Abstract
The aim of this study was to report on the clinical and radiographic results 5 years following treatment of intrabony defects with guided tissue regeneration (GTR) in combination with deproteinized bovine bone (DBB) (Bio-Oss). Fifteen patients, with at least one intrabony periodontal defect with probing pocket depth (PPD)≥7 mm and radiographic presence of an intrabony component (IC)≥4 mm, were treated with a PLA/PGA bioabsorbable membrane. Prior to placement of the membrane, the defect was filled with DBB impregnated with gentamicin sulfate 2 mg/ml. Standardized intraoral radiographs were taken prior to treatment and at the control examinations after 1 and 5 years. At baseline, the average PPD was 9.2±1.1 mm, and the average probing attachment level (PAL) was 10.1±1.6 mm; the radiographic bone level (RBL) was 10.4±2.45 mm, and an IC of 6.2±2.3 mm was present. One year after membrane placement, treatment had resulted in a PAL gain of 3.8±1.8 mm, a residual PPD of 4.2±1.3 mm, an RBL gain of 4.7±2.0 mm, and a residual IC of 2.1±1.2 mm. At the 5-year examination, two patients did not show up, and two patients had lost the treated tooth. However, both teeth were endodontically treated, and progressive periodontal destruction might not necessarily have been the reason for extraction. At the 5-year control (11 patients), the PAL gain was 4.1±1.6 mm, and the residual PPD was 4.6±1.2 mm; an RBL gain of 4.9±2.7 mm and a residual IC of 1.8±0.8 mm were observed. Statistically significant clinical improvements had occurred between baseline and the 1- and 5-year controls, whereas there were no significant differences between the 1- and 5-year results. The results of GTR with bioabsorbable membranes in combination with Bio-Oss in the treatment of periodontal intrabony defects are basically stable on a long-term basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is ample evidence (i.e., human histologic specimens, controlled clinical trials, controlled animal experiments, a biologic concept) that by means of the guided tissue regeneration (GTR) technique, regeneration of the periodontal attachment apparatus can be achieved (for review see [16]). It has also been documented that the clinical improvements obtained (i.e., probing attachment level (PAL) gain, probing pocket depth (PPD) reduction, bone fill) after GTR treatment of, for instance, periodontal intrabony defects can be preserved on a long-term basis, irrespective of the nature of the barrier material used (nonbioabsorbable/bioabsorbable) [1, 6–10, 13, 17, 26, 31, 37]
Although conflicting results have been reported following the use of adjuncts to the GTR technique regarding an added effect of the combination treatment, bone grafts and/or bone graft substitutes are often placed underneath the membrane aiming to support the barrier material, and thereby prevent its collapse, and/or to promote bone regeneration (for review see [29]). Commercially available deproteinized bone of bovine (DBB) origin (e.g., Bio-Oss, Geistlich AB, Wohlhusen, Switzerland), for instance, has been broadly used as an adjunct to GTR in the treatment of a variety of periodontal defects, and clinically successful results have been reported [2, 3, 18, 21, 25, 30]. However, these studies have only presented results up to 1 year after treatment, while to the best of our knowledge, there are no publications reporting about the long-term outcome of the combined treatment regimen. Thus, the aim of the present paper is to report on the clinical and radiographic results 5 years following treatment of intrabony defects with GTR in combination with DBB (Bio-Oss).
Material and methods
Fifteen interproximal intrabony defects in 15 adult patients presenting for treatment at the Department of Periodontology and Oral Gerontology, Royal Dental College, University of Aarhus, Denmark, were included in the study. Approximately 2 months after the initial periodontal treatment, which consisted of oral hygiene instruction and scaling and root planing, the defects presented the following characteristics: (a) PPD≥7 mm and radiographic evidence of an intrabony component (IC)≥4 mm (Fig. 1a and b), which did not include a furcation involvement; (b) the site had not been treated surgically within the last year before initiation of the study; and (c) systemic antibiotics had not been used within the last 6 months prior to treatment.
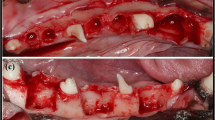
a A deep pocket is probed on the distal side of 46 at baseline. b A deep defect with evidence of an IC>4 mm is observed in the baseline x-ray. c The presence of a predominantly 2-wall defect, with an IC≥4 mm, is confirmed during surgery after removal of the granulation tissue. d Prior to the final placement and stabilization of the membrane, Bio-Oss impregnated with gentamicin sulfate 2 mg/ml is loosely packed into the defect without overfilling it. e The clinical result 1 year after treatment. f Resolution of the defect with RBL gain and slight crestal resorption is observed in the x-ray 1 year after treatment. g The clinical result 5 years after treatment. Note the slight improvement on the soft tissue conditions when compared with the 1-year result. h Radiographically stable conditions are observed 5 years after treatment
The defects were treated as follows. After local anesthesia, intrasulcular incisions were made on the buccal and oral aspects of the jaw at the defect site and extended to the adjacent teeth mesially and distally. Care was taken to preserve as much as possible the interdental tissues at the defect site. Full thickness mucoperiosteal flaps were then raised at both the buccal and oral aspect of the teeth. The defect was debrided, and the roots were scaled and planed and rinsed with sterile saline. It was then assessed whether the defect was ≥4 mm deep (Fig. 1c). A bioabsorbable barrier membrane made of a PLA/PGA copolymer (Resolut XT, W.L. Gore & Associates, Flagstaff, AZ, USA) was trimmed and adapted so that it totally covered the defect and extended at least 3 mm beyond its margins. Prior to the final placement of the membrane, DBB impregnated with gentamicin sulfate 2 mg/ml (Garamycin, Schering-Plough A/S, Farum, Denmark) for approximately 10 min was loosely packed into the defect without overfilling it (Fig. 1d). Then, the membrane was fixed by means of a bioabsorbable ligature around the neck of the adjacent teeth, and the mucoperiosteal flaps were coronally displaced to fully cover the membrane. To avoid tension on the tissues, horizontal split thickness and/or vertical releasing incisions were made as needed. The flaps at the defect site were sutured by means of vertical mattress and single interdental 4.0 Teflon sutures (Gore-Tex suture material, W.L.Gore & Associates). The sutures were removed 2–3 weeks later.
The patients received systemic antibiotic therapy with a combination of amoxicillin 750 mg and metronidazol 250 mg thrice a day for 5 days, starting 1 h before surgery. Chemical plaque control (0.2% chlorhexidine digluconate rinsing twice a day) was instituted for a period of 6 weeks, after which mechanical oral hygiene measures including interproximal tooth cleaning were reinstituted. In addition, the patients were recalled for control and professional prophylaxis with supragingival polishing with a rubber cup, once every week during the first 6 weeks after treatment. At these visits, it was recorded whether the membrane had become exposed. The patients were then examined once per month for the following 5 months of the study period, where calculus, if present, was removed and the teeth were polished. Deep subgingival instrumentation and probing were avoided at the GTR treated sites for the first year after surgery. One year after surgery, a control examination was made, and the patients were transferred to their own private dentist, where each of them followed an individualized maintenance program. All patients were recalled for another control of the membrane treated sites 5 years after treatment.
At the day of surgery (baseline), and after 1 and 5 years, the following clinical parameters were recorded at each GTR-treated site (both from the buccal and the palatal/lingual aspect) to the closest millimeter by means of a manual periodontal probe with a round tip of 0.5 and 1 mm marked increments (Hu-Friedy LL 20): (a) PPD, the distance from the gingival margin to the level of probe-tip penetration; (b) gingival recession (REC), the distance from the cemento–enamel–junction (CEJ) to the gingival margin—in case that the CEJ was difficult to distinguish or absent, the margin of a restoration or crown was used as the coronal reference point; (c) PAL= PPD+REC. In addition, presence or absence of plaque [plaque index (PI)] and presence or absence of bleeding on probing (BOP) were assessed.
Information about the patients' smoking habits was collected at the 1- and 5-year control visits. The frequency of dental visits at their own dentist since the 1-year control and the kind of treatment administered during these visits were also recorded according to information given by the patients. A single investigator performed all the surgeries and made the recordings at baseline and after 5-years, while another (previously calibrated) investigator made all the recordings during the 1-year control.
Standardized periapical radiographs were taken prior to surgery (baseline) and at the 1- and 5-year control. An individual, custom-made silicon bite index and a film-holder device consisting of a metal bar connected to a muff, in which the x-ray tube fitted, were utilized to ensure reproducible projection angles. Identical exposure parameters were used at all examinations, and the films were processed automatically [28]. The radiographs were digitized by means of a scanner with a transparency module at 300 dpi and fed to a computer-imaging device that recognized the scanner signal. By means of an image analysis program (PorDios, Institute of Orthodontic Computer Science Ltd., Aarhus, Denmark) [12], the following parameters were estimated on the images: (a) the distance from CEJ to the bottom of the intrabony defect (BD), representing the radiographic bone level (RBL); (b) the distance from CEJ to the bone crest (BC); and (c) the distance from BC to BD, representing the IC of the lesion. The BD was defined as the most coronal point where the periodontal ligament space showed a continuous width. A single investigator made the analysis of the pre and posttreatment radiographs. The intraexaminer reproducibility of this method of analysis of the radiographs has been evaluated previously [30].
Significance of differences for PI and BOP between baseline, 1-year, and 5-year data was evaluated with the McNemar's test. Significance of differences between baseline, 1-year, and 5-year clinical data was evaluated by means of the Student's t test for paired observations. Patients declaring that they smoked regularly (i.e., at least five cigarettes on a daily basis) at both the 1- and the 5-year control were classified as smokers. Patients receiving a dental control/and or professional prophylaxis at least every 4 months after the 1-year control were classified as being frequently controlled. Presence of plaque at the treated site in both the 1- and the 5-year control was acknowledged as evidence of poor oral hygiene. Treated sites that bled after probing in both the 1- and the 5-year control were classified as showing frequent BOP. Association of smoking, frequency of dental controls, oral hygiene, and BOP with sites that showed PAL loss was evaluated with Fisher's exact test, with the threshold to characterize sites loosing attachment between the 1- and the 5-year control set to (a) PAL loss≥1 mm and (b) PAL loss≥2 mm. The level of significance was set at P<0.05. All calculations were performed with the SPSS for Windows (version 10.0.5) software package (SPSS Inc., Chicago, IL, USA).
Results
During surgery, the presence of an IC≥4 mm was confirmed; in four patients the defects were primarily of 1-wall, whereas in the rest of the patients, the defects had primarily 2-bone walls. All surgically treated sites healed without significant problems (Fig. 1e,f). Membrane exposure, however, was a common event; 13 membranes became exposed to the oral environment. In most of these cases, the exposure presented as an “opening” (separation) of the interdental papillae that occurred 2–3 weeks after surgery. The exposed membranes were not associated with any signs of excessive inflammation, and none of them was removed. Usually, the exposed portion of the membranes was resorbed after approximately 2 weeks, disclosing new immature tissue formed underneath the barrier. Occasionally, graft particles incorporated in the newly formed tissue could be observed. The site (buccal or oral) of the interproximal defect with the deepest PPD value at baseline was chosen as the site of analysis. In case baseline PPD values did not differ, the site (buccal or oral) with the deepest PPD after 1 year was chosen as the site of analysis. At the 5-year examination, two patients did not come for the control due to general health problems. They informed (over the telephone) that they had not experienced any problems with the treated teeth. Two patients that came for the 5-year control had lost their treated tooth. One of these teeth was removed 3 years after, and the other tooth, 4.5 years after surgery due to “persistent periodontitis” (according to the patients). Both these teeth were endodontically treated by a general dentist at some point after GTR surgery and exhibited a rather deep PPD (6 mm) at the 1-year control. Thus, at the 5-year control, 11 sites were available for evaluation. According to the patients, regular maintenance care (i.e., professional tooth cleaning) was the only periodontal treatment administered to these teeth.
The clinical and radiographical data from baseline and from the 1- and 5-year control are presented in Table 1. On average, treatment resulted in statistically significant clinical improvements (i.e., PPD reduction, PAL gain) 1 year after surgery, which were preserved during the following 4-year observation period. PPD had increased to a minor extend (0.4 mm) from the 1- to the 5-year control visit, but the average amount of residual PPD was not significantly different between the two observation periods. On the other hand, REC had decreased from 1- to 5 years and the gingival margin was no longer at a significantly different level from that at baseline (Fig. 1g). The clinical measurements of each individual patient at baseline and at the 1- and 5-year control are presented in Table 2. Out of the 11 sites available for analysis at the 5-year control, only a few had lost part of the PAL gain obtained 1-year after GTR surgery (36.6% with PAL loss≥1 mm and 9.1% with PAL loss≥2 mm), whereas 63.7% of the sites presented with PAL gain≥4 mm (Table 3).
To avoid that radiographs, which due to their projection geometry presented a large dimensional distortion from the real anatomical features included in the analysis, those with an RBL 1.5 mm smaller than the corresponding PAL were excluded. Radiographs where the artificial CEJ of a restoration was not distinguishable or the periodontal ligament space could not be identified were also excluded. Thus, radiographs from only ten patients were available for appraisal at baseline (Table 1). The evaluation of the radiographs showed that a statistically significant gain in RBL was obtained 1 year after treatment (Fig. 1f), and that this gain was preserved during the rest of the observation period (Fig. 1h). In addition, a statistically significant reduction in IC depth was observed at both the 1- and the 5-year control (Table 1) compared with baseline. In most of the cases, the grafted defect space was still discernible on the 1-year radiographs due to its more radio-opaque appearance compared with the adjacent (host) alveolar bone. After 5 years, the original defect space was barely distinguishable, i.e., the new tissue resembled more the neighboring bone with only few exemptions.
Out of the 11 patients, 5 were smokers (1 of the patients that smoked at the 1 year control had quit smoking, whereas no patient had started smoking regularly during the follow-up period), 10 of them had good oral hygiene, but only 3 had gone to control visits frequently. Out of the 11 sites, only 3 bled on probing at both the 1- and the 5-year control. There was no statistically significant association among the above-mentioned parameters and the sites with PAL loss≥1 mm or PAL loss≥2 mm (data not shown).
Discussion
The results of the present study show that the clinical improvements obtained 1 year after the GTR treatment with PLA/PGA bioabsorbable membranes in combination with DBB in periodontal intrabony defects can be preserved for at least 4 additional years. Only two teeth were lost (13.3%) due to persistent periodontal destruction (according to the patients). However, care should be taken regarding self-reporting of periodontal health status [11, 15], and because both of these teeth were endodontically treated at some point after GTR therapy, they might in fact have been lost due to a continuing/recurrent pathological condition of endodontic etiology. The findings in the present study are in accordance with previous publications about the long-term effect of GTR treatment in intrabony defects, where various types of bioabsorbable membranes without the use of bone grafts/substitutes were employed [17, 26, 31]. Stavropoulos and Karring [31], for example, reported that only 0.2 mm from an average of 3.8 mm PAL gain obtained 1 year after GTR treatment with polylactic acid/citric acid ester copolymer bioabsorbable membranes was lost 6 to 7 years after surgery. Similarly, Sculean et al. [26] reported that only 0.3 mm of the average PAL gain (3.2 mm) obtained 1 year after GTR treatment with PLA/PGA bioabsorbable membranes was lost over an additional 3-year follow-up period, whereas Kim et al. [17] failed to detect any difference in PAL gain (3.0 mm) from 1 to 5 years after GTR surgery. Comparable results have been reported following GTR treatment with nonbioabsorbable membranes, where the majority of defects/patients remained relatively stable over various long-term observation periods [1, 6–8, 13, 37]. In a recent retrospective analysis of 175 deep intrabony defects treated by means of GTR using both types of membranes with or without various grafts, tooth retention was greater than 96% and only one third of all treated sites had lost more than 1 mm in PAL over a 15-year period [9].
In the present group of patients, the graft particles were impregnated with gentamicin sulfate 2 mg/ml prior to implantation. Preliminary data from an experimental study, published only a few months earlier than the time these patients were treated, had suggested that gentamicin might promote early vascularization of bone grafts [14]. Recognizing the importance of angiogenesis in bone formation [23] and periodontal regeneration [38], it was thought that impregnation of DBB with gentamicin might promote healing of intrabony periodontal defects. The improvements of the clinical and radiographical parameters 1 year after treatment in the present group of patients, however, were not found to be significantly better than those obtained after GTR+Bio-Oss without gentamicin in a randomized controlled clinical trial set-up [30]. In addition, an experimental study in rats using a “discriminating” capsule model failed to disclose an added effect of gentamicin on bone formation produced by Bio-Oss and GTR [32]. Inasmuch as gentamicin does not seem to enhance bone healing, it is also unlikely that it has played a role in the long-term stability of the clinical improvements observed in the present study.
The improvements of the clinical (PAL gain=3.8 mm, residual PPD=4.2 mm) and radiographical (RBL gain=4.7 mm, residual IC=2.1 mm) parameters 1 year after treatment in the present group of patients are similar to those reported by others after GTR treatment with bioabsorbable membranes in combination with Bio-Oss grafting in intrabony defects [25, 36], but in some reports, an even larger PAL gain (range, 5.0–5.5 mm) was observed [3, 18, 21]. The aim of supplementing the GTR technique with a bone graft and/or a biomaterial placed underneath the membrane is to support the barrier material and prevent collapse (i.e., to avoid a compromised healing result due to reduced/limited space for tissue ingrowth) and/or to promote bone regeneration (i.e., to enhance periodontal regeneration). Conflicting results, however, have been reported when the GTR+Bio-Oss combination regimen was evaluated against only GTR in the treatment of intrabony defects. In a recent study [21], a significantly larger PAL gain was observed after GTR+DBB as compared with GTR with a collagen membrane only (5.1 vs 4.0 mm, respectively), but in another recent randomized controlled clinical trial, the improvements obtained with these two treatment modalities were not significantly different [30]. The results of a recently published systematic review evaluating a variety of bone grafts and/or substitutes as adjuncts to GTR have also failed to find any added clinical benefit of the combination treatment regimen over that achieved by the use of only a membrane [20]. On the other hand, in a recent experimental study in dogs [39], where surgically induced 2-wall intrabony defects were treated with either a bioabsorbable collagen membrane+Bio-Oss or with GTR alone, the authors reported that periodontal regeneration occurred to a statistically significant greater extend (higher level) in sites receiving the combination regimen as compared with those treated only with a membrane. In addition, recent histological evidence confirmed that the GTR+Bio-Oss combination indeed produced various amounts of periodontal regeneration in humans [3, 19, 22, 27]. However, the results from recent controlled studies in experimental animals have questioned the potential of Bio-Oss to enhance bone regeneration in association with GTR [4, 5, 33, 34]. Stavropoulos et al. [33, 34], for example, observed that in Bio-Oss grafted hemispherical Teflon capsules (placed on the lateral side of the mandibular ramus in rats), bone formation was rather limited and occurred only in the proximity of the pristine mandibular ramus, whereas the majority of the capsule space was filled with graft particles embedded in loose connective tissue even after prolonged periods of time. Nongrafted capsules, on the other hand, were consistently filled with voluminous amounts of new bone. Based on these results and the results of Carmagnola et al. [4, 5], Stavropoulos et al. [35] suggested that the outcome of treatment in terms of bone fill in a Bio-Oss grafted defect depends on the configuration (i.e., number of bone walls) and the dimensions of the defect.
The above-mentioned observations question whether bone grafts and/or substitutes are beneficial as adjuncts to GTR in the treatment of intrabony periodontal defects. However, an important aspect in such a discussion should also be the long-term effect of the combination treatment. To date, there is no histological evidence on the long-term result of GTR+Bio-Oss in periodontal defects, but it has been observed that Bio-Oss particles remain in augmented bone defects for a rather long period of time—1.5 years in rats [35] and up to 6 years in humans [24]. It is reasonable to expect that Bio-Oss particles will also be present in treated periodontal sites for quite a long time. The present results suggest that the mere presence of Bio-Oss particles in the reformed periodontal tissues may have no influence on the stability of the improved clinical conditions.
Loss of the PAL gain obtained 1 year after GTR treatment has been associated with smoking, poor oral hygiene [6, 9], and lack of compliance with a supportive periodontal program [7, 37]. In contrast to these observations, smoking, oral hygiene, BOP, and the frequency of dental controls did not seem to play a role for the stability of PAL gain in the present study. This discrepancy may be due to the fact that a rather few sites in the present study lost part of the PAL gain obtained 1 year after GTR treatment (36.6% if PAL loss≥1 mm and 9.1% if PAL loss≥2 mm) and/or the rather limited total number of sites (11 sites) and not due to an increased resistance in periodontal destruction due to Bio-Oss grafting. In fact, there is no information on how periodontal and/or bone tissue regenerated by GTR+Bio-Oss reacts in the event of recurrent inflammation.
In conclusion, the findings of the present case series, although based on a small number of observations, suggest that the clinical improvements in the periodontal conditions of intrabony defects treated with GTR+Bio-Oss are basically stable on a long-term basis.
References
Becker W, Becker BE (1993) Treatment of mandibular 3-wall intrabony defects by flap debridement and expanded polytetrafluoroethylene barrier membranes. Long-term evaluation of 32 treated patients. J Periodontol 64:1138–1144
Camargo PM, Lekovic V, Weinlaender M, Nedic M, Vasilic N, Wolinsky LE, Kenney EB (2000) A controlled re-entry study on the effectiveness of bovine porous bone mineral used in combination with a collagen membrane of porcine origin in the treatment of intrabony defects in humans. J Clin Periodontol 27:889–896
Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, Nevins M (1998) Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodontics Restor Dent 18:321–331
Carmagnola D, Adriaens P, Berglundh T (2003) Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res 14:137–143
Carmagnola D, Berglundh T, Lindhe J (2002) The effect of a fibrin glue on the integration of Bio-Oss with bone tissue. A experimental study in labrador dogs. J Clin Periodontol 29:377–383
Cortellini P, Paolo G, Prato P, Tonetti MS (1996) Long-term stability of clinical attachment following guided tissue regeneration and conventional therapy. J Clin Periodontol 23:106–111
Cortellini P, Pini-Prato G, Tonetti M (1994) Periodontal regeneration of human infrabony defects (V). Effect of oral hygiene on long-term stability. J Clin Periodontol 21:606–610
Cortellini P, Stalpers G, Pini PG, Tonetti MS (1999) Long-term clinical outcomes of abutments treated with guided tissue regeneration. J Prosthet Dent 81:305–311
Cortellini P, Tonetti MS (2004) Long-term tooth survival following regenerative treatment of intrabony defects. J Periodontol 75:672–678
De Sanctis M, Zucchelli G (2000) Interleukin-1 gene polymorphisms and long-term stability following guided tissue regeneration therapy. J Periodontol 71:606–613
Gilbert AD, Nuttall NM (1999) Self-reporting of periodontal health status. Br Dent J 186:241–244
Gotfredsen E, Kragskov J, Wenzel A (1999) Development of a system for craniofacial analysis from monitor-displayed digital images. Dentomaxillofacial Radiol 28:123–126
Gottlow J, Nyman S, Karring T (1992) Maintenance of new attachment gained through guided tissue regeneration. J Clin Periodontol 19:315–317
Holck DE, Dutton JJ, Proia A, Khawly J, Mittra R, Dev S, Imami N (1998) Rate of vascularization of coralline hydroxyapatite spherical implants pretreated with saline/gentamicin, rTGF-beta 2, and autogenous plasma. Ophthalmic Plastic Reconstr Surg 14:73–80
Kallio P, Nordblad A, Croucher R, Ainamo J (1994) Self-reported gingivitis and bleeding gums among adolescents in Helsinki. Community Dent Oral Epidemiol 22:277–282
Karring T, Lindhe J, Cortellini P (2003) Regenerative periodontal therapy. In: Lindhe J, Karring T, Lang NP (eds) Clinical periodontology and implant dentistry. Blackwell, Oxford, pp 650–704
Kim TS, Holle R, Hausmann E, Eickholz P (2002) Long-term results of guided tissue regeneration therapy with non-resorbable and bioabsorbable barriers. II. A case series of infrabony defects. J Periodontol 73:450–459
Lundgren D, Slotte C (1999) Reconstruction of anatomically complicated periodontal defects using a bioresorbable GTR barrier supported by bone mineral. A 6-month follow-up study of 6 cases. J Clin Periodontol 26:56–62
Mellonig JT (2000) Human histologic evaluation of a bovine-derived bone xenograft in the treatment of periodontal osseous defects. Int J Periodontics Restor Dent 20:18–29
Murphy KG, Gunsolley JC (2003) Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol 8:266–302
Paolantonio M (2002) Combined periodontal regenerative technique in human intrabony defects by collagen membranes and anorganic bovine bone. A controlled clinical study. J Periodontol 73:158–166
Paolantonio M, Scarano A, Di Placido G, Tumini V, D'Archivio D, Piattelli A (2001) Periodontal healing in humans using anorganic bovine bone and bovine peritoneum-derived collagen membrane: a clinical and histologic case report. Int J Periodontics Restor Dent 21:505–515
Rhinelander FW, Wilson JW (1982) Blood supply to developing, mature and healing bone. In: Sumner-Smith G (ed) Bone in clinical orthopaedics. W.B. Saunders, Philadelphia, USA, pp 81–158
Schlegel AK, Donath K (1998) BIO-OSS—a resorbable bone substitute? J Long-Term Eff Med Implants 8:201–209
Sculean A, Berakdar M, Chiantella GC, Donos N, Arweiler NB, Brecx M (2003) Healing of intrabony defects following treatment with a bovine-derived xenograft and collagen membrane. A controlled clinical study. J Clin Periodontol 30:73–80
Sculean A, Donos N, Miliauskaite A, Arweiler N, Brecx M (2001) Treatment of intrabony defects with enamel matrix proteins or bioabsorbable membranes. A 4-year follow-up split-mouth study. J Periodontol 72:1695–1701
Sculean A, Stavropoulos A, Windisch P, Keglevich T, Karring T, Gera I (2004) Healing of human intrabony defects following regenerative periodontal therapy with a bovine-derived xenograft and guided tissue regeneration. Clin Oral Investig 8:70–74
Sewerin I (1990) Device for serial intraoral radiography with controlled projection angles. Tandlaegebladet 94:613–617
Stavropoulos A (2002) Guided tissue regeneration in combination with deproteinized bovine bone and gentamicin. Ph.D. Thesis. Department of Periodontology, Royal Dental College, University of Aarhus, p 163
Stavropoulos A, Karring ES, Kostopoulos L, Karring T (2003) Deproteinized bovine bone and gentamicin as an adjunct to GTR in the treatment of intrabony defects: a randomized controlled clinical study. J Clin Periodontol 30:486–495
Stavropoulos A, Karring T (2004) Long-term stability of periodontal conditions achieved following guided tissue regeneration with bioresorbable membranes: case series results after 6–7 years. J Clin Periodontol 31:939–944
Stavropoulos A, Kostopoulos L, Mardas N, Nyengaard JR, Karring T (2003) Gentamicin used as an adjunct to GTR. J Clin Periodontol 30:455–462
Stavropoulos A, Kostopoulos L, Mardas N, Nyengaard JR, Karring T (2001) Deproteinized bovine bone used as an adjunct to guided bone augmentation: an experimental study in the rat. Clin Implant Dent Relat Res 3:156–165
Stavropoulos A, Kostopoulos L, Nyengaard JR, Karring T (2003) Deproteinized bovine bone (Bio-Oss) and bioactive glass (Biogran) arrest bone formation when used as an adjunct to guided tissue regeneration (GTR). An experimental study in the rat. J Clin Periodontol (in press)
Stavropoulos A, Kostopoulos L, Nyengaard JR, Karring T (2004) Fate of bone formed by guided tissue regeneration with or without grafting of Bio-Oss or Biogran. An experimental study in the rat. J Clin Periodontol 31:30–39
Tonetti MS, Cortellini P, Lang NP, Suvan JE, Adriaens P, Dubravec D, Fonzar A, Fourmousis I, Rasperini G, Rossi R, Silvestri M, Topoll H, Wallkamm B, Zybutz M (2004) Clinical outcomes following treatment of human intrabony defects with GTR/bone replacement material or access flap alone. A multicenter randomized controlled clinical trial. J Clin Periodontol 31:770–776
Weigel C, Bragger U, Hammerle CH, Mombelli A, Lang NP (1995) Maintenance of new attachment 1 and 4 years following guided tissue regeneration (GTR). J Clin Periodontol 22:661–669
Wikesjo UM, Selvig KA (1999) Periodontal wound healing and regeneration. Periodontol 2000 19:21–39
Yamada S, Shima N, Kitamura H, Sugito H (2002) Effect of porous xenographic bone graft with collagen barrier membrane on periodontal regeneration. Int J Periodontics Restor Dent 22:389–397
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stavropoulos, A., Karring, T. Five-year results of guided tissue regeneration in combination with deproteinized bovine bone (Bio-Oss) in the treatment of intrabony periodontal defects: a case series report. Clin Oral Invest 9, 271–277 (2005). https://doi.org/10.1007/s00784-005-0002-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-005-0002-7