Abstract
Many in vitro studies have confirmed the corrosion of dental alloys. However, in vivo corrosion studies, for example, recording of the release of metal ions into saliva, are scarce, and data on the repeatability of the metal content measurements of saliva are lacking. The present study examined the metal content of saliva of patients with and without metal restorations and assessed the repeatability of these data. The composition of each patient’s oral cast alloys was analyzed using the energy-dispersive X-ray analysis of metal biopsy specimens and was compared to the metals found in saliva. Saliva analysis was performed using atomic absorption spectroscopy. Chemical analysis comprised the metals Ag, Au, Co, Cr, Cu, Fe, Ga, In, Ni, Pd, Pt, Sn, and Zn. The metals Ag, Cr, Cu, Fe, Ni, and Zn were found in saliva of patients without metal restorations, but these data showed statistically significant differences in the metal content between consecutively performed samples per patient. The metals Ag, Au, Cr, Cu, Fe, Ni, and Zn were identified in saliva of patients with metal restorations being higher in concentration than in control patients. In 77% of the cases at least one metal of the restoration was found in the patient’s saliva. However, the metal content showed statistically significant differences between replicate samples of the same patient taken at different times. The metal content of saliva is affected among other things by intraoral metal restorations, but present data do not support the idea that it is a reliable indicator for the systemic exposure to metals released from dental alloys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental cast alloys left in the oral cavity for a prolonged period of time may cause adverse effects as a consequence of corrosion [8, 9]. The corrosion products can accumulate in the oral tissues and reach the gastrointestinal tract via saliva [13, 14]. The release of metal ions from various alloys by corrosion has been thoroughly investigated in vitro [9, 12]. However, in vitro corrosion tests do not necessarily reflect the in vivo situation. Biological factors such as bacteria, saliva composition, food, and wear may contribute to corrosion of dental cast alloys [2, 9]. In vivo corrosion tests with saliva as the prime corrosion medium better reflect the complex intraoral conditions [12]. Using atomic absorption spectroscopy (AAS) in a study group of 32 patients with dental alloys, Wirz et al. [15] found correspondence between the components of the alloys and the metal content of saliva. The amounts of Ag, Au, Pd, Cu, Sn, Co, and Cr were higher than those in a control group without metal restorations. De Melo et al. [5] measured the metal release from Co-Cr partial dentures in 30 patients. More than 80% of the saliva samples showed higher amounts of Cr and Co with the denture inserted in the mouth than without the denture; newer dentures released more than older ones and larger dentures more than smaller ones. Directly after insertion of Ni-Cr-Mo alloys in 36 patients, Pfeiffer and Schwickerath [11] found an increased salivary concentration of Ni. After 7 days the amounts for most alloys decreased to those before insertion. Some authors (http://www.amalgam-info.ch/tastu2.htm) even claim that saliva metal content can be used for a quantitative measurement of metal exposure of the patient. However, only few in vivo studies have been published on the corrosion behavior of dental alloys. This may be due to the wide variety of alloys on the market today [4] and to the fact that the components of the alloys inserted may be unknown. Furthermore, there is a lack of information on the repeatability of the metal content measurements of saliva, and therefore the diagnostic value of saliva analysis has not yet been demonstrated.
Therefore the aim of the present study was to examine the metal content of saliva of patients with and without metal restorations and to investigate the repeatability of these data. Furthermore, the composition of all oral cast alloys was analyzed to compare the metals found in saliva to those of the cast alloys. The patients with metal restorations formed a patient group of a defined geographical area (eastern Bavaria, Germany) claiming local adverse effects from dental alloys.
Materials and methods
Patients
In 1995 all dentists in eastern Bavaria, an area with about one million inhabitants, were asked to refer patients with suspected adverse effects from dental alloys, except amalgams, to the Department of Operative Dentistry and Periodontology of the University of Regensburg. Over the next 3 years 250 persons contacted our Department by telephone. Of these, 86 fulfilled the selection criteria and participated in the study. Selection criteria were intraoral complaints or symptoms, including gingivitis, taste irritation, dry mouth, and burning mouth in relation to metal restorations, except amalgams. Patients with exclusively general (nonoral) symptoms were excluded. Standardized anamnesis questionnaires and clinical examination procedures have been described in detail previously [7]. Of the 86 patients 85 provided saliva samples comprising the study group including three smokers. As a control group saliva samples of 20 patients without metal restorations (one smoker) were used for background metal content determination. Age matching was not possible because age-matched patients without metal restorations were not available. The study protocol was approved by the ethics committee of the University of Regensburg in accordance with the Declarations of Helsinki (1975) and Tokyo (1983). All participants gave informed consent.
Saliva and alloy analysis
From each patient of the study group (n=85) and control group (n=20) 1–2 ml unstimulated saliva was collected in the morning before breakfast, tooth brushing, and smoking using polypropylene (PP) tubes (Greiner, Frickenhausen, Germany). In the study group the procedure was repeated once after 2 days. In the control group 11 patients collected one saliva sample and 9 collected saliva three times on 3 consecutive days. The samples were then transferred into perfluoralkoxy bottles (AHF, Tübingen, Germany) and solubilized with about the same volume of 69.5% nitric acid (TraceSelect, Fluka, Neu-Ulm, Germany). After a storage period of 3 days at room temperature the saliva solution was diluted for technical reasons to about 1:10. The AAS analysis of the metals Ag, Au, Co, Cr, Cu, Fe, Ga, In, Ni, Pd, Pt, Sn, and Zn in saliva and the alloy analysis using a modification of the method of Wirz et al. [16] have been described in detail previously [8].
Data treatment and statistical analysis
Saliva data treatment
The data treatment of the AAS values is thoroughly described for the analysis of biopsy specimens adjacent to dental alloys elsewhere [8]. As the same saliva volume was collected from each patient, the detection limit for a single metal did not vary with the sample size. The measured metal contents (micrograms of metal per gram of tissue) were transformed to an ordinal scale by a logarithmic transformation resulting in scores, designated S. Where xg and xppm denote the detection limit and the measured metal content, respectively, the score S was assigned as follows:
The metal content of the study group samples was defined as elevated if the metal content in scores was higher than the highest score of the metal content of the control group.
Alloys data treatment
To identify metals in intraoral alloys three randomly selected particles of each alloy biopsy sample were analyzed. A metal was defined as present if it was detected in at least one of the particles.
Statistical analysis
Medians with 25th and 75th quantiles were used to describe the central tendency and the variations of values. The χ2 test was used to analyze the repeatability of the metal content per patient in consecutive saliva samples and to analyze differences between groups at the α=0.05 level of significance.
Results
Original data
The original data from the AAS pooled for all patients and all times for all measured values not below the detection limit are summarized in Table 1. The total number of cases was 170 for the study group (2 samples of 85 patients), and 38 for the control group (3 samples of 9 patients, 1 sample of 11 patients). The detection limit varied from 2.0 µg metal per gram of saliva for Ag to 500.0 µg Sn. All values of the control group were below the detection limit for Au, Co, Ga, In, Pd, Pt, and Sn. For the other metals tested the number of cases in the control group not below the detection limit ranged from 12 (Ni) to 38 (Cu, Fe, Zn). For the study group the number of cases not below the detection limit ranged from 1 (Ga) to 170 (Fe, Zn). The maximum value of the study group was measured for Sn (13410.0 µg metal per g saliva). The maximum value of the control group was measured for Fe (8410.0 µg). The medians of the study group ranged from 8.0 (Cr) to 8875.0 (Sn). The medians of the control group ranged from 2.0 (Ag) to 631.0 (Zn).
Metal content of saliva of the control group
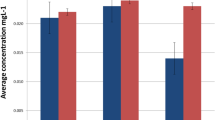
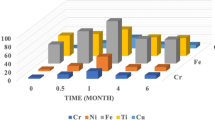
The median metal content in scores of the control group is presented in Fig. 1. The metals Ag, Cr, Cu, Fe, Ni, and Zn were detected in saliva. The scores of these metals ranged from 0 (Cr and Ni) to 3.5 (Fe). The scores for Cu and Zn were 3, and for Ag 0.5. The metal content of the three consecutive samples in scores is shown as an example in Fig. 2 (A) for one randomly selected individual (control s). This shows the wide variation between the repeated measurements, for example, the scores of Cr varied from score 1 (detection limit <metal content ≤ twofold detection limit) to score 3 (eightfold detection limit) and the score of Ni ranged from score 0 (value below detection limit) to score 4 (16-fold detection limit). Statistical analysis on the repeatability showed significant differences in metal content between the three tests performed for all controls.
Metal content of saliva of the study group
The median metal content in scores of the study group is shown in Fig. 1. The metals Ag, Au, Cr, Cu, Fe, Ni, and Zn were detected. The scores (median) of these metals ranged from 0 (Ni) to 4 (Fe and Zn). The metal content in scores for Cu and Ag was 3 and 2, and for Au and Cr the value was 1. The metal content of the two consecutive samples in scores is shown as an example in Fig. 2 (B) for one randomly selected patient (patient 24). In this example the metal content differed significantly between the two tests. Again, this figure shows the wide variation between repeated measurements, for example, the scores of Ag and Au varied from score 0 to score 6 (Ag) and to score 5 (Au). In 82 of the 85 patients in the study group the metal content again differed significantly between the two tests.
Comparison of saliva of control and study group
The metal content of saliva was uniformly higher in the study than in the control group (Fig. 1). The χ2 test revealed a significant difference between control and study group. The frequency distribution of metals with elevated metal content in the study group (scores higher than maximum score in control group) is presented in Fig. 3. Metals with elevated scores in study group were predominately Au (59%) and Ag (48%) followed by Pd, In, Co, Zn, Cr, Sn, Pt, Cu (≤ 8%), and Fe, Ga, Ni (0%).
Comparison of the metal content of saliva and intraoral alloys
The frequency distribution of the number of metals detected in the intraoral alloys and that were elevated in saliva samples in the study group is shown in Fig. 4. In 23% of 85 cases no metal was a component of the cast alloys and was also elevated in saliva. In 77% of 85 cases at least one of the metals was a component of the cast alloys and was also elevated in saliva. In 39% at least two and in 9% at least three metals were components of the cast alloys and were elevated in the saliva samples.
Discussion
The metal content of saliva showed wide variations between the patients in the present study. These variations have also been found in previous reports [5, 10, 15]. The detection limit varied for each analyzed metal. Therefore the metal contents were transformed to an ordinal scale by a logarithmic transformation resulting in scores to compare data from different metals. The data treatment of the AAS values and the inherent limitations of the AAS are thoroughly discussed earlier [8]. According to information of the manufacturer the measurement error of the AAS device is within 3–5% (relative standard deviation).
An age-matched control group would have been desirable in the present study. This was not possible, however, because older patients (50–59 years) predominated in the study group [7], and no age-matched control group without metal restorations was available.
The effect of smoking on the metal content of saliva is controversial [3, 6]. To be on the safe side, in the present study the patients were asked to avoid smoking before saliva collecting. Furthermore, the number of smokers was very low (three in the study and one in the control group), and therefore the results of the metal content analysis are not separately presented for smokers and nonsmokers.
In saliva samples of the control group (without any metal restorations) the metals Ag, Cr, Cu, Fe, Ni, and Zn were found, indicating that other factors than metal restorations may affect saliva metal content. The composition and properties of saliva may be affected by many physiological variables such as nutrition, diet, and salivary flow [10, 11]. According to Edgar and O’Mullane [6], hormones, drugs, and various diseases also influence saliva composition. Furthermore, concerning the data on Fe it should be considered that Fe may be released from hemoglobin and thus can be regarded as endogenous. The data for the ubiquitous metal Zn is an uncontrolled variable because of its possible contamination, for example, through air, despite a thorough hygiene regimen. In agreement with our data, Wirz et al. [15] found the metals Ag, Cr, Cu, Ni, and Zn in the saliva of a control group of 33 individuals without metal restorations. In contrast to the data of the present study, the authors [15] also detected Au, Co, In, Pd, and Sn in saliva samples of the control group (the AAS analysis did not include Fe, Ga, and Pt). Using AAS for Au analysis Björkman et al. [1] did not find Au in samples from individuals without gold alloys in contrast to the patients with gold alloys, which is in line with our data. In summary, metals are present in the saliva of patients without metal restorations, depending on a number of variables.
In saliva samples of the study group the metals Ag, Au, Cr, Cu, Fe, Ni, and Zn were detected, and the metal content was significantly higher than in the control group. The comparison of the metal content of saliva and the intraoral cast alloys revealed that in most cases (77%) at least one metal was a component of the alloys and was also elevated in saliva compared to the control group. These data indicate that components of dental alloys are released into saliva. In this context amalgam fillings in the oral cavity of the study group patients must be considered as an additional source of metal exposure as well. With the AAS Wirz et al. [15] found higher values for Ag, Au, Cr, Cu, Pd, Sn, and Co in a study group of 32 patients with dental alloys than in a control group without metal restorations. For Ag, Au, Cr, and Cu these data are in line with the results of the present study. The authors [15] concluded that metal analysis of saliva makes sense, and that the detected metals give information on the composition of the incorporated alloy. Using AAS De Melo et al. [5] measured Co and Cr in saliva of 30 patients with and without Co-Cr partial dentures. In more than 80% of the patients the amounts of Co and Cr increased when the dentures were incorporated. The median increase in Cr was approximately twice that of Co. Stenberg [13] determined the release of Co from a Co-Cr alloy construction in ten patients. In contrast to the data of the present study AAS revealed an increase in the median Co concentration after insertion of the alloy construction. The data of all these studies show that saliva metal content is influenced by the intraoral metal restorations, although a direct estimation of the composition of the intraoral alloys is not possible from the metal content of saliva.
However, considering the metal content of consecutive saliva samples a wide variation was shown per individual in the present study, despite standardizing saliva collection as consistently as possible. As ruled out above, a possible error of the AAS device may not explain these variations. The metal contents differed significantly between consecutive samples per individual in the control group and in the study group. These variations are difficult to explain and may have the same causes as discussed above for the variation in metal content of patients without metal restorations. Furthermore, it can be speculated that factors such as temporary bruxism affect the metal content of saliva. However, such variations complicate the use of metal analysis for metal exposure estimation of patients. This implies that saliva metal analysis does not add relevant information for the diagnosis and treatment of patients claiming adverse effects from dental alloys. Furthermore, the method of chemical analysis (AAS) does not distinguish between organic and inorganic metal compounds and does not provide information on the electric charge and oxidation level, which, as described earlier [8], affect the biological activity of the metal. For saliva no data are available on the concentration limit for a safe exposure. As a consequence, the determination of the metal content in saliva being advocated in the literature appears as a rather unreliable method so far.
Conclusion
Even in patients without metal restorations metals can be found in saliva. In patients with metal restorations the saliva metal content is significantly higher, and alloy components can be found in saliva. However, repeatability of the recorded data is so low that, including other aspects, metal analysis of saliva is still considered to be a rather unreliable indicator for the metal exposure of patients with metal restorations.
References
Björkman L, Ekstrand J, Lind B (1998) Determination of gold released from dental alloys into saliva (abstract). J Dent Res 77:765
Bumgardner JD, Johansson BI (1998) Effects of titanium-dental restorative alloys galvanic couples on cultured cells. J Biomed Mater Res 43:184–191
Catalanatto FA, Sunderman FW Jr (1977) Nickel concentrations in human parotid saliva. Ann Clin Lab Sci 7:146–151
Datenbank für dentale Metallegierungen (1997) Von Praktikern für Praktiker; für (fast) alle Legierungen, die Zahnärzte in Deutschland einsetzen können, Version 1.0. Spitta, Balingen
De Melo JF, Gjerdet NR, Erichsen ES (1983) Metal release from cobalt-chromium partial dentures in the mouth. Acta Odontol Scand 41:71–74
Edgar WM, O’Mullane DM (1996) Saliva and oral health, 2nd edn. British Dental Journal, London
Garhammer P, Schmalz G, Hiller KA, Reitinger T (2001) Patients with local adverse effects from dental cast alloys: frequency, complaints, symptoms, allergy. Clin Oral Investig 5:240–249
Garhammer P, Schmalz G, Hiller KA, Reitinger T (2003) Metal content of biopsies adjacent to dental cast alloys. Clin Oral Investig 7:92–97
Geurtsen W (2002) Biocompatibility of dental casting alloys. Crit Rev Oral Biol Med 13:71–84
Keruso H, Moe G, Hensten-Pettersen A (1997) Salivary nickel and chromium in subjects with different types of fixed orthodontic appliances. Am J Orthod Dentofacial Orthop 111:595–598
Pfeiffer P, Schwickerath H (1990) Nickellöslichkeit von Dentallegierungen im Speichel. Dtsch Zahnarztl Z 45:492–494
Schmalz G, Garhammer P (2002) Biologic interactions of dental cast alloys with oral tissues. Dent Mater 18:396–406
Stenberg T (1982) Release of cobalt from cobalt chromium alloy constructions in the oral cavity of man. Scand J Dent Res 90:472–479
Wataha JC (2000) Biocompatibility of dental casting alloys: a review. J Prosthet Dent 83:223–234
Wirz J, Dillena P, Schmidli F (1992) Metalle im Speichel. Quintessenz 43:869–874
Wirz J, Schmidli F, Jäger K (1992) Splittertest. Quintessenz 43:1017–1023
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garhammer, P., Hiller, KA., Reitinger, T. et al. Metal content of saliva of patients with and without metal restorations. Clin Oral Invest 8, 238–242 (2004). https://doi.org/10.1007/s00784-004-0281-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-004-0281-4