on odontoblast-like cells. Rev Odontol Bras Central 18:15–21, Abstract
Coldebella CR, Ribeiro AP, Sacono NT, Trindade FZ, Hebling J, Costa CA (2009) Indirect cytotoxicity of a 35% hydrogen peroxide bleaching gel on cultured odontoblast-like cells. Braz Dent J 20:267–274
Marson FC, Sensi LG, Strassler H, Miraziz L, Riehl H, Reis R (2008) In-office bleaching gel application times: clinical evaluation. J Dental Res (Special Issue) Abstract #1028
Marson FC, Sensi LG, Strassler Riehl, H, & Reis R (2008) In-office bleaching gel application time evaluation(3 × 15 min × 1 × 45 min): pilot studies. Journal of Dental Research 87(Special Issue B) Abstract #1027.
Thomé T, Melara R, Salaverry A, Brandalise C, Goulart M, Conceição EM, Erhardt MC, Coelho-de-Souza FH, Rolla JN, Conceição AB (2011) Clinical evaluation of in-office bleaching gel application times. J Dent Res 90 (Special Issue A) Abstract #561
Al-Qunaian TA, Matis BA, Cochran MA (2003) In vivo kinetics of bleaching gel with three-percent hydrogen peroxide within the first hour. Oper Dent 28:236–241
Matis BA, Gaiao U, Blackman D, Schultz FA, Eckert GJ (1999) In vivo degradation of bleaching gel used in whitening teeth. J Am Dent Assoc 130:227–235
Kwon SR, Oyoyo U, Li Y (2013) Effect of light activation on tooth whitening efficacy and hydrogen peroxide penetration: an in vitro study. J Dent 41:39–45
Jacques P, Hebling J (2005) Effect of dentin conditioners on the microtensile bond strength of a conventional and a self-etching primer adhesive system. Dent Mater 21:103–109
Soares DG, Ribeiro APD, Vargas FS, Hebling J, Costa CAS (2013) Efficacy and cytotoxicity of a bleaching gel after short application times on dental enamel. Clin Oral Investig 17:1901–1909
Mottola HA, Simpson BE, Gorin G (1970) Absorptiometric determination of hydrogen peroxide in submicrogram amounts with leuco crystal violet and peroxidase as catalyst. Anal Chem 42:410–411
Hanks CT, Sun ZL, Fang DN, Edwards CA, Wataha JC, Ritchie HH, Butler WT (1998) Cloned 3T6 cell line from CD-1 mouse fetal molar dental papillae. Connect Tissue Res 37:233–249
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Reis A, Tay LY, Herrera DR, Kossatz S, Loguercio AD (2011) Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 36:590–596
Marson FC, Gonçalves RS, dos Santos PH, Silva CO, Pascotto RC, Cintra LTA, Briso ALF (2014) Penetration of hydrogen peroxide and degradation rate of different bleaching product. Oper Dent. doi:10.2341/13-270-L (in press).
Price RB, Sedarous M, Hiltz GSJ (2000) The pH of tooth-whitening products. Can Dent Assoc 66:421–426
Trentino AC, Mondelli RFL, Azevedo LM, Wang L, Ishikiriama SK, Atta MT (2011) Variation of pH bleaching gels and roughness on bovine enamel. J Dent Res 90 (Special Issue A) Abstract #2038
Krause F, Jepsen S, Braun A (2008) Subjective intensities of pain and contentment with treatment outcomes during tray bleaching of vital teeth employing different carbamide peroxide concentrations. Quintessence Int 39:203–209
Dietschi D, Rossier S, Krejci I (2006) In vitro colorimetric evaluation of the efficacy of various bleaching methods and products. Quintessence Int 37:515–526
Matis BA, Cochran MA, Franco M, Al-Ammar W, Eckert GJ, Stropes M (2007) Eight in-office tooth whitening systems evaluated in vivo: a pilot study. Oper Dent 32:322–327
Basting R, Amaral F, França F, Flório F (2012) Clinical comparative study of the effectiveness of and tooth sensitivity to 10% and 20% carbamideperoxide home-use and 35% and 3% hydrogen peroxide in-office bleaching materials containing desensitizing agents. Oper Dent 37:464–473
Jones AH, Diaz-Arnold AM, Vargas MA, Cobb DS (1999) Colorimetric assessment of laser and home bleaching techniques. J Esthet Dent 11:87–94
Haywood VB (1996) Achieving, maintaining and recovering successful tooth bleaching. J Esthet Dent 8:31–38
Giannini M, Silva AP, Cavalli V, Paes Leme AF (2006) Effect of carbamide peroxide-based bleaching agents containing fluoride or calcium on tensile strength of human enamel. J Appl Oral Sci 14:82–87
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255
Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN (2007) Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim Biophys Acta 1773:93–104
Saito Y, Nishio K, Ogawa Y, Kimata J, Kinumi T, Yoshida Y, Noguchi N, Niki E (2006) Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res 40:619–630
Sato C, Rodrigues FA, Garcia DM, Vidal CM, Pashley DH, Tjäderhane L, Carrilho MR, Nascimento FD, Tersariol IL (2013) Tooth bleaching increases dentinal protease activity. J Dent Res 92:187–192
Sauro S, Pashley DH, Montanari M, Chersoni S, Carvalho RM, Toledano M, Osorio R, Tay FR, Prati C (2007) Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dent Mater 23:705–713
Vongsavan N, Matthews B (1991) The permeability of cat dentine in vivo and in vitro. Arch Oral Biol 36:641–646
Esposito P, Varvara G, Murmura G, Terlizzi A, Caputi S (2003) Ability of healthy and inflamed human dental pulp to reduce hydrogen peroxide. Eur J Oral Sci 111:454–456
Acknowledgments
The authors acknowledge the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; Grant numbers 2010/10378-2 and 2010/17637-3) and the Fundação para o Desenvolvimento da Unesp (Fundunesp; Grant number 0113610) for financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Almeida, L.C.A.G., Soares, D.G., Gallinari, M.O. et al. Color alteration, hydrogen peroxide diffusion, and cytotoxicity caused by in-office bleaching protocols. Clin Oral Invest 19, 673–680 (2015). https://doi.org/10.1007/s00784-014-1285-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1285-3