Abstract
Objectives
The aim of our study was to analyse whether the irradiation time and/or the modulation of irradiation time influence the degree of conversion (DC) and the amount of elutable substances from modern nano-hybrid resin-based composites (RBCs).
Materials and methods
The DC was recorded in real time for 5 min by means of attenuated total reflectance–Fourier transform infrared spectroscopy (n = 5) on the lower surface of 2-mm-thick samples irradiated with continuous and modulated irradiation times for 20 s and 40 s. The modulated times comprise a short polymerisation (2 s or 5 s) followed by a rest period of 1 min and an additional polymerisation to complete 20 s and 40 s of polymerisation (2 s + 18 s, 5 s + 15 s, 2 s + 38 s and 5 s + 35 s). After storing the specimens in ethanol/water for 7 days at 37 °C, the eluates were analysed by gas chromatography–mass spectrometry. Results were statistically analyzed using a one-way ANOVA analysis (α = 0.05).
Results
The effect of irradiation time on DC is similar in all three analyzed materials, showing a significant increase in DC by increasing irradiation time from 20 s to 40 s, while the DC is not influenced within one irradiation time (20 s or 40 s) by the modulation of time.
Conclusions
The type and amount of eluates are strongly dependent from the material and the irradiation protocol.
Clinical relevance
An interrupt irradiation of RBCs is clinically feasible, reducing in general the amount of elutable substances at similar DC as the corresponding continuous polymerisation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An increased number of resin-based dental restorations were placed over the past decades, inquiring the potentially adverse effects of released material components into the oral environment [1–5]. Owing to a large diversity and variation of eluates detected in different resin-based composites (RBCs) [6, 7] including monomers, co-monomers, initiators, stabilisers, decomposition products or contaminants, the biocompatibility and the allergenic potential of these restoratives were revealed to be material dependent [6]. A high amount of leachable species were shown to elute from RBCs already within the first 3 h of storage (50 % by soaking in water and 75 % in an ethanol/water mixture), while the elution of nearly all leachable components was stated in older studies to be nearly complete within a 24-h storage period in either solvents [7]. Studies on modern RBCs found, however, a continuous elution of leachable components also 28 days after polymerisation [8, 9], while the eluted compounds and their amount was strongly dependent on the immersion media [10, 11].
Since elution is generally thought to occur via diffusion of molecules through the resin matrix, it is therefore dependent upon the size and chemical characteristics of the leachable species [12, 13]. It was observed that the chemical structure of resin monomers in a RBC directly affects the amount of eluted monomers and the time needed for the elution of this amount, while the elution process seems not to be influenced by the presence of filler [14]. Apart from improvements in the filler system, modern restoratives, like the nano-hybrid RBCs, are characterised by new monomer-matrix formulations with enlarged molecular size. Besides traditional monomers like BisGMA (bisphenol A diglycidyl ether dimethacrylate), BisEMA (bisphenol A polyethylene glycol diether dimethacrylate), UDMA (urethane dimethacrylate) or TEGDMA (triethyleneglycol dimethacrylate), a series of new monomers such as dimer acid-based monomers [15], tricyclodecane urethane [16], the DuPont monomer DX-511 or ormocers [17] are either completely replacing the traditional monomers or only merged in a traditional monomer formulation. While in terms of mechanical properties and aging stability no noteworthy advantages of RBCs with novel formulations were attested, when compared to traditional formulated nano-hybrid RBCs [18, 19], the degree of conversion (DC) of the former is higher and decrease slower with increased incremental thickness [20].
The quantity of leachable substances from RBCs has been directly correlated to the DC of the polymer network [12, 21]. Further developments of light curing units [22] and the continuous demands of dental practitioners to reduce the time needed to place a restoration are suggesting that RBCs can be adequately cured also at short polymerisation times. Despite these trends, numerous in vitro investigations have demonstrated that a polymerisation time of 20 s or more is necessary to properly polymerise modern RBCs not only at filling’s surface but also in deeper layers [9, 20, 23]. Moreover, curing fast for a short period of time with a plasma arc light unit resulted in four to seven times higher monomer elution compared to the elution from specimens cured with a regular halogen unit [24].
Besides, it is still equivocally whether modulated polymerisation times, in terms of polymerising for few seconds followed by a rest period and an additional polymerisation to complete the requested polymerisation time, would negatively affect the polymerisation process in modern RBC restoratives. This modulated irradiation time might be an issue in improving the shaping of a restoration by subtending RBCs slumping, or with regard to an easier elimination of luting material surplus, when indirect restorations are inserted.
Therefore, the aim of our study was to investigate whether the DC and the amount of elutable substances from modern nano-hybrid RBCs are influenced by the irradiation time and/or the modulation of irradiation time. The null hypotheses tested was that both parameters—DC and amount of eluted substances—would neither be affected when the irradiation time increase from 20 s to 40 s, nor by modulating this irradiation times.
Materials and methods
All solvents and reagent products were obtained from Merck (Darmstadt, Germany) and are of highest purity available. Three nano-hybrid RBCs—Tetric Evo Ceram® (Ivoclar Vivadent, Ellwangen, Germany), Venus® Diamond (Heraeus Kulzer, Hanau, Germany) and Filtek™ Supreme XTE (3M ESPE, Seefeld, Germany; Table 1)—were investigated
Degree of conversion
The measurements of the DC (n = 5) were made in real time with a Fourier transform infrared (FTIR) spectrometer with an attenuated total reflectance (ATR) accessory (Nexus; Thermo Nicolet, Madison, WI, USA). Therefore, the un-polymerised composite paste (approximately 30 mg) was put directly on the diamond ATR crystal in a mould 2-mm high and with a diameter of 3 mm with a resulting sample surface of 32.99 mm2 and a volume of 14.13 mm3. The mould was filled in one increment, covered by a transparent foil (US-120 KE; Frasaco, Tettnang, Germany) and cured by applying the curing unit (LED light source Freelight2, 3M ESPE) directly on the sample surface. The irradiance of the used curing unit (1,241 mW/cm2) was measured by means of a calibrated fibre optic spectrally resolving radiometer equipped with an integrating sphere (S2000; Ocean Optics, USA).
The FTIR spectra were recorded in real time for 5 min, with two spectra per second, on the lower surface of the samples, with continuous and modulated irradiation times for 20 s and 40 s. The modulated time comprised a short polymerisation (2 s or 5 s) followed by a rest period of 1 min and an additional polymerisation to complete 20 s and 40 s of polymerisation (2 s + 18 s, 5 s + 15 s, 2 s + 38 s and 5 s + 35 s).
The diameter of the measured surface was 800 μm, the wave number range of the spectrum was 4,000–650 cm−1 and the FTIR spectra were recorded with four scans at a resolution of 8 cm−1.
To determine the percentage of the remained unreacted double bonds, the DC was assessed as the variation of the absorbance intensities peak height ratio of the methacrylate carbon carbon double bond (peak at 1,634 cm−1) and those of an internal standard (aromatic carbon carbon double bond; peak at 1,608 cm−1) during polymerisation, in relation to the uncured material:
Specimens were incubated 5 min after completing DC measurements (thus 10 min after starting the irradiation) in an ethanol/water (3:1) solution at 37 °C for 7 days. Caffeine (CF; 0.1 mg/ml) was added to the eluates and each aliquot was analysed by gas chromatograph coupled with mass spectrometry (GC–MS).
GC–MS analysis
The analysis of the eluates (n = 5) was performed on a Finnigan Trace GC ultra gas chromatograph connected to DSQ mass spectrometer (Thermo Electron, Dreieich, Germany). A FactorFour® capillary column (length 25 m, inner diameter 0.25 mm, coating 0.25 μm; Varian, Darmstadt, Germany) was used as capillary column for GC. The GC oven was heated from 50 °C (2 min isotherm) to 300 °C (5 min isotherm) with a rate of 10 °C/min and 1 μl of the solution was injected with a split of 1:30. Helium was used as carrier gas at a constant flow rate of 1 ml/min. The temperature of the split–splitless injector as well as of the direct coupling to the MS was 250 °C. The MS was operated in electron ionisation mode (EI, 70 eV); the ion source temperature was 200 °C; only positive ions were scanned. Scan ran over the range m/z 50–500 at a scan rate of 1 scan/s for scans operated in full scan mode to qualify analytes. The results were referred to an internal CF standard (0.1 mg/ml CF = 100 %), which allows to determine the relative quantities of substances released from various resin-based materials. For absolute quantification of the elutable substances, calibration curves were used by integration of the mass signal with the best signal-to-noise ratio. All eluates were analysed five times. The integration of the chromatograms was carried out over the base peak of the compounds, and the results were normalised by means of the internal CF standard. The identification of the various substances was achieved by comparison of their mass spectra with those of reference compounds/spectra, spectra from the NIST library and by chemical analysis of their fragmentation patterns [25]. Reference retention times, reference spectra and calibration curves for each substance were obtained by using the pure substances from Sigma-Aldrich. For methodical reasons, monomers with a higher molecular weight like BisGMA and UDMA cannot be quantified with GC–MS.
Calculations and statistics
The results are presented as means ± standard error of mean (SEM). The statistical significance (p < 0.05) of the differences between the experimental groups was tested using one-way ANOVA and Tukey HSD post hoc test (α = 0.05) (SPSS Inc., Chicago, IL, USA, version 20.0).
Tetric Evo Ceram® specimens
The DC is statistically significantly increasing by increasing the curing time from 20 s to 40 s. Within one polymerisation time (20 s or 40 s), the modulation of time (2 s + 18 s or 5 s + 15 s, and 2 s + 38 s or 5 s + 35 s) has no influence on DC (Table 2, Fig. 1).
Elutable substances from polymerised specimens (n = 5) from Tetric Evo Ceram® after 7 days in ethanol/water (3:1) as elution medium. MAA methacrylic acid, EGDMA ethylene glycol dimethacrylate, CQ camphorquinone, DMABEE 4-N,N-dimethylaminobenzoic acid ethyl ester, TEGDMA triethylene glycol dimethacrylate, BisEMA bisphenol A polyethylene glycol dimethacrylate
No statistically significantly different amount of camphorquinone (CQ) in the elution medium was measured after polymerising for 20 s, with or without time modulation, and the continuous 40-s polymerisation. For a 40-s polymerisation, the time modulation caused a gradual decrease in the amount of eluted CQ from 12.1 ± 1.9 μmol/l after a continuous polymerisation to 8.0 ± 0.7 μmol/l after a modulated polymerisation (5 s + 35 s).
The amount of BisEMA in the elution medium was similar for both continuous polymerisation times (20 s and 40 s), whereas a modulation of polymerisation time induced, for both 20 s and 40 s, a significantly lower amount of eluted substance. Increasing the polymerisation time from 20 s to 40 s increased significantly the amount of eluted methacrylic acid (MAA), whereas the modulation of polymerisation time resulted in significantly lower eluted values.
Venus® Diamond specimens
The DC is statistically significantly increasing by increasing the curing time from 20 s to 40 s. Within one polymerisation time (20 s or 40 s), the modulation of polymerisation time (2 s + 18 s or 5 s + 15 s, and 2 s + 38 s or 5 s + 35 s) has no influence on DC (Table 2, Fig. 2).
Elutable substances from polymerized specimens (n = 5) from Venus® Diamond after 7 days in ethanol/water (3:1) as elution medium. EGA ethylene glycol acrylate, HEMA 2-hydroxyethyl methacrylate, CQ camphorquinone, TEGDMA triethylene glycol dimethacrylate, DMABEHE 4-dimethylaminobenzoic acid 2-ethylhexyl ester
No statistically significantly different amount of CQ in the elution medium was measured after polymerising for 20 s, with or without time modulation, and the continuous 40-s polymerisation. For a 40-s polymerisation, the time modulation caused a gradual decrease in the amount of eluted CQ from 6.6 ± 2.1 μmol/l after a continuous polymerisation to 4.0 ± 0.6 μmol/l after a modulated polymerisation (5 s + 35 s).
The amount of TEGDMA in the elution medium was statistically similar for a 20-s and 40-s continuous polymerisation. For both polymerisation times (20 s and 40 s), modulating the polymerisation time significantly decreased the amount of eluted TEGDMA up to one half. The way how the time was modulated—2 s + 18 s, 5 s + 15 s, 2 s + 38 s or 5 s + 35 s—had no significant effect on the amount of eluted TEGDMA.
Filtek™ Supreme XTE specimens
The DC is statistically significantly increasing by increasing the curing time from 20 s to 40 s. Within one polymerisation time (20 s or 40 s), the modulation of polymerisation time (2 s + 18 s or 5 s + 15 s, and 2 s + 38 s or 5 s + 35 s) has no influence on DC (Table 2, Fig. 3).
The amount of CQ in the elution medium was significantly higher when the material was polymerised for 20 s compared to 40 s, with no significant differences within a given polymerisation time at modulated time.
The amount of EGDMA was affected neither by polymerisation time nor by the modulation of time. Similarly, it is also valid for TEGDMA, except the comparison between the groups 2 s + 18 s and 40-s polymerisation, with a significantly lower amount of eluted TEGDMA in the latter case.
The amount of CQ in the elution medium for Venus® Diamond and Filtek™ Supreme XTE specimens was statistically similar (5.3 ± 1.5 μmol/l vs. 6.5 ± 2.5 μmol/l) and significantly lower when compared to Tetric Evo Ceram® (11.8 ± 2.6 μmol/l).
Discussion
RBCs used in dentistry are predominantly methacrylate-based materials, containing monomers with two or more carbon carbon double bonds able to build up a three-dimensional and highly cross-linked polymer network. A high DC of the carbon carbon double bonds in the methacrylate group is directly associated with improved mechanical properties [26], better colour stability [27], higher biocompatibility as well as a lower water solubility and amount of leachable substances [12, 28]. The effect of polymerisation time on the measured DC in this study is similar in all three analysed nano-hybrid RBCs, showing a significant increase in DC by increasing the irradiation time from 20 s to 40 s and not being influenced within one polymerisation time (20 s or 40 s) by the modulation of time (2 s + 18 s or 5 s + 15 s, and 2 s + 38 s or 5 s + 35 s). At modulated polymerisation regimes (Fig. 4b), the initial short irradiation (2 s or 5 s) starts the polymerisation and activates only a small amount of CQ molecules. The polymer chains continue to grow during the dark period, while the following rest of irradiation activates again CQ at a higher extent due to the longer irradiation, continuing the polymerisation reaction [29]. At a continuous polymerisation time (Fig. 4a), with high irradiance, thus with a high number of photons, it must be considered that the efficiency of an activated initiator is limited by a deactivation mechanism. An activated initiator cannot only start a polymerisation reaction but also can stop it when recombined with other activated initiators or with activated polymer chains (termination of chain growth) [29]. Consequently, the higher the number of methacrylate radicals, the higher will be the amount of termination of chain growth. A high number of activated initiator molecules at the same time would be less effective to integrate monomers in the polymer network than the same amount of activated initiator molecules dispersed over a longer irradiation time, as offered in the modulated irradiation protocols. It must, however, also be considered that when the chain growth is terminated by a disproportionation mechanism, a new carbon carbon double bond is formed, which cannot be separated in the FTIR analysis from the monomer carbon carbon double bonds [30].
Proposed models for the polymerisation mechanism of a continuous polymerisation (a) (high amount of short chains, low cross-linkage, higher amount of rest monomers) and irradiation time modulated polymerisation (b) (long polymer chains, high cross-linkage and a lower amount of unpolymerised monomers)
An additional plausible explanation for the different amount of eluted substances within one material at similar DC could be a different architecture of the three-dimensional polymer network, induced by different irradiation protocols. At modulated irradiation times, the lower amount of activated initiator molecules induces less nuclei of polymerisation, thus facilitating the building of longer polymer chains [29]. The final high-intensity light irradiation after the dark period binds these chains to a closer meshed network, which would act at the sample’s surface as a better isolator, impeding the diffusion of unreacted monomers from the depth. Both proposed mechanisms are in accordance with the measured results, showing at equivalent DC less methacrylate elution at modulated irradiation time.
These results are of great clinical relevance since many practitioners are in doubt whether an interrupted polymerisation, which might occur within a restoration process, would negatively affect RBC properties. Apart from no detrimental effects on DC, different soft start curing protocols involving also a combination of low initial energy density followed by a lag period (2 min) previous to a final high-intensity light irradiation were proved to provide a remarkable reduction of polymerisation contraction stresses in dental composites [31]. Moreover, the marginal gap and the stress developed at the dental tissue–composite interface may benefit from the modulation of irradiation [31, 32].
The amount of eluted substances from the analysed materials was determined in our study by using an ethanol/water 3:1 elution media since water or artificial saliva were proved to be less effective for this purpose. Furthermore, an ethanol/water 3:1 media is additionally considered by the United States Food and Drug Administration as a food/oral simulating liquid of clinical relevance [33, 34]. Irrespective of the neutral effect on DC, the amount of eluted substances was, in general, lowered by time modulation in Tetric Evo Ceram® specimens. The amount of eluted MAA and BisEMA was significantly reduced during the modulation of time at both 20-s and 40-s irradiation times. At the same time, however, the amount of DMABEE (4-N,N-dimethylaminobenzoic acid ethyl ester) was slightly but significantly increased. Since the amount of eluted MAA increased when the irradiation time increased from 20 s to 40 s, it cannot be excluded that MAA was formed during the polymerisation process. This aspect was described also in other studies, as the intra- and intermolecular reactions during the polymerisation were shown to be able to influence the polymer network formation, leading to reaction products like MAA [35]. The modulation of time, however, was able to reduce the elution of MAA event to a higher extent at high irradiation time (from 386.5 μmol/l at 40 s to 55.09 μmol/l at 5 s + 35 s and from 282.8 μmol/l at 20 s to 47.5 at 5 s + 15 s μmol/l, respectively).
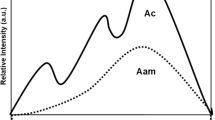
In Venus® Diamond, a modulated polymerisation time significantly lowered the amount of all detected eluates. The chemical composition of this material comprise exclusively aliphatic methacrylates, merging in its monomer matrix UDMA and TCD-urethane (tricyclodecane urethane). TCD-urethane is a hydrophobic acrylic acid ester prepared by reaction of hydroxyalkyl (meth)acrylic acid esters with diisocyanates and subsequent reaction with polyols [16]. Acrylates, lacking the methyl group of the methacrylates, are less sterically hindered, thus the monomer is described to have a high reactivity, reaching higher DC, a good biocompatibility[36] and a very good mechanical stability also after aging [19, 37]. The low viscosity of the monomer allows to eliminate additional diluents, which is positively reflected in a reduced polymerisation shrinkage when compared to traditional BisGMA-based RBCs [38] or even with the low shrinkage silorane-based RBC [39]. Nevertheless, in the elution medium of Venus® Diamond, HEMA, a monomer with low viscosity used as diluent, was found. Two sources are plausible for the elution of HEMA, either as an impurity from the TCD-urethane synthesis from hydroxyalcyl methacrylates or as a reaction product formed during the polymerisation process [40]. Previously to the elution tests made in the polymerised samples, a screening of the unpolymerised material was also made, which was not able to detect HEMA, thus strengthening our second hypothesis for the presence of HEMA as eluate (Fig. 5).
a Reference chromatogram of HEMA (retention time 5.23 min; internal standard caffeine at a retention time of 9.93 min). b Reference mass spectra from HEMA: main signals are at m/z 41, 69, 86 and 100. c Gas chromatogram from the ethanol/water 3:1 eluate from specimen from Venus® Diamond after 20 s of polymerisation time. The signal at a retention time of 5.32 min indicates HEMA. The mass spectra (d) verifies that the signal is HEMA (see mass spectra downwards). The main peaks from the reference spectra are also in the mass spectra from the eluate
As for Filtek™ Supreme XTE, the material was less sensitive influenced in its elution by the polymerisation time and the modulation of time, except for the MAA amount, which increased by time modulation.
The photoinitiating systems used in all three analysed materials are primarily based on CQ and amines. Although used in very small amounts (ppm), the yellow-coloured CQ significantly influences the final composite colour [41], thus the dosage of the CQ amount in a RBC must be carefully balanced. Moreover, non-irradiated CQ was shown to induce oxidative stress, DNA damage and cytotoxicity in primary human gingival fibroblasts at concentrations ranging between 0.05 mM and 2.5 mM [42], being also one of the most easily eluted substances in RBCs [25]. The amount of eluted CQ was at all irradiation protocols and in all analysed material by far less as the values indicated above. Nevertheless, an increased irradiation time (40 s, Filtek™ Supreme XTE) or the modulation of time at 40 s (Tetric Evo Ceram®, Venus® Diamond) were able to significantly reduce the amount of eluted CQ.
For methodical reasons, monomers with a higher molecular weight like BisGMA or UDMA cannot be quantified with GC–MS, limiting thus the statements of the study. However, when compared to LC–MS methods (liquid chromatograph connected to a mass spectrometer), the method used in our study (GC–MS) allows for detecting low molecular weight substances, which are the most elutable substances from RBCs.
Conclusions
The effect of irradiation time on DC is similar in all three analysed materials; DC increased significantly by increasing the curing time from 20 s to 40 s and was not influenced within one polymerization time (20 s or 40 s) by the modulation of time. Since the modulation of time is in all analysed material generally associated with a lower amount of eluted substances, an interrupted irradiation during a restoration procedure with RBCs is tolerable. The evaluated parameters—DC and amount of eluted substances—emphasise the importance of a prolonged polymerisation.
References
Geurtsen W, Lehmann F, Spahl W, Leyhausen G (1998) Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res 41:474–480
Schweikl H, Spagnuolo G, Schmalz G (2006) Genetic and cellular toxicology of dental resin monomers. J Dent Res 85:870–877
Di Pietro A, Visalli G, La Maestra S, Micale R, Baluce B, Matarese G, Cingano L, Scoglio ME (2008) Biomonitoring of DNA damage in peripheral blood lymphocytes of subjects with dental restorative fillings. Mutat Res 650:115–122
Schwengberg S, Bohlen H, Kleinsasser N, Kehe K, Seiss M, Walther UI, Hickel R, Reichl FX (2005) In vitro embryotoxicity assessment with dental restorative materials. J Dent 33:49–55
Goon AT, Bruze M, Zimerson E, Goh CL, Soo-Quee Koh D, Isaksson M (2008) Screening for acrylate/methacrylate allergy in the baseline series: our experience in Sweden and Singapore. Contact Dermatitis 59:307–313
Michelsen VB, Lygre H, Skalevik R, Tveit AB, Solheim E (2003) Identification of organic eluates from four polymer-based dental filling materials. Eur J Oral Sci 111:263–271
Ferracane JL, Condon JR (1990) Rate of elution of leachable components from composite. Dent Mater 6:282–287
Manojlovic D, Radisic M, Vasiljevic T, Zivkovic S, Lausevic M, Miletic V (2011) Monomer elution from nanohybrid and ormocer-based composites cured with different light sources. Dent Mater 27:371–378
Polydorou O, Trittler R, Hellwig E, Kummerer K (2007) Elution of monomers from two conventional dental composite materials. Dent Mater 23:1535–1541
Seiss M, Langer C, Hickel R, Reichl FX (2009) Quantitative determination of TEGDMA, BHT, and DMABEE in eluates from polymerized resin-based dental restorative materials by use of GC/MS. Arch Toxicol 83:1109–1115
Zhang Y, Xu J (2008) Effect of immersion in various media on the sorption, solubility, elution of unreacted monomers, and flexural properties of two model dental composite compositions. J Mater Sci Mater Med 19:2477–2483
Ferracane JL (1994) Elution of leachable components from composites. J Oral Rehabil 21:441–452
Kim JG, Chung CM (2005) Elution from light-cured dental composites: comparison of trimethacrylate and dimethacrylate as base monomers. J Biomed Mater Res B Appl Biomater 72:328–333
Sideridou ID, Achilias DS (2005) Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J Biomed Mater Res B Appl Biomater 74:617–626
Stansbury J, Bowman N, Trujillo M (2008) Dimer acid-derived dimethacrylates and use in dental restorative compositions. United States Patent Application US 20080318188 assignee: The Regents of the University of Colorado, Boulder, CO, USA
Utterodt A et al (2008) Dental composites with Tricyclo[5.2.02.6]decane derivatives. European Patent EP1935393 assignee: Heraeus Kulzer GmbH
Moszner N, Gianasmidis A, Klapdohr S, Fischer UK, Rheinberger V (2008) Sol–gel materials 2. Light-curing dental composites based on ormocers of cross-linking alkoxysilane methacrylates and further nano-components. Dent Mater 24:851–856
Schmidt C, Ilie N (2013) The effect of aging on the mechanical properties of nanohybrid composites based on new monomer formulations. Clin Oral Investig 17:251–257
Schmidt C, Ilie N (2012) The mechanical stability of nano-hybrid composites with new methacrylate monomers for matrix compositions. Dent Mater 28:152–159
Frauscher KE, Ilie N (2012) Degree of conversion of nano-hybrid resin-based composites with novel and conventional matrix formulation. Clin Oral Investig [Epub ahead of print]
Durner J, Obermaier J, Draenert M, Ilie N (2012) Correlation of the degree of conversion with the amount of elutable substances in nano-hybrid dental composites. Dent Mater 28:1146–1153
Rueggeberg FA (2011) State-of-the-art: dental photocuring—a review. Dent Mater 27:39–52
Rencz A, Hickel R, Ilie N (2012) Curing efficiency of modern LED units. Clin Oral Investig 16:173–179
Munksgaard EC, Peutzfeldt A, Asmussen E (2000) Elution of TEGDMA and BisGMA from a resin and a resin composite cured with halogen or plasma light. Eur J Oral Sci 108:341–345
Spahl W, Budzikiewicz H, Geurtsen W (1998) Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J Dent 26:137–145
Ferracane JL, Greener EH (1986) The effect of resin formulation on the degree of conversion and mechanical properties of dental restorative resins. J Biomed Mater Res 20:121–131
Shin DH, Rawls HR (2009) Degree of conversion and color stability of the light curing resin with new photoinitiator systems. Dent Mater 25:1030–1038
Daronch M, Rueggeberg FA, De Goes MF (2005) Monomer conversion of pre-heated composite. J Dent Res 84:663–667
Odian G (2004) Principles of polymerization. Wiley, New York
Catalgil-Giz H, Giz A, Oncul-Koc A (1999) Termination mechanism of poly(methyl methacrylate) and polystyrene studied by ultrasonic degradation technique. Polymer Bulletin (Berlin) 43:215–222
Lim BS, Ferracane JL, Sakaguchi RL, Condon JR (2002) Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater 18:436–444
Asmussen E, Peutzfeldt A (2003) Two-step curing: influence on conversion and softening of a dental polymer. Dent Mater 19:466–470
United States Food and Drug Administration (1988) Recommendations for chemistry data for indirect food additives petitions. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, DC
Moreira Fdo C, Antoniosi Filho NR, Souza JB, Lopes LG (2010) Sorption, solubility and residual monomers of a dental adhesive cured by different light-curing units. Braz Dent J 21:432–438
He H, Li L, Lee LJ (2008) Photopolymerization and structure formation of methacrylic acid based hydrogels: the effect of light intensity. React Funct Polym 68:103–113
Utterodt A, Ruppert K, Schaub M, Diefenbach C, Reischl K, Hohmann A, Eck M, Schoenhof N (2008) Dental composites with decreased toxicity containing as crosslinkers tricyclo[5.2.02.6]decane (TCD) derivatives of urethane group-containing (meth)acrylic acid. Application: EP: Heraeus Kulzer GmbH
Frauscher KE, Ilie N (2012) Depth of cure and mechanical properties of nano-hybrid resin-based composites with novel and conventional matrix formulation. Clin Oral Investig 16:1425–1434
Kurokawa R, Finger WJ, Hoffmann M, Endo T, Kanehira M, Komatsu M, Manabe A (2007) Interactions of self-etch adhesives with resin composites. J Dent 35:923–929
Marchesi G, Breschi L, Antoniolli F, Di Lenarda R, Ferracane J, Cadenaro M (2010) Contraction stress of low-shrinkage composite materials assessed with different testing systems. Dent Mater 26:947–953
Reiners J, Podszun W, Winkel J (1988) Urethane group-containing (meth)acrylic acid derivatives of tricyclo[5.2.1.02,6]decane as intermediates in the manufacture of dental materials. Application: DE patent 1987-3703120
Janda R, Roulet JF, Kaminsky M, Steffin G, Latta M (2004) Color stability of resin matrix restorative materials as a function of the method of light activation. Eur J Oral Sci 112:280–285
Volk J, Ziemann C, Leyhausen G, Geurtsen W (2009) Non-irradiated campherquinone induces DNA damage in human gingival fibroblasts. Dent Mater 25:1556–1563
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilie, N., Obermaier, J. & Durner, J. Effect of modulated irradiation time on the degree of conversion and the amount of elutable substances from nano-hybrid resin-based composites. Clin Oral Invest 18, 97–106 (2014). https://doi.org/10.1007/s00784-013-0934-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-013-0934-2