Abstract
Objectives
The objective of this study was to evaluate the in vitro effect of low-fluoride (F) gels supplemented with sodium trimetaphosphate (TMP) on enamel demineralization.
Materials and methods
Bovine enamel blocks (n = 160) were selected based on surface hardness (SH) and divided into eight treatment groups (n = 20 per group): no F or TMP (placebo), 3 % TMP (3 %TMP), 5 % TMP (5 %TMP), 4,500 μg F/g (4,500), 4,500 μg F/g + 3 % TMP (4,500 3 %TMP), 4,500 μg F/g + 5 % TMP (4,500 5 %TMP), 9,000 μg F/g (9,000), and 12,300 μg F/g (acid gel). Blocks were subjected to demineralization/remineralization cycling for 5 days. Subsequently, surface hardness (SH1) and integrated loss of subsurface hardness (ΔKHN) were assessed, and the concentrations of loosely bound (CaF2-like) and firmly bound (FA-like) formed and retained F were determined.
Results
The 4,500 5 %TMP and acid gel groups showed similar results and had the lowest mineral loss (SH1 and ∆KHN). The acid gel group had the highest concentration of CaF2-like F, but the formation and retention of FA-like F was greater in the 4,500 5 %TMP group than in the acid gel group (p < 0.05).
Conclusion
It is possible to inhibit enamel demineralization with low-F gels supplementing these gels with 5 % TMP.
Clinical relevance
The low-F gel containing TMP can be regarded as a safer alternative for clinical use from a toxicological point of view since it contains half of the amount of a conventional formulation while promoting similar anticaries effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effectiveness of topical fluoride (F) as a cariostatic agent is well established; the anticaries effect of topical products with high F concentration is related to the deposition of CaF2 on enamel, which is dependent on concentration and pH [1, 2], although F can also be incorporated into the enamel crystal, forming a fluorapatite-like mineral and improving the ability of enamel to resist acid challenge [1, 3]. Products with high F concentrations have proven efficacy and are used in the form of solutions, gels, foams, and varnishes [4, 5]. Topical application of F is also performed by dental professionals to interrupt active caries lesions, mainly in patients at high risk of developing this disease [4–6]. In addition, dental care programs for children aged 0–3 years [7, 8] recommend the use of products with high F concentrations for cases of active caries. A university-based study in Brazil recommended the use of F varnish in such cases because of its better safety profile [9]. However, F varnish is costly, and its availability is restricted at the public health level (mainly in developing countries); therefore, F gels are most commonly used.
However, even professionally applied products have a high F concentration, which makes the risk of intoxication a concern. The patient may ingest the product during application, and young children do not have fully developed complex sputum [4]. Therefore, safer products and methods of F application are required to prevent gastric irritation, nausea, and vomiting [10, 11]. In addition to clinical precautions to reduce the intake of the product during application, a reduction in the F concentration in the gels could also impact F intake. With this concern, some authors [12] showed that 0.62 and 0.32 % acidulated gels may be utilized for caries prevention in children, even though caries preventive effects are not as high as after treatment with 1.25 % APF gel.
The addition of a phosphate with anticaries activity [13, 14], such as sodium trimetaphosphate (TMP), may enable a reduction in the F concentration of some products [14–17]. Some studies have demonstrated the anticaries action of phosphate salts [13, 14, 18, 19] based on the evaluation of its effectiveness after addition to rat diets. The addition of TMP to the diet resulted in a protective effect as well as delay in caries development [20–22]. Clinical trials testing chewing gum containing 1.5 % TMP [23] or dentifrices with 3 % TMP showed a reduction of caries [19]. In contrast, a 3 % TMP dentifrice had significantly higher caries when compared to a fluoride dentifrice [24]. When the 3 % TMP was associated to sodium, fluoride dentifrices (1,000–1,500 μg F/g) did not improve the anticariogenic action when compared to dentifrices without TMP [25]. However, the addition of TMP to dentifrices with low F concentrations has in vitro anticaries activity that is similar or superior to that of a standard dentifrice containing 1,100 μg F/g [26]. Although some studies suggest that TMP reduces demineralization, its mechanism of action remains unclear [21, 27].
Thus far, no studies have evaluated the efficacy of TMP-supplemented low-F gels in combating demineralization. Studies of new gels with these characteristics are important to assess the efficacy of these gels in reducing both enamel demineralization and the risk of intoxication. The aim of this study was to evaluate the ability of TMP-supplemented low-F gels to inhibit in vitro enamel demineralization. The null hypothesis was that low-F gels associated to TMP would present the same ability to reduce the enamel demineralization when compared to low-F gel without TMP.
Material and methods
Experimental design
Bovine incisors were stored in a 2 % formaldehyde solution (pH 7.0) for 30 days at room temperature after extraction. To obtain enamel blocks (4 × 4 mm, n = 160), the vestibular surfaces of the bovine incisors were fixed to acrylic plates and sectioned longitudinally and transversally along the flat portion using a precision saw (IsoMet 1000, Buehler, Lake Bluff, IL, USA) with two diamond disks (series 15HC 11–4243—Diamond, Buehler) separated by a spacer disk (thickness, 4 mm) under refrigeration with distilled/deionized water. The enamel surfaces were sequentially polished using 600-, 800-, and 1,200-grade water-cooled silicon carbide paper disks (Buehler), with a final polish using a felt disk (Buehler Polishing Cloth 40–7618) moistened with a 1-μm diamond polishing suspension (Extec Corp., Enfield, CT, USA). Blocks with a surface hardness (SH) between 320 and 380 KHN were selected and randomized into eight groups (n = 20 per group): (a) gel without F or TMP (placebo), (b) gel containing 3 % TMP (3 %TMP), (c) gel containing 5 % TMP (5 %TMP), (d) gel containing 4,500 μg F/g (4,500), (e) gel containing 4,500 μg F/g + 3 % TMP (4,500 3 %TMP), (f) gel containing 4,500 μg F/g + 5 % TMP (4,500 5 %TMP), (g) gel containing 9,000 μg F/g (9,000), and (h) gel containing 12,300 μg F/g (acid gel). Each block was sealed with nail varnish, leaving the enamel exposed. The gels were then applied once to the enamel surfaces of the appropriate group. After the gel treatment, ten blocks from each group were used to determine the concentrations of loosely bound (CaF2-like) and firmly bound (FA-like) F formed in the enamel. The other ten enamel blocks from each group were treated with the respective gels under the same conditions and then subjected to pH cycling for 5 days. After pH cycling, surface hardness (SH1) and cross-sectional hardness were assessed to calculate the integrated loss of subsurface hardness (ΔKHN). The depths of the surface layer and the lesion were then calculated by polarized light microscopy (PLM). The concentrations of CaF2-like and FA-like F retained in the enamel were also determined (Fig. 1).
Flow chart of the in vitro experimental study design. a Enamel blocks: sequentially polished with 600-, 800-, and 1,200-grade water-cooled silicon carbide [SiC] paper disks and felt disk wetted with solutions of 1 μm diamond. b Surface hardness test (five indentations, 25-g load, 10 s). c Gel treatments. d Demineralization pH cycling (De demineralizing and Re remineralizing). e Surface hardness analysis after demineralization pH cycling (SH1). f Section longitudinally through the center of the enamel block. g Half of each block was embedded in acrylic resin. h Cross-sectional hardness analyses were made (5-g load and 10 s). i Blocks sealed with wax. j Analysis CaF2-like F formed and retained (fluoride analysis). k Microabrasion. l Dissolution of enamel powder in acid for analysis FA-like formed and retained in enamel. m Analysis utilizing fluoride electrode
Gel formulation and determination of fluoride and pH in products
An experimental gel with a neutral pH was prepared in a laboratory using the following ingredients: carboxymethylcellulose (Synth, Diadema, São Paulo, Brazil), sodium saccharin (Vetec, Duque de Caxias, Rio de Janeiro, Brazil), glycerol (Merck, Darmstadt, Germany), peppermint oil (Synth), and water. Fluoride (NaF; Merck) was added to the gel at concentrations of 0, 4,500, or 9,000 μg F/g. Subsequently, TMP (Sigma-Aldrich Co., St. Louis, MO, USA) was added at concentrations of 3 or 5 % to the gels with F concentrations of 0 and 4,500 μg F/g. A commercial acidic gel was used as a positive control (12,300 μg F/g, acid gel, pH = 4.5, DFL Indústria e Comércio S.A., Rio de Janeiro, RJ, Brazil). The F concentration in the gels was determined using a specific electrode for the F ion (9609 BN; Orion Research Inc., Beverly, MA, USA) attached to an ion analyzer (Orion 720 A+; Orion Research Inc.) and calibrated with standards containing 0.125–2.000 μg F/g. Approximately 100 mg of each product was dissolved in deionized water and transferred to a volumetric flask. The volume was then adjusted to 100 ml using deionized water. For each product, three dilutions were prepared. Subsequently, two samples of 1 ml were buffered with total ionic strength adjustment buffer II (TISAB II) [28]. The pH of the experimental gels in the suspensions (100 mg of gel in 100 ml of deionized water) was checked electrometrically using an Orion 720 A+ (Orion Research Inc.) calibrated with standard solutions of pH 4.0 and 7.0.
Gel treatments and demineralization/remineralization cycling
The treatments were applied for 1 min to each gel block (3 g/block) (Fig. 1c) [29–31]. After treatment, the gel was removed, and the blocks were washed with deionized water. Thereafter, ten blocks from each group were subjected to five pH cycles at 37 °C during a procedure that lasted for 7 days [32]. The blocks were then placed in a demineralizing solution (DE; 2.0 mmol/l Ca and P in 75 mmol/l acetate buffer, pH 4.7; 0.04 μg F/ml; 2.2 ml/mm2). After 6 h, the blocks were transferred to a remineralizing solution (RE; 1.5 mmol/l Ca, 0.9 mmol/l P, and 150 mmol/l KCl in 0.1 mol/l cacodylic buffer, pH 7.0; 0.05 mg F/ml; 1.1 ml/mm2) for 18 h (Fig. 1d). The blocks were rinsed with deionized water between each step. The blocks were kept in fresh RE solution during the last 2 days of the procedure.
Hardness measurements
The hardness of the enamel surface of each block was determined before the topical application of the gels (SH, Fig. 1b) and after pH cycling (SH1, Fig. 1e) using a Shimadzu HMV-2000 microhardness tester (Shimadzu Corp., Kyoto, Japan) with a Knoop diamond indenter under a 25-g load for 10 s. Five indentations spaced 100 μm apart were created in the center of the enamel block (SH). After pH cycling, five indentations spaced 100 μm from the baseline indentations were created for determination of surface hardness (SH1).
For cross-sectional hardness measurements, the enamel blocks were longitudinally sectioned through their center (Fig. 1f) and embedded in acrylic resin with the cut face exposed (Fig. 1g). They were then gradually polished until the enamel was totally exposed. Three sequences of 14 indentations were created 100 μm apart at different distances (5, 10, 15, 20, 25, 30, 40, 50, 70, 90, 110, 130, 220, and 330 μm) from the surface of the enamel in the central region using a Micromet 5114 hardness tester (Buehler and Mitutoyo Corp., Kanagawa, Japan) and Buehler OmniMet software (Buehler) with a Knoop diamond indenter under a 5-g load for 10 s (Fig. 1h). The mean values at all three measuring points at each distance from the surface were then averaged. The integrated hardness (IH; KHN × μm) of lesions in sound enamel was calculated by the trapezoidal rule (GraphPad Prism, version 3.02) and subtracted from the IH for sound enamel to obtain the integrated area of the subsurface region in the enamel, which was termed integrated loss of subsurface hardness (ΔKHN; KHN × μm) [33]. Hardness measurement can be used to quantify mineral loss since it presents a positive correlation with mineral content [34]. To analyze the patterns of demineralization, differential hardness profiles were calculated by subtracting the hardness values of the placebo group from those of the TMP groups (i.e., placebo group values minus the 3 %TMP and 5 %TMP group values) and by subtracting the hardness values of the 4,500 group from those of the F and F + TMP groups (i.e., 4,500 group values minus the 4,500 3 %TMP, 4,500 5 %TMP, 9,000, and acid gel group values) at each depth. These differential profiles were then integrated over two depth zones in the lesion (zone A, 5–70 μm; zone B, 70–130 μm) and underlying sound enamel to yield ΔIH values.
Polarized light microscopy
Next, a diamond saw was used to prepare sections of approximately 600-μm thickness from half of each block embedded in acrylic resin. The tooth slices were then ground and polished to a thickness of 100 μm using a BETA grinder polisher (Buehler). Enamel sections were mounted on slides in distilled/deionized water and covered with a cover glass, the edges of which were sealed with synthetic resin (Entellan, Merck, Darmstadt, Germany) [26]. The sections were examined by PLM (AxioPhot; Zeiss, Oberkochen, Germany) at×400 magnification. Three areas in the central regions of the slices were analyzed using Axiovision Software Rel. 4.3 to verify the presence and thickness (micrometer) of the superficial enamel layer (superficial PLM) and the demineralization depth (depth PLM).
Analysis of the CaF2-like F concentration in enamel
The concentration of CaF2-like F in enamel (Fig. 1j) was analyzed to evaluate the amount of CaF2-like F present after the application of the F gel (CaF2-like F formed) and after pH cycling (CaF2-like F retained). A digital caliper (Mitutoyo CD-15B; Mitutoyo Corp., Japan) was used to measure the surface area of the enamel [35, 36]. The surface of each specimen, except for the enamel, was coated with wax. The specimens were then immersed in 0.5 ml of 1.0 mol/l KOH solution for 24 h under constant agitation. The solution was then neutralized with 0.5 ml of 1.0 mol/l HCl and buffered with 1.0 ml of TISAB II. An ion analyzer (720A; Orion Research, USA) and a combined ion-selective electrode (9609 BN; Orion Research, USA) previously calibrated with standards of 0.0625, 0.125, 0.250, 0.500, and 1.0 μg F/ml were used. Data were obtained in mV and were converted to microgram F per square centimeter using Microsoft Excel.
Analysis of the FA-like F concentration in enamel
After extraction of the CaF2-like F, an enamel biopsy was performed (Fig. 1l) [37]. Blocks measuring 2 × 2 mm (n = 160) were obtained from half of the longitudinally sectioned blocks. To measure depth, the blocks were fixed to a mandrel and attached to a handpiece (N 270; Dabi-Atlante, Ribeirão Preto, SP, Brazil) fixed to the top of a modified microscope with a micrometer (Pantec, Sao Paulo, SP, Brazil). Self-adhesive polishing disks (diameter, 13 mm) and 400-grit silicon carbide (Buehler) were fixed to the bottom of polystyrene crystal tubes (J-10; Injeplast, Sao Paulo, SP, Brazil). One layer of 100 μm was removed from each enamel block. A total of 0.5 ml of 0.5 mol/l HCl was added to the enamel powder retained on the polishing disk fixed to the polystyrene crystal tube. This mixture was then agitated for 1 h, and then, 0.5 ml of 0.5 mol/l NaOH was added [38]. For the FA-like F analysis (FA-like F formed and retained), a specific electrode (Orion 9609) was connected to an ion analyzer (Orion 720+) and TISAB II. A 1:1 ratio (TISAB/sample) was used (Fig. 1m).
Statistical analysis
For statistical analysis, SigmaPlot software version 12.0 (SigmaPlot, Systat Software Inc., San Jose, CA, USA) was used, and the significance limit was set at 5 %. Experimental groups (placebo, 3 %TMP, 5 %TMP, 4,500, 4,500 3 %TMP, 4,500 5 %TMP, 9,000, and acid gel) were considered as fixed factors. After proving homogeneity, the variables SH1, ∆KHN, superficial PLM, and depth PLM were analyzed using analysis of variance (one-way) followed by Bonferroni post hoc tests. The CaF2-like F and FA-like F (log transformation) concentrations in enamel and the ΔIH values were analyzed using analysis of variance (two-way) followed by Bonferroni tests. The Pearson correlation coefficient between ΔKHN and depth PLM was also calculated.
Results
The mean (SD) F ion concentration in the placebo, 3 %TMP, 5 %TMP, 4,500, 4,500 3 %TMP, 4,500 5 %TMP, 9,000, and acid gel groups was 113.8 (1.9), 150.5 (43.6), 99.9 (3.3), 4,733.9 (77.3), 4,100.9 (22.3), 4,400.1 (11.92), 8,940.9 (218.9), and 11,247.0 (54.0) μg F/g, respectively. The mean (SD) SH for all blocks was 373.4 (0.5) KHN, and no significant difference was observed among the groups (p = 0.610). All gels, except for the acid gel, had a neutral pH.
The SH1 results (Table 1) showed that the placebo and 5 %TMP groups had similar hardness values (p = 0.491), as did the 4,500 5 %TMP, 9,000, and acid gel groups (p = 0.073). The F and TMP groups (4,500 3 %TMP and 4,500 5 %TMP) differed from each other (p = 0.007). The ∆KHN results (Table 1) indicated that groups without F (placebo, 3 %TMP, and 5 %TMP) had the highest values when compared to the other groups (p < 0.001). The results for the 4,500 5 %TMP and acid gel groups were similar (p = 0.170) in terms of lesion area (ΔKHN). PLM (Table 1) showed that enamel blocks treated with the acid gel and 4,500 5 %TMP had shallower lesions (p = 0.877) than those of the other groups (p < 0.001). There was no difference in the thickness of the superficial layer of enamel between the groups (p = 0.134). Subsurface lesions were observed regardless of treatment but were present to a lesser extent in the F groups (Fig. 2). A positive correlation was observed between ΔKHN and depth PLM (Pearson’s r value = 0.911; R 2 = 0.829; p < 0.0001).
a Polarized light photomicrograph of lesion formed after treatment with placebo gel (no fluoride, no TMP); b polarized light photomicrograph of lesion formed after treatment with gel containing 5 %TMP; c polarized light photomicrograph of lesion formed after treatment with acid gel; d polarized light photomicrograph of lesion formed after treatment with gel containing 4,500 5%TMP (×400)
The acid gel group had higher concentrations of formed CaF2-like F (after topical application) than did the other groups (p < 0.001). After pH cycling, all groups had similar concentrations of retained CaF2-like F (p > 0.05). The concentration of formed FA-like F in enamel (Table 2) (after topical application) was similar among the 4,500, 4,500 3 %TMP, 4,500 5 %TMP, and 9,000 groups (p > 0.05). There was no change in the FA-like F concentration after pH cycling in these groups (retained FA-like F; p > 0.05), except that the 9,000 group had greater concentrations of retained FA-like F than formed FA-like F (p = 0.003). The acid group also had a higher concentration of retained FA-like F versus formed FA-like F (p < 0.001).
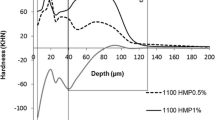
Differential hardness profiles (Fig. 3) provided evidence of different subsurface lesion patterns among the TMP and F groups. The demineralization process was more pronounced in zone A (5–70 μm, Fig. 3a) than in zone B among the TMP groups (p < 0.001, Table 3). The 4,500 5 %TMP group had lower demineralization in zone B (70–130 μm) than did the other groups (p = 0.001, Table 3). Other comparisons are shown in Table 3.
a Differential hardness profiles (hardness vs depth) calculated by subtracting no fluoride gels profiles from placebo. b Differential hardness profiles calculated by subtracting fluoride gels profiles from 4,500 group (Table 3)
Discussion
The application of products with high F concentrations is recommended for patients who have a high risk of developing dental caries. The use of these products in young children [7–9] must be carefully monitored because of the risk of acute intoxication. The greatest risk of F intoxication may be related to the concentrated F preparations used in professionally applied treatments. Although some dental fatalities [11, 39] related to F poisoning have been reported, the doses used for prevention of dental caries most often lead to mild symptoms of gastric irritation because of the corrosive effect of hydrofluoric acid on the epithelial lining of the gastrointestinal tract. The results of this study demonstrate that it may be possible to reduce the amount of F intake after topical application. The association of TMP with F enables the formulation of a gel with a low F concentration (4,500 μg F/g) without altering the efficacy of treatment; this result was not observed in another study, to which reducing the concentration of sodium fluoride gels acidified not obtain satisfactory results against the ability to inhibit the demineralization of tooth enamel [12]. The ΔKHN and ΔIH values observed in this study confirm previous findings that TMP reduces mineral loss deep in the enamel (Fig. 3 and Table 3) [13, 21].
The current study shows that TMP affects the processes of enamel de- and remineralization and that the mode of action of TMP is related to the development of lesions deep in the enamel. Different demineralization patterns were observed in the lesions of the TMP only and F groups, which is consistent with the findings of other authors [16]. Lower demineralization was observed deep in the enamel when TMP was present in the gel (Table 3 and Fig. 3). Analysis of all outcomes (Table 1, ΔKHN values) suggested that TMP alone does not reduce demineralization; demineralization is reduced only when TMP is combined with F. However, the differential calculation (placebo group values minus the TMP gels F-free group values) showed that TMP alone (3 or 5 %) reduced mineral loss deep in the enamel (Table 3 and Fig. 3). In this study, the gels used were applied once for 1 min, and it was possible to observe the inhibitory effect of TMP on demineralization. The linear chain polyphosphates show higher adsorption on enamel but lower substantivity. The TMP remains bound to enamel for a longer period than do linear polyphosphates [40], and this may help explain the observed outcomes.
The difference between the 4,500 and 9,000 groups indicates that increasing the F concentration of the gel had an effect on the outer layer of the enamel (Table 3). It is well established that the effect obtained with acid gel was due to the large deposits of CaF2 (Tables 1 and 2) [12]. This effect was visible in the superficial enamel layers rather than in the deeper layers (Fig. 3 and Table 3). In the presence of TMP, a greater Ca flux inside the lesions in the deeper layers can block the interprismatic pores at the lesion front, thus reducing acid diffusion to the underlying sound enamel [16]. As a result, TMP preserves the stability and integrity of the enamel mineral surface [12]. According to some authors [16], an F-rich precipitate in the lesion does not block acid diffusion to deeper layers.
The anticaries effect of topical products with high F concentrations is related to the deposition of CaF2 on enamel [1, 12], which is dependent on concentration and pH. Data relating to CaF2 formation in the present study showed a positive correlation between the experimental placebo, 4,500, and 9,000 gels (r = 0.982; R 2 = 0.927). CaF2-like F formation was four times higher when an acid gel was used than when a neutral gel was used (9,000 group). The decrease (90 %) in CaF2-like F formation in the 9,000 group during pH cycling (which resulted in a 70 % increase in FA-like F in enamel) may have produced a similar effect (SH1) to that in the acid gel group (Table 1), being limited to the superficial layer of the enamel. A smaller subsurface lesion observed in the acid gel group (Fig. 3b and Table 3) was related to greater deposition of CaF2-like F formed in the enamel because FA-like F present in enamel after pH cycling was similar between treatments with and without F (Table 2). The combined use of F and TMP did not alter the adsorption pattern of CaF2-like F in enamel. Furthermore, the pattern of CaF2-like F loss was lower with 5 %TMP (50 %) than with the other F gels alone (70–90 %). Therefore, the FA-like F formed in the enamel (the 4,500 5 %TMP groups had three times more F than did the acid gel group) may contribute to reduced demineralization.
Although TMP and F act independently and simultaneously [13, 16], the molar proportion of TMP/NaF is important for optimizing the anticaries action [15, 17]. The suggested molar proportion of TMP/NaF is between 1.24:1 and 3.72:1 [15, 17]. The molar proportion in the 4,500 5 %TMP group was 0.7, which is close to the lowest value described above. A concentration of 7 % TMP may increase the efficiency of the F gel. Considering that a topical gel may be applied in the presence of the salivary pellicle and plaque, the effect of the gel on the enamel may be reduced because polyphosphates can be adsorbed by organic and inorganic components of the biofilm [41]. Thus, the effect of TMP on enamel can be limited. However, this hypothesis needs to be investigated. Therefore, in situ studies must be performed to determine the effect of the salivary pellicle or plaque. The results of this study cannot be considered definitive because although the chemical model simulates the development of caries under controlled conditions, it still has limitations. All in vitro protocols are not able to reproduce the complex intraoral conditions or to mimic solid surface area/solution ratios; the substrate utilized is bovine enamel; the time periods of de- and remineralization are much faster; they are not able to adequately simulate clearance of products and the saliva/plaque fluid composition [42]. But, the in vitro studies can estimate the role of new anticaries compounds and to screening tests.
Conclusions
On the basis of the findings of this in vitro study, we conclude that the addition of 5 %TMP to gels with a reduced concentration of F (4,500) can inhibit enamel demineralization.
References
Moreno EC (1993) Role of Ca–P–F in caries prevention: chemical aspects. Int Dent 43:71–80
Hicks J, Garcia-Godoy F, Flaitz C (2004) Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remineralization (part 3). J Clin Pediatr Dent 28:203–214
Chow LC (1990) Tooth-bound fluoride and dental caries. J Dent Res 69(Spec No):595–600
van Rijkom HM, Truin GJ, van’t Hof MA (1998) A meta-analisys of clinical studies on caries-inhibiting effect of fluoride gel treatment. Caries Res 32:83–92
Marinho VC, Higgins JP, Logan S, Sheiham A (2002) Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2:CD002280
Ammari JB, Baqain ZH, Ashley PF (2007) Effects of programs for prevention of early childhood caries: a systematic review. Med Princ Prac 16:437–442
Cunha RF, Delbem AC, Percinoto C, Saito TE (2000) Dentistry for babies: a preventive protocol. ASDC J Dent Child 67:89–92
Melhado FL, Cunha RF, Nery RS (2003) Influence of dental care for infants on caries prevalence: a comparative study. J Dent Child (Chic) 70:120–123
Elias GP, Assunção LRS, Cunha RF (2008) Profile of the use of topical fluoride for babies adopted by Brazilian universities dental. Pesqui Bras Odontopediatric Clín Integr 8:78–81
Lecompte EJ (1987) Clinical application of topical fluoride products: risks, benefits and recommendations. J Dent Res 66:1066–1071
Spack CJ, Sjöstedt S, Eleborg L, Vereu B, Perbeck L, Ekutrand L (1990) Studies of human gastric mucosa after application of 0,42% fluoride gel. J Dent Res 69:426–429
Wiegand A, Krieger C, Attin R, Hellwing E, Attin T (2005) Fluoride uptake and resistance to further demineralization of demineralised enamel after application of differently concentrated acidulated sodium fluoride gels. Clin Oral Invest 9:52–57
Gonzalez M (1971) Effect of trimetaphosphate ions on the process of mineralization. J Dent Res 50:1056–1064
Gonzalez M, Jeansonne BG, Feagin FF (1973) Trimetaphosphate and fluoride actions on mineralization at the enamel-solution interface. J Dent Res 52:261–266
Moretto MJ, Magalhães AC, Sassaki KT, Delbem AC, Martinhon CC (2010) Effect of different fluoride concentrations of experimental dentifrices on enamel erosion and abrasion. Caries Res 44:135–140
Takeshita EM, Exterkate RA, Delbem AC, ten Cate JM (2011) Evaluation of different fluoride concentrations supplemented with trimetaphosphate on enamel de- and remineralization in vitro. Caries Res 45:494–497
Manarelli MM, Vieira AE, Matheus AA, Sassaki KT, Delbem AC (2011) Effect of mouth rinses with fluoride and trimetaphosphate on enamel erosion: an in vitro study. Caries Res 45:506–509
Harris RS, Niizel AE, Walsh BN (1967) The effect of phosphate structure on dental caries development in rats. J Dent Res 46:290–294
Ständler P et al (1996) The effect of sodium trimetaphosphate on caries: a 3-year clinical toothpaste trial. Caries Res 30:418–422
Larson RH, Spalter CD, Clemmer BA, Scherp HW (1972) Continuous versus intermittent feedings of different levels of trimetaphosphate in relation to caries development in the rat. Arch Oral Biol 17:1537–541
Navia JM, Lopez H, Harris RS (1968) Cariostatic effects of sodium trimetaphosphate when fed to rats during different stages of tooth development. Arch Oral Biol 13:779–786
Shaw JH (1980) Influence of sodium, calcium and magnesium trimetaphosphates on dental caries activity in the rat. J Dent Res 59:644–650
Finn SB, Frew RA, Leibowitz R, Morse W, Manson-Hing L, Brunelle J (1978) The effect of sodium trimetaphosphate (TMP) as a chewing gum additive on caries increments in children. J Am Dent Assoc 96:651–655
Andlaw RJ, Palmer JD, King J, Kneebone SB (1983) Caries preventive effects of toothpastes containing monofluorophosphate and trimetaphosphate: a 3-year clinical trial. Community Dent Oral Epidemiol 11:143–147
O’Mullane DM, Kavanagh D, Ellwood RP, Chesters RK, Schafer F, Huntington E et al (1997) A three-year clinical trial of a combination of trimetaphosphate and sodium fluoride in silica toothpastes. J Dent Res 76:1776–781
Takeshita EM, Castro LP, Sassaki KT, Delbem AC (2009) In vitro evaluation of dentifrice with low fluoride content supplemented with trimetaphosphate. Caries Res 43:50–56
Henry CA, Navia JM (1969) Sodium trimetaphosphate influence on the early development of rat caries and concurrent microbial changes. Caries Res 3:326–338
Delbem AC, Sassaki KT, Castro AM, Pinto LM, Bergamaschi M (2003) Evaluation of fluoride content mouthwashes and gels and risk of acute. Rev ABO Nac 11:188–193
Delbem ACB, Cury JA (2002) Effect of application time of APF and NaF gels on microhardness and fluoride uptake of in vitro enamel caries. Am J Dent 15:169–172
Villena RS, Tenuta LM, Cury JA (2009) Effect of APF gel application time on enamel demineralization and fluoride uptake in situ. Braz Dent J 20:37–41
Calvo AFB, Tabchoury CPM, Del Bel Cury AA, Tenuta LMA, da Silva WJ, Cury JA (2012) Effect of acidulated phosphate fluoride gel application time on enamel demineralization of deciduous and permanent teeth. Caries Res 46:31–37
Vieira AE, Delbem AC, Sassaki KT, Rodrigues E, Cury JA, Cunha RF (2005) Fluoride dose response in pH-cycling models using bovine enamel. Caries Res 39:514–520
Spiguel MH, Tovo MF, Kramer PF, Franco KS, Alves KM, Delbem AC (2009) Evaluation of laser fluorescence in the monitoring of the initial stage of the de-/remineralization process: an in vitro and in situ study. Caries Res 43:302–307
Kielbassa AM, Wrbas KT, Schulte-Mönting J, Hellwig E (1999) Correlation of transversal microradiography and microhardness on in situ-induced demineralization in irradiated and nonirradiated human dental enamel. Arch Oral Biol 44:243–251
Caslavska V, Moreno EC, Brudevold F (1975) Determination of the calcium fluoride formed from in vitro exposure of human enamel to fluoride solutions. Arch Oral Biol 20:333–339
Delbem AC, Danelon M, Sassaki KT, Vieira AE, Takeshita EM, Brighenti FL, Rodrigues E (2010) Effect of rinsing with water immediately after neutral gel and foam fluoride topical application on enamel remineralization: an in situ study. Arch Oral Biol 55:913–918
Weatherell JA, Robinson C, Strong M, Nakagaki H (1985) Micro-sampling by abrasion. Caries Res 19:97–102
Alves KM, Pessan JP, Brighenti FL, Franco KS, Oliveira FA, Buzalaf MA, Sassaki KT, Delbem AC (2007) In vitro evaluation of the effectiveness of acidic fluoride dentifrices. Caries Res 41:263–267
Bayless JM, Tinanoff N (1985) Diagnosis and treatment of acute fluoride toxicity. J Am Dent Assoc 110:209–211
McGaughey C, Stowell EC (1977) Effects of polyphosphates on the solubility and mineralization of HA: relevance to a rationale for anticaries activity. J Dent Res 56:579–587
van Dijk JW, Borggreven JM, Driessens FC (1980) The effect of some phosphates and a phosphonate on the electrochemical properties of bovine enamel. Arch Oral Biol 25:591–595
Buzalaf MA, Hannas AR, Magalhães AC, Rios D, Honório HM, Delbem AC (2010) pH-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: strengths and limitations. J Appl Oral Sci 18:316–334
Acknowledgments
We thank the technicians from the laboratory of Pediatric Dentistry of the Araçatuba School of Dentistry, UNESP, and Maria dos Santos Fernandes for the laboratorial assistance in this study. We also thank FAPESP (The State of São Paulo Research Foundation, 2008/08913-7) for the concession of a scholarship to the first author.
Conflict of interests
The corresponding author has filed an application for patenting a product used in the study at the National Institute of Industrial Property (INPI/SP) on April 29, 2008 under the number 018080026091, PI0801811-1 and published on January 11, 2011. The other authors have no financial or personal conflicts of interest in relation to this study. All authors approved the publication of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danelon, M., Takeshita, E.M., Peixoto, L.C. et al. Effect of fluoride gels supplemented with sodium trimetaphosphate in reducing demineralization. Clin Oral Invest 18, 1119–1127 (2014). https://doi.org/10.1007/s00784-013-1102-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-013-1102-4