Abstract
Objectives
To evaluate human dental pulp stem cell viability and capacity to recover from experimental dental bleaching techniques.
Material and methods
Enamel/dentin disks adapted to trans-wells were positioned on previously cultivated dental pulp stem cells. Bleaching gels containing 35, 17.5, 10, and 8 % hydrogen peroxide (H2O2) were applied one or three times (each application lasting 15 min) on enamel. Cell viability (MTT assay) and morphology (SEM) were evaluated immediately (T1) or 72 h (T2) post-bleaching.
Results
The 35 % H2O2 gel promoted intense reduction in viability (93–97 %) and morphological alterations of the cells at T1, irrespective of frequency of application, with absence or limited capacity for recovery being observed at T2. The other bleaching gels presented significant lower toxicity when compared with the 35 % H2O2 gel, in a time/concentration fashion. In T1, no significant difference was observed between the negative control (without bleaching) and the 8 and 10 % H2O2 gels applied on enamel for 15 min, in which the cells presented elevated viability and morphology similar to the negative control at T2.
Conclusions
Bleaching gels with 8 and 10 % H2O2 in their composition cause limited immediate toxic effect on pulp stem cells, which recover their viability 3 days after treatment.
Clinical relevance
This study presents proposals for in-office dental bleaching to be performed with limited aggressive effect on dental pulp stem cells. Therefore, we are able to offer interesting clinical alternatives for bleaching vital teeth, under professional supervision, maintaining the integrity and reparative capacity of pulp-dentin complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional in-office bleaching is based on high concentrated hydrogen peroxide (H2O2) gels, applied for relative short periods (30–45 min) on dental enamel. This type of clinical esthetic procedure, which is performed under professional supervision, results in rapid color change of the dental structure [1–4]. However, the high prevalence of post-bleaching tooth sensitivity, which intensity varies from moderate to intolerable, is the main adverse effect of this technique [1–3]. Recent clinical studies have demonstrated that this sensitivity is limited to the anterior teeth [5, 6], with it being more intense in those teeth that have a small enamel/dentin thickness, such as the incisors [6]. In a histological analysis of human mandibular incisors submitted to this therapy, the occurrence of areas of partial coronal pulp necrosis associated with an inflammatory reaction in the subjacent tissue was demonstrated [7]. These clinical data corroborate laboratory studies, in which researchers demonstrated the occurrence of intense trans-enamel and trans-dentinal cytotoxicity to pulp cells when bleaching gels with elevated concentrations of H2O2 were applied to tooth enamel [4, 8].
Up to the present time, at-home bleaching using 10 % carbamide peroxide (CP) has been considered the safest bleaching therapy for the pulp-dentin complex [8–11]. However, this technique has several limitations, such as the fact that the professional does not directly supervise the entire treatment and the long bleaching time needed to achieve significant color improvement as well as the risk of gel application over exposed dentin and soft tissue [10]. Therefore, alternative professional bleaching therapies, with the goal of minimizing the toxic effect on pulp cells, have been the target of diverse contemporary researches [4, 8]. Recently, it was demonstrated that a reduction from 35 to 17.5 % in the concentration of H2O2 in a bleaching gel, and a reduction in the time of the product in contact with enamel, resulted in effective bleaching associated with a reduction of around 60 to 95 % in H2O2 diffusion through the tooth structure, when compared with the traditional therapy [14]. Consequently, there was significant reduction in trans-enamel and trans-dentinal cytotoxicity to pulp cells when these protocols were tested. However, the dental pulp stem cells of human teeth, which are fundamental for the maintenance of homeostasis and the repair capacity of the dentin-pulp complex, continued to be shown to be very sensitive to the techniques tested [4]. Therefore, in the present study, the immediate cytotoxicity and capacity for recovery of human dental pulp stem cells exposed to the experimental in-office bleaching techniques, with variations in the concentration of H2O2 and frequency of applying the product on enamel, were evaluated. The hypothesis of this study is that the concentration of H2O2 in the bleaching gel and its contact time with dental structure has significant effect on the immediate trans-enamel and trans-dentinal toxicity of the bleaching procedure as well as on pulp cells proliferative capability through time.
Materials and methods
Obtaining the specimens
Enamel/dentin disks, from bovine incisors, measuring 5.6 mm in diameter and 3.5 mm thick were used in this study, as previously described [4, 8, 11]. The enamel surface of the disks was cleaned with solution of pumice stone and water using a low-speed hand piece. The dentin surface was regularized by wear with 400 and 600 grit water abrasive papers (T469-SF- Norton, Saint-Gobam Abrasivos Ltda., Jundiaí, SP, Brazil), and the smear layer created at the site was removed by application of an EDTA solution (0.5 N) for 30 s. After the disks were abundantly washed with water, and they were individually adapted in an acrylic trans-well devices (Corning Inc., Corning, NY, USA) with the aid of light polymerizing fluid resin (Top-dam®; FGM, Joinville, SC, Brazil) [4], and the set was sterilize with ethylene oxide.
Cell culture
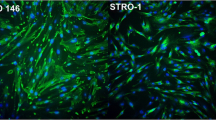
A primary culture of human dental pulp cells was obtained by enzymatic digestion of the pulp tissue from the third molars of volunteers, after they had signed the term of free and informed consent (Proc. No. 13/11; approved by the Research Ethics Committee of the Araraquara Dental School/UNESP, São Paulo, Brazil). Immediately after the teeth were extracted, the pulp tissue was aseptically removed, fragmented, and incubated in DMEM culture medium (Dulbecco’s Modified Eagle’s Medium; Sigma Chemical Co., St. Louis, MO, USA) containing 10 % fetal bovine serum (FBS; GIBCO, Grand Island, NY, USA), 100 IU/mL and 100 μg/mL of penicillin and streptomycin, respectively, 2 mmol/L of glutamin (GIBCO), and 200 UI/mL of collagenase (Worthington Biochemical Corporation, Lakewood, NJ, USA), at 37 °C and 5 % CO2, for the period of 24 h. After this period, the cells were centrifuged and subcultured in cell culture bottles in complete DMEM, without collagenase. Stem cell characterization was performed by immunofluorescence assay. Cells in the third passage were seeded in 96-well plates in a pattern of 80 % confluence. After this, the cells were fixed in 4 % buffered paraformaldehyde, then permeabilized with 0.1 % Triton (Sigma Chemical Co.) and blocked in 5 % BSA (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Next, the cells were incubated with the following primary antibodies: Nanog, Oct 3/4, STRO-1, CD-44, and CD-146 (1:50; Santa Cruz Biotechnology) at 4 °C for 12 h, followed by incubation for 1 h with secondary antibody conjugated with FITC (fluorescein isothiocyanate. 1:1000; Santa Cruz Biotechnology) at room temperature. After this, the cells were incubated with Hoechst (1:5000; Invitrogen San Francisco, CA, USA) for nuclear contra-fluorescence. The cells were analyzed in the IN Cell Analyser 2000 (GE Healthcare Life Sciences, Freiburg, Germany), with six images being obtained per compartment, and the percentage of positively marked cells was obtained by means of the IN Cell Investigation software program (GE Healthcare Life Sciences). Positive marking was observed in 57.7 % for CD-146; 83.8 % for Nanog; 97.2 % for Oct 3/4; 72.8 % for STRO-1; and 83.9 % for CD-44. These data demonstrated that the primary culture of pulp cells used represented a culture with the predominance of stem cells [15, 16].
Experimental procedure
The cells (passage 3) were seeded in 24-well plates (60,000 cells per well) and remained in an incubator at 37 °C and 5 % CO2 for 24 h, obtaining 80 % confluence. After this period had elapsed, the culture medium was replaced with 300 μL of DMEM without FBS, and the disk/trans-well set was placed on the compartment in which the cells were seeded, so that the dentin surface was kept in intimate contact with the culture medium and the enamel surface remained exposed in order to receive the bleaching gels. Gels with decreasing concentrations of H2O2 were obtained by diluting the liquid of a 35 % H2O2 bleaching gel (Whiteness HP, FGM, Joinvile, Brazil), immediately before the bleaching procedure, thereby obtaining the following experimental groups: G1—negative control (without treatment), G2—35 % H2O2 gel applied for 3 × 15 min, G3—35 % H2O2 gel applied for 1 × 15 min, G4—17.5 % H2O2 gel applied for 3 × 15 min, G5—17.5 % H2O2 gel applied for 1 × 15 min, G6—10 % H2O2 gel applied for 3 × 15 min, G7—10 % H2O2 gel applied for 1 × 15 min, G8—8 % H2O2 gel applied for 3 × 15 min, and G9—8 % H2O2 gel applied for 1 × 15 min. A total of 16 disk/trans-well set were used for each experimental group. Twelve of them were used to evaluate the cell viability and the other 4 were assigned to cell morphology analysis. Immediately after conclusion of the bleaching procedure, the disk/trans-well set was removed, the culture medium was aspired, and the cells were washed with buffered saline solution (PBS). Cell viability and morphology were evaluated in two distinct time intervals: T1—immediately after the bleaching procedure and T2—72 h after conclusion of bleaching. For the cells evaluated at T2, the culture medium with 10 % FBS was changed every 24 h. For each time interval, six disk/trans-well set were used for cell viability and two were used for cell morphology analyses.
Cell viability
A total of 6 disk/trans-well sets were used for each experimental group in each time interval of analysis (n = 6). For each period, the cells were incubated with MTT solution (5 mg/mL) diluted in DMEM (1:10), and were incubated at 37 °C and 5 % CO2 for 4 h. Next, the formazan crystals formed in the viable cells were dissolved in acidified isopropanol, and the absorbance was measured in an Elisa reader at 570 nm (Tp Reader, Thermoplate, Nanshan District, Shenzhen, China) [8, 9]. The mean absorbance value of the negative control group in time interval T1 was considered 100 % cell viability, with the percentage cell viability for each experimental group at T1 and T2 being calculated from this parameter.
Cell morphology
In order to perform this analysis, the cells were seeded on glass slides placed at the bottom of 24-well plates. In each time interval of analysis (n = 2), the cells were fixed in 2.5 % glutaraldehyde (Sigma Chemical Co.), and then post-fixed in 1 % osmium tetroxide (Sigma Chemical Co.), dehydrated in increasing concentrations of alcohol (30, 50, 70, 90, and 100 %) and submitted to chemical drying in HMDS (1,1,1,3,3,3-hexamethyldisilazane; Sigma Chemical Co.). Lastly, the slides with the cells were fixed on metal stubs, kept in a desiccator for 72 h, sputter-coated with gold and finally analyzed by scanning electron microscopy (JEOL JSM 6610; JEOL Ltd., Akishima, Tokyo, Japan).
Statistical analysis
Two independent experiments were performed to demonstrate the reproducibility of the results, and the data of cell viability were complied and submitted to statistical analysis. The effect of the factors “time interval of evaluation”, “H2O2 concentration,” and “frequency of application” on the response variable (cell viability) was evaluated in the following manner: initially, the different H2O2 concentrations were compared within each time interval and frequency of application. Next, the effect of frequency (one vs. three applications) was analyzed for each time interval and concentration; finally, the effect of time interval (immediate vs. 72 h) was investigated within each concentration and frequency of application. The Kruskall Wallis and Mann-Whitney statistical test were applied at a level of significance of 5 %. The statistical software program SPSS 20.0 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA) was used for the analyses.
Results
The cell viability values are shown in Table 1. It was possible to observe that the factors H2O2 concentration and frequency of application had a significant effect on stem cell viability immediately after performing tooth bleaching. In general, the higher the concentration and longer the frequency of bleaching gel application on the tooth surface, the more intense was the reduction in cell viability at T1 and the lower the cell capacity to recover at T2.
At T1 (immediately after the bleaching procedure), the 35 % H2O2 bleaching gel promoted intense reduction in cell viability when a single 15-min application of the product was made on enamel, with this effect being even greater when three applications were made. The reduction in H2O2 concentration to 17.5 % in the gel diminished the toxic effect of the product in both techniques tested, with significant difference being observed when compared with the 35 % H2O2 gel. A statistically similar result to that of the negative control was observed for the gels with concentrations of 8 and 10 % H2O2 when one 15-min application was made. It should be pointed out that there was no statistical difference when the two gels were compared with one another. Nevertheless, the increase in frequency of application (3 × 15 min) resulted in a greater toxic effect, seeing that the cell viability values for both groups (8 and 10 % H2O2) were significantly lower than those for the negative control group. However, the two gels applied according to the tested techniques presented a significantly higher cell viability than that observed for the 35 % H2O2 gel.
At T2 (72 h after the bleaching procedure), it was possible to observe that the cells that had remained viable immediately after bleaching presented the capacity to recover, since the cell viability values were significantly higher than those observed at T1, with the exception of the treatment performed with 35 % H2O2 gel applied three times × 15 min. All the groups presented a significantly lower cell viability value than the negative control group. Cell viability of over 100 % was observed for the 8 and 10 % H2O2 gels applied on enamel for 15 min. When the 8 % H2O2 gel was applied three times × 15 min, the cell viability value in T2 was similar to that found for the negative control in T1.
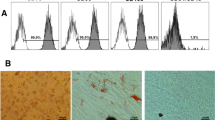
Representative images of cell morphology resulting from the tooth bleaching protocols tested in the two time intervals of analysis are represented in Fig. 1. At T1, it was possible to observe changes in the cell morphology for the groups submitted to the bleaching protocols tested. In these groups, the number of cells that remained adhered to the glass substrate exhibited contraction of the cytoplasm, characterizing smaller cells than those observed for the control group. The most intense morphological alterations in the cells occurred when the 35 % H2O2 bleaching gel was applied three times × 15 min, particularly in T2. In the other bleached groups, the cells presented ample cytoplasm in T2, almost covering the glass substrate. The groups bleached with 8 and 10 % H2O2 gels applied on the enamel one time for 15 min exhibited a cell morphology at T2, similar to that observed in the negative control at T1.
SEM Images MEV (×500) representative of the control and bleached groups, for each hydrogen peroxide concentration, and frequency of application (3 × 15 min or 1 × 15 min), in both time intervals of analysis, immediate (T1) and 72 h post-treatment (T2). A-1 The cells exhibited ample cytoplasm, covering the entire glass slide (horizontal arrows). A-2 A larger number of cells may be observed. B-1 The cells featured intense alterations in morphology, characterized by contraction in cytoplasm (oblique arrows) and reduction in the number of adhered cells, and it is possible to observe exposed areas of the glass slide (asterisk). B-2 A similar feature as B-1 is observed, demonstrating that cells presented no recovery in the morphology at T2. C-1, D-1 contraction in cytoplasm (oblique arrows) and large cells free areas (asterisk) are observed. C-2, D-2 The cells exhibit ample cytoplasm, covering almost the entire glass surface. E-1, F-1, G-1, H-1, I-1 The cells exhibit contraction on cytoplasm, and discrete reduction on the number of cells is observed, exposing some areas of the glass slide (asterisk). E-2, F-2, H-2 Cells with a wide cytoplasm, almost covering the entire glass surface, are observed. G-2, I-2 a great number of cells with wide cytoplasm are observed, featuring the same morphology as the control group at T1 (A-1)
Discussion
Dental pulp is a highly specialized connective tissue almost completely covered by mineralized tissue [17]. At the periphery of pulp, in an intimate relationship with dentin, there are the odontoblasts, terminal cells, whose main function is the physiological deposition and mineralization of the dentin matrix, or in response to external injuries [18]. In addition, these cells participate in regulation of the immune/inflammatory response of pulp tissue [19]. Thus, the odontoblasts are considered the central cells in the process of repair and maintenance of the dentin-pulp complex vitality [18]. As is the case with the odontoblasts, the stem cells present in the pulp are also primordial for the homeostasis of this tissue. Under normal conditions, these cells are capable of differentiating to replace cells that die by apoptosis [20]. However, when the pulp suffers an intense injury capable of causing the death of a large number of odontoblasts, the reparative dentinogenesis process is triggered, which involves recruitment, proliferation, and differentiation of stem cells into odontoblast-like cells [17, 20]. Therefore, the pulp stem cells are intimately related to the capacity of pulp tissue repair in the face of traumas of different origins [20]. Diverse studies have demonstrated that these cells express Nanog, Oct3/4, STRO-1, CD-44, and CD-146 markers [15, 16]. Therefore, positive response of around 58 to 97 % for these markers in the cell culture used in this study confirms the presence of a dominant population of stem cells, validating the scientific data obtained.
In vivo studies have demonstrated that in addition to the primary odontoblasts, pulp stem cells are lethally attacked when the traditional in-office bleaching therapy is performed on the healthy tooth surface [7, 21, 22]. The occurrence of damage of such magnitude promotes early pulp tissue aging, since a large number of stem cells, which would be used for odontoblast replacement throughout the life of the dental organ, are lost, thereby reducing the healing capacity of the dentin-pulp complex [20]. Therefore, new parameters for in-office tooth bleaching have been the target of various contemporary researches, with the aim of minimizing the damage to pulp cells [4, 8, 11], and the consequent tooth sensitivity related by patients [12, 13]. Recently, Soares et al. [4] demonstrated that some factors, such as (1) reduction in the time of 35 % H2O2 bleaching gel application from 45 to 15 or 5 min, and (2) a 50 % reduction in the concentration of H2O2 present in this type of gel, are capable of significantly diminishing the toxicity of the bleaching procedure to pulp cells in culture. However, the authors showed that human pulp cells were highly sensitive to the 35 and 17.5 % H2O2, applied for 45, 15, or 5 min, with the cell viability reduced by 65 to 97 %. In this study, the authors showed that when 17.5 % H2O2 bleaching gel was used, a greater reduction on H2O2 diffusion (60–95 %) through enamel and dentin occurred compared to the traditional protocol (35 % H2O2; 3 × 15 min). However, when the contact time was reduced to 5 min, the bleaching effectiveness was intensely impaired [14]. Also, even with this intense reduction on H2O2 diffusion, the immediate cytotoxicity of the 17.5 % H2O2 gel to the human dental pulp cells was high. However, the cells presented an interesting proliferative capability through time, which was up to 50 % 3 days after bleaching [4]. Therefore, in the present study, the effect of bleaching gels containing even lower concentrations of H2O2 in their composition (8 and 10 %), on the vitality, and recovery of human dental pulp stem cells was evaluated as well as the effect of the frequency of application of these gels within these parameters, and data was compared to the 35 and 17.5 % H2O2 gels previously investigated.
According to the results obtained, the dental pulp stem cells presented an intense reduction in viability with the 35 % H2O2 gel was used, irrespective of the frequency of application. After 72 h, a small recovery in cell viability was observed only when this gel was applied for 15 min. These data were corroborated by the SEM images obtained, in which intense alteration in cell morphology was observed, in addition to a reduction in the quantity of cells that remained adhered to the glass substrate; these results determined that part of the cells in contact with the H2O2 that diffused through the enamel and dentin died and became detached from the substrate on which they were cultivated (glass slides). Various other studies, using a methodology similar to that used in this investigation, have demonstrated that when a bleaching gel containing 35 % H2O2 was applied on enamel for 45 min, it caused intense damage to odontoblast-like cells, with the toxicity being proportional to the time the product was in contact with the tooth surface [4, 8, 11]. However, the reduction in the contact time from 45 to 15 min led to a reduction in the toxic effect, since the odontoblast-like cells presented around 70 to 99 % cell viability [8, 11]. In the present study, a reduction in cell viability of around 97 % was observed when dental pulp stem cells were submitted to these therapies, demonstrating the greater susceptibility of these cells to bleaching treatment when compared with cells with the odontoblastic phenotype, as observed in previously studies [4, 8].
It has been demonstrated that the cytotoxicity of bleaching gels to pulp cells in culture is caused by the direct action of the H2O2 and its byproducts (relative oxygen species—ROS), and by oxidative cell stress induced by these ROS, whose intensity is proportional to the time the product is in contact with the tooth surface and the H2O2 concentration in the bleaching gel [4]. These parameters have a direct influence on the diffusion of H2O2 and its degradation byproducts through the mineralized structures of the tooth [4, 14]. Depending on the intensity of the oxidative stress generated, the process of lipid peroxidation is initiated, due to the reaction of ROS with the fatty acids of cell membranes [23, 24]. Consequently, the cells suffer rupture of these membranes, which induces cell death by necrosis [4]. In the in vivo situation, this oxidative stress triggers an inflammatory tissue reaction [7, 21, 22], and increase in the expression of metalloproteinases and cathepsins by local pulp cells, promoting degradation of the cell matrix [25]. Also, the cells that die by necrosis release their lysosomal enzymes, which lead to cell matrix decomposition, resulting in extensive tissue damage [23, 24], such as was demonstrated recently in the pulp of anterior teeth submitted to professional bleaching treatment [7]. Therefore, there is a clear risk of performing the in-office bleaching technique traditionally used in teeth with reduced enamel and dentin thickness, such as incisors, which allows intense diffusion of H2O2 and its degradation byproducts into the pulp chamber, with consequent extensive and irreversible tissue damage [7, 22].
The reduction in concentration of the bleaching gel in the present study caused a significant drop in the toxic effect when compared with the gel containing 35 % H2O2. The 17.5 % H2O2 gel promoted a reduction in toxicity of around 3 to 4 times when compared with the gel containing 35 % H2O2, whereas the gels containing 8 and 10 % H2O2, promoted a reduction of around 8 to 12 times in this toxic effect. For the three experimental concentrations tested, the frequency of bleaching gel application on enamel showed a direct effect on toxicity. In the period of 72 h post-bleaching, it was observed that the cells submitted to the experimental protocols with gels containing 17.5, 10, and 8 % H2O2 presented a greater capacity for recovery than that observed for the gel containing 35 % H2O2 applied on enamel for 15 min. The 8 and 10 % H2O2 gels showed the best results, with cell viability values close to or higher than 100 % being observed, in addition to morphology similar to that of the negative control at T1. In negative control group, high cell viability was observed in T2 (203.7 %). This was expected since the cells were not exposed to toxic solutions and consequently had their proliferative potential unchanged with time. Nevertheless, in spite of the cell viability observed for the 8 and 10 % H2O2 gels did not reached the same value as negative control at T2, values similar to control group at T1 were observed, demonstrating that cells exposed to these gels were able to recover its initial viability.
Previous studies have demonstrated that low concentrations of H2O2 are capable of stimulating cells to express proteins related to the deposition and mineralization of dentin matrix, in addition to intensifying the deposition of mineralization nodules; these positive cellular effects were associated with the low intensity oxidative stress generated by the reduced quantity of H2O2 [26, 27]. Therefore, it may be speculated that this ROS at low concentration may stimulate the process of odontoblastic differentiation, and consequently, the healing capacity of the dentin-pulp complex. These data demonstrated that human dental pulp cells are capable of regulating low intensity oxidative stress, which results in an increase in their rate of proliferation and modulation of their secretory activity [26, 27].
In spite of the interesting results observed in the present study when low concentrations of H2O2 were used, it must be pointed out that the efficacy of the bleaching procedure may be compromised in a short-term analysis [11]. Nevertheless, Soares et al. [14] have demonstrated that the gel containing 17.5 % H2O2 obtained the same bleaching effect observed for gel with 35 % H2O2 when the number of sessions was increased. Furthermore, Sulieman et al. [28] observed that gels with 25, 10, 15, and 5 % H2O2 were capable of achieving the same standard of bleaching (B1, VITA scale) as the gel with 35 % H2O2 by increasing the number of sessions. Other in vivo studies observed a bleaching effect similar to that of the conventional protocol when gels with 15–20 % H2O2 were used, with the advantage of a reduction in the prevalence and intensity of tooth sensitivity [12, 13]. Therefore, the use of bleaching gels with low concentrations of H2O2 appears to be an interesting alternative for professional tooth bleaching and is able to be used for teeth with a small enamel and dentin thickness, such as the mandibular incisors, which are more susceptible to the damage resulting from the traditional therapy [7]. Additionally, based on the fact that the cells subjected to the bleaching protocols with gels containing low concentrations of H2O2 exhibited a greater capacity for recovery compared to the gel containing 35 % H2O2, it may be speculated that in clinical situation, the slight initial damage caused by the gels containing 17.5, 10, and 8 % H2O2 to pulpal cells would be significantly minimized, with pulp tissue presenting regenerative capability through time.
Although interesting, the scientific data obtained in this study must be carefully analyzed, considering that in in vivo situations, the presence of organic and inorganic components within the dentinal tubules, as well as the exudation of dentinal fluid may reduce the trans-enamel and trans-dentinal diffusion of toxic products released from dental materials [29]. Similarly, the regenerative capacity of pulp cells in vivo is greater than when these cells are cultivated in a laboratory, which may be explained by the fact that the presence of extracellular matrix in vital pulp tissue minimizes the direct damage caused by toxic agents to these specific types of cells [30]. Because of the limitations previously cited, the results of the present investigation cannot be directly extrapolated to the clinical situation. Therefore, further in vivo studies are needed to evaluate the bleaching effectiveness, tooth sensitivity, as well as the response of pulp tissue to different bleaching therapies. This is justified due to the fact that tooth bleaching is a well-established esthetic clinical procedure worldwide; nevertheless, safer and effective bleaching techniques that prevent post-treatment sensitivity must be established, benefiting patients and appreciating the technical skill and scientific knowledge of dentists.
Conclusions
According to the results of the present investigation, gels with 17.5, 10 and 8 % of H2O2 minimized significantly the immediate trans-enamel and trans-dentinal cytotoxicity of bleaching to the dental pulp stem cells compared to the traditional in-office protocol (35 % H2O2 3 × 15 min). This immediate cytotoxicity was concentration dependent, and the lower the H2O2 concentration, the higher the late proliferative capability of pulp cells.
References
Reis A, Tay LY, Herrera DR, Kossatz S, Loguercio AD (2011) Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 36:590–596. doi:10.2341/10-173-C
Tay LY, Kose C, Herrera DR, Reis A, Loguercio AD (2012) Long-term efficacy of in-office and at-home bleaching: a 2-year double-blind randomized clinical trial. Am J Dent 25:199–204
He LB, Shao MY, Tan K, Xu X, Li JI (2012) The effects of light on bleaching and tooth sensitivity during in-office vital bleaching: a systematic review and meta-analysis. J Dent 40:644–653. doi:10.1016/j.jdent.2012.04.010
Soares DG, Basso FG, Hebling J, de Souza Costa CA (2014) Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent 42:185–198. doi:10.1016/j.jdent.2013.10.021
de Almeida LC, Costa CA, Riehl H, dos Santos PH, Sundfeld RH, Briso AL (2012) Occurrence of sensitivity during at-home and in-office tooth bleaching therapies with or without use of light sources. Acta Odontol Latinoam 25:3–8
Bonafé E, Bacovis CL, Iensen S, Loguercio AD, Reis A, Kossatz S (2013) Tooth sensitivity and efficacy of in-office bleaching in restored teeth. J Dent 41:363–369. doi:10.1016/j.jdent.2013.01.007
de Souza Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:59–64. doi:10.1016/j.tripleo.2009.12.002
de Oliveira Duque CC, Soares DG, Basso FG, Hebling J, de Souza Costa CA (2013) Bleaching effectiveness, hydrogen peroxide diffusion, and cytotoxicity of a chemically activated bleaching gel. Clin Oral Investig. doi:10.1007/s00784-013-1147-4
Soares DGS, Ribeiro APD, Sacono NT, Coldebella CR, Hebling J, de Souza Costa CA (2011) Transenamel and transdentinal cytotoxicity of carbamide peroxide bleaching gels on odontoblast-like MDPC-23 cells. Int Endod J 44:116–125. doi:10.1111/j.1365-2591.2010.01810.x
Boushell LW, Ritter AV, Garland GE, Tiwana KK, Smith LR, Broome A, Leonard RH (2012) Nightguard vital bleaching: side effects and patient satisfaction 10 to 17 years post-treatment. J Esthet Restor Dent 24:211–219. doi:10.1111/j.1708-8240.2011.00479.x
Soares DG, Ribeiro AP, da Silveira Vargas F, Hebling J, de Souza Costa CA (2013) Efficacy and cytotoxicity of a bleaching gel after short application times on dental enamel. Clin Oral Investig 17:1901–1909. doi:10.1007/s00784-012-0883-1
Ozcan M, Abdin S, Sipahi C (2013) Bleaching induced tooth sensitivity: do the existing enamel craze lines increase sensitivity? A clinical study. Odontology. doi:10.1007/s10266-013-0104-7
Reis A, Kossatz S, Martins G, Loguercio A (2013) Efficacy of and effect on tooth sensitivity of in-office bleaching gel concentrations: a randomized clinical trial. Oper Dent 38:386–393. doi:10.2341/12-140-C
Soares DG, Basso FG, Pontes EC, Garcia LD, Hebling J, de Souza Costa CA (2013) Effective tooth-bleaching protocols capable of reducing H2O2 diffusion through enamel and dentine. J Dent. doi:10.1016/j.jdent.2013.09.001
Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV et al (2006) Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissue Organs 184:105–116. doi:10.1159/000099617
Lizier NF, Kerkis A, Gomes CM, Hebling J, Oliveira CF, Caplan AI et al (2012) Scaling-up of dental pulp stem cells isolated from multiple niches. PLoS One 7:e39885. doi:10.1371/journal.pone.0039885
Goldberg M, Kulkarni AB, Young M, Boskey A (2011) Dentin: structure, composition and mineralization. Front Biosci 3:711–735
Couve E, Osorio R, Schmachtenberg O (2013) The amazing odontoblast: activity, autophagy, and aging. J Dent Res 92:765–772. doi:10.1177/0022034513495874
Farges JC, Keller JF, Carrouel F, Durand SH, Romeas A, Bleicher F et al (2009) Odontoblasts in the dental pulp immune response. J Exp Zool B Mol Dev Evol 312B:425–436. doi:10.1002/jez.b.21259
Simon SR, Berdal A, Cooper PR, Lumley PJ, Tomson PL, Smith AJ (2011) Dentin-pulp complex regeneration: from lab to clinic. Adv Dent Res 23:340–345. doi:10.1177/0022034511405327
Seale NS, McIntosh JE, Taylor AN (1981) Pulpal reaction to bleaching of teeth in dogs. J Dent Res 60:948–953
Cintra LT, Benetti F, da Silva Facundo AC, Ferreira LL, Gomes-Filho JE, Ervolino E et al (2013) The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 39:1576–1580. doi:10.1016/j.joen.2013.08.007
Squier TC (2001) Oxidative stress and protein aggregation during biological aging. Exp Gerontol 36:1539–1550. doi:10.1016/S0531-5565(01)00139-5
Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM et al (2007) Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim Biophys Acta 1773:93–104. doi:10.1016/j.bbamcr.2006.08.039
Sato C, Rodrigues FA, Garcia DM, Vidal CM, Pashley DH, Tjäderhane L et al (2013) Tooth bleaching increases dentinal protease activity. J Dent Res 92:87–92. doi:10.1177/0022034512470831
Min KS, Lee HJ, Kim SH, Lee SK, Kim HR, Pae HO (2008) Hydrogen peroxide induces Heme Oxygenase-1 and dentin sialophosphoprotein mRNA in human pulp cells. J Endod 34:983–989. doi:10.1016/j.joen.2008.05.012
Matsui S, Takahashi C, Tsujimoto Y, Matsushima K (2009) Stimulatory effects of low-concentration reactive oxygen species on calcification ability of human dental pulp cells. J Endod 35:67–72. doi:10.1016/j.joen.2008.08.034
Sulieman M, Addy M, MacDonald E, Rees JS (2004) The effect of hydrogen peroxide concentration on the outcome of tooth whitening: an in vitro study. J Dent 32:295–299. doi:10.1016/j.jdent.2004.01.003
Sauro S, Pashley DH, Montanari M, Chersoni S, Carvalho RM, Toledano M et al (2007) Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dent Mater 23:705–713. doi:10.1016/j.dental.2006.06.010
Wataha JC (2012) Predicting clinical biological responses to dental materials. Dent Mater 28:23–40. doi:10.1016/j.dental.2011.08.595
Acknowledgments
The authors acknowledge the Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (grants # 2011/12938-8 and 2011/09385-7), Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (grant # 301029/2010-1), and FUNDUNESP (grant # 0024/021/13-PROPE-CDC) for financial support.
Conflict of interest
The authors affirm that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soares, D.G., Basso, F.G., Hebling, J. et al. Immediate and late analysis of dental pulp stem cells viability after indirect exposition to alternative in-office bleaching strategies. Clin Oral Invest 19, 1013–1020 (2015). https://doi.org/10.1007/s00784-014-1321-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1321-3