Abstract
Single case reports indicate that components of dental alloys accumulate in the adjacent soft tissue of the oral cavity. However, data on a wider range of dental alloys and patient groups are scarce. Therefore, the aim of the present study was to examine the metal content of oral tissues adjacent to dental alloys showing persisting signs of inflammation or other discoloration (affected sites) and of healthy control sites with no adjacent metal restoration in 28 patients. The composition of the adjacent alloys was analyzed and compared to the alloy components in the affected sites. Tissue analysis was performed using atomic absorption spectroscopy. Alloy analysis was performed with energy-dispersive X-ray analysis. In the affected sites, the metals Ag, Au, Cu, and Pd prevailed compared to control sites, reflecting the frequency distribution of single metals in the adjacent alloys. In most cases (84%), at least one of the analyzed metals was a component of the alloy and also detected in the tissue. Metal components from almost all dental cast alloys can be detected in adjacent tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental cast alloys are widely used in different applications, e.g., crowns, bridges, and removable dentures. They come into close contact with oral tissues for prolonged periods of time. An essential prerequisite for the assumption that adjacent alloys cause adverse tissue reactions may be considered as the presence of these elements in the alloys [15, 20]. Corrosion of dental cast alloys results in the release of metal ions, which may cause toxic effects or allergic reactions [21, 22, 23]. Many studies have been devoted to allergies to metals and alloys, being verified by skin tests with the corresponding metal salts or alloy disks [8, 11, 14]. However, only single case reports indicate so far that metals such as Ag, Ni, or Cu released from dental cast alloys accumulate in adjacent tissue and are claimed to be the toxic cause of adverse effects such as hyperplasia and gingivitis [12, 22, 25]. In two of these studies, sensitive analytic techniques were used to demonstrate the presence of components of metal restorations in adjacent tissue. Atomic absorption spectroscopy (AAS) was described as a suitable method for analysis of metal content in oral tissues [12, 25]. Wirz et al. [25] showed single cases of adverse effects (inflammation of the adjacent gingiva) related to the metal restoration, in which alloy components were released into the adjacent tissue and the inflammations had not disappeared after plaque removal. Kratzenstein et al. [12] found gingivitis or hyperplasia adjacent to dental alloys in three out of seven cases reporting adverse effects. Metal analysis of these tissues revealed that all metal components of the adjacent alloys could be found in the tissue. However, no data are available on a wider range of dental cast alloys and patient groups, which would allow an estimation of the diagnostic value of analyzing the metal content of biopsies in general. This may be due to the large variety of different alloys on the market today [4] and that the composition of the inserted alloys might be unknown in single patients.
Therefore, the aim of the present study was to examine the metal content of oral tissues showing persistent signs of inflammation or other discoloration adjacent to dental cast alloys and of healthy control sites in the same individual, in a patient group from a defined geographic area of Germany (east Bavaria) claiming local adverse effects from dental alloys. Furthermore, the composition of the cast alloys adjacent to these affected sites was analyzed in order to compare the alloy components found in the affected tissues to those of adjacent alloys.
Materials and methods
Patients
In 1995, all dentists in eastern Bavaria, a region with about one million inhabitants, were asked to refer patients with suspected adverse effects from all dental alloys except amalgam to the Department of Operative Dentistry and Periodontology of the University of Regensburg. Two hundred fifty persons contacted our department by telephone during 3 years. Of these, 86 fulfilled the selection criteria and participated in the study. Selection criteria were intraoral complaints or symptoms such as gingivitis, taste irritation, dry mouth, or burning mouth in relation to metal restorations except amalgam. Patients with exclusively general symptoms were excluded. Standardized anamnesis questionnaires and clinical examination procedures have been described in detail [8]. Professional plaque removal with oral hygiene instructions was performed, and rinsing with chlorhexidine (0.1%) for 1 week was prescribed to reduce the effects of bacteria as causes of inflammation. Twenty-eight of 86 examined patients still showed inflammation (gingivitis, red palate, or denture stomatitis) or discoloration (grayish, whitish, or lichenoid lesion) of the tissue (affected site) adjacent to dental cast alloys after this mechanical and chemical plaque removal (Fig. 1). These 28 patients comprised the study group.
The study protocol was approved by the ethics committee of the University of Regensburg in accordance with the Declarations of Helsinki (1975) and Tokyo (1983). All participants gave informed consent.
Tissue analysis
From each patient (n=28), biopsies of the affected sites (n=31) and healthy control sites without adjacent restorations (n=28) were taken after local anesthesia. These 28 patients correspond to 31 cases, since in two patients multiple biopsies at affected sites were taken because they had different affected sites adjacent to different alloys. The biopsies were dried at 37°C up to weight constancy. Subsequently, the oven-dry mass (dry weight) was determined with an analytic balance (Micro MC 210 P) (Sartorius, Göttingen, Germany). The dried samples were then transferred to a perfluoralkoxy (PFA) bottle (AHF, Tübingen, Germany) and treated with 1–2 ml of 69.5% nitric acid (TraceSelect) (Fluka, Neu-Ulm, Germany). After a storage period of 3 days at room temperature, the solution was diluted to a total volume of 10 ml. The content of the metals Ag, Au, Co, Cr, Cu, Fe, Ga, In, Ni, Pd, Pt, Sn, and Zn was determined in each sample using AAS (Spectr AA-300 Plus, GTA-96 with autosampler, QCP software) (Varian, Darmstadt, Germany) with the standard addition method. For calibration of the metal elements certified, high-purity, inductively coupled plasma (ICP) standards (Merck, Darmstadt, Germany) were used. Measurements of each sample were performed twice.
Alloy analysis
To analyze the composition of the alloys adjacent to affected sites, a modification of the method described by Wirz et al. [25] was used: grinding dust was taken from crowns, bridges, or removable dentures with a new carbide rose-headed burr for each restoration (H1S 204, 014-123) (Komet/Brasseler, Lemgo, Germany) (Fig. 2). These metal "biopsies" were collected using an adhesive foil (G3347, 12 mm, Plano) (Planet, Marburg, Germany) on aluminum stubs (Balzers, Wiesbaden, Germany). After taking samples, the restorations were polished. Alloy analysis was performed with energy-dispersive X-ray analysis (Link AN 100000) (Cambridge Instruments, Nussloch/Heidelberg, Germany).
The analyzed alloys were divided into the following groups according to relevant standards [5, 6, 7] or recommendations of Wirz and Schmidli [24]:
-
High-gold: Au>65% and Au+Pt+Pd+Ir+Rh+Ru+Os>75%
-
Gold-reduced: 25%≤Au+Pt+Pd+Ir+Rh+Ru+Os≤75%, Au being the main component
-
Pd-based: 25%≤Au+Pt+Pd+Ir+Rh+Ru+Os≤75%, Pd being the main component
-
Cr-Co: Cr>25%, Cr+Co>85%, and Mo>4%
Data treatment and statistical analysis
Tissues
All measured values from the AAS were given in µg of metal per g tissue. The AAS analysis of each sample was performed twice (see above) and the higher value was selected as representative for each sample. The smallest metal content we were able to measure in biopsies with the AAS technique (detection limit) varied for a single metal in a biopsy with the size of the tissue samples. In order to compare data from different biopsies, the following procedure of scoring the metal content was applied. In a first step, the detection limit of a particular metal in a single patient was defined as the maximum value of the corresponding detection limits for the affected and control sites. If, in a second step, results of a patient group for any single metal tested were compared, the detection limit was defined as the maximum value of all limits of the group considered. The measured metal contents (µg metal per g tissue) were transformed to an ordinal scale, resulting in scores by a logarithmic transformation. If x g and x ppm denote the detection limit and measured metal content, the score s was assigned as follows:
Alloys
For alloy analysis, three randomly selected particles of each alloy biopsy were analyzed and, for each metal component, the arithmetic mean of the detected proportions was calculated. These proportions were subjected to a specially developed dBase IV database system (Ashton Tate, Chicago, Ill., USA) in order to select matching commercially available alloys. This database included all dental cast alloys available on the German market at the time of the study [2]. The composition of the alloys was given up to 0.1%.
Medians with 25% and 75% quantiles were used to describe the central tendency and variations in values. Discriminate statistics were performed using Wilcoxon's test at the α=0.05 level of significance.
Results
The original data from the AAS are depicted in Table 1. The detection limit (median) varies from 0.7 µg metal per g tissue for Ag to 83.3 µg metal per g tissue for Sn. The values (minimum, maximum, and median) at the affected and control sites were below the detection limits for Co and Pt. The maximum value at the affected site was measured for Ag (5,355.0 µg per g tissue). The maximum value at the control site was measured for Zn (1,376.0 µg per g tissue). Medians at the affected sites ranged from 0.7µg (Cr) to 398.0 µg (Fe) and at the control sites from 0.9 µg (Ag) to 348.0 µg (Fe). For Ag, Au, Cr, Cu, Fe, Ga, In, Ni, and Pd, the maximum value for the controls was higher than the minimum value of the affected sites.
Metal content of the affected tissues and control sites
The metal content of each case and tissue origin was calculated as the sum of scores of the analyzed metals to serve as an indicator for total metal exposure. The median (with 25% and 75% quantiles) of the total metal content (sum of scores) of the affected sites of all biopsies was 14 g (range 10–19). The median of the total metal content of the control sites of all biopsies was 8 g (range 6–10). The difference was statistically significant.
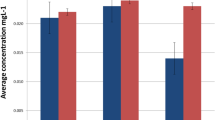
For each metal and tissue origin, the median metal contents are depicted in Fig. 3. In the affected sites, Ag, Au, Cu, Fe, Pd, and Zn were detected, whereas in the controls only Cu, Fe, and Zn were found. The Ag, Au, Cu, and Pd contents differed significantly between the affected and control sites.
The number of cases with higher metal content in affected than control sites is described in Fig. 4, left bars. In 13 or more cases, Ag, Cu, Au, Fe, and Pd showed higher content in affected than in control sites, and Zn, Ni, Cr, Ga, In, Sn, Co, and Pt had higher content in eight cases or fewer.
Composition of the adjacent alloys
The frequency distribution of single metals in adjacent alloys is depicted in Fig. 5. The metals occurring most frequently as components of the adjacent alloys were Au, Pd, Ag, Pt, and Cu. The frequency distribution of alloy groups is shown in Fig. 6, left bars. According to the classification system for cast alloys (see above), 11 high-gold, six gold-reduced, ten Pd-based, and four Cr-Co alloys were found adjacent to affected tissues.
Comparison of the metal content of affected tissues and adjacent alloys
In 26 out of 31 cases, at least one of the metals was a component of the cast alloy and also detected in the adjacent tissue. In 14 cases at least two metals, in ten cases at least three, and in four cases at least four were components of the cast alloy and also detected in the adjacent tissue.
Figure 4 (right bars) shows for each metal the number of cases with higher metal content in affected than control sites when the metal was also a component of the adjacent alloy. The comparison of right to left bars (Fig. 4) reveals that, of the cases with higher metal content in affected than control tissue, Au, Pd, Ga, and In were in the adjacent alloy in most cases.
Figure 6 (right bars) describes in how many cases per alloy group at least one alloy component was also found in adjacent tissue. For all gold-reduced and Pd-based alloys, at least one alloy component was found in the adjacent tissue. For high-gold and Cr-Co alloys, alloy components were found in adjacent tissue in eight of 11 cases and two of four cases, respectively.
Discussion
According to the patient's clinical situation, biopsy size varied in the patients of the study group and therefore, due to dilution effects, the detection limits varied accordingly. When designing the study, it was expected that AAS values of samples might range near the detection limit, and duplicate measurements were performed. There is quite a large variety of possibilities in calculating the descriptive value of these two measurements. The maximum value was used because it usually includes originally measured values in most cases, and any average calculated from data including values below the detection limit would require the definition of further variables. Furthermore, the detection limit for a single metal in a biopsy varied with each metal. To compare data from different biopsies with different detection limits, the metal contents were transformed to an ordinal scale, resulting in scores by a logarithmic transformation. Using one value for the detection limit of a specific metal for all biopsies made it possible to compare reliably the metal content of different tissues.
An independent control group comprised of patients with no symptoms related to dental alloys divided in two groups (one without metal restorations and one with) would have been desirable in this study. However, taking biopsies from healthy patients is considered very problematic for ethical reasons. Therefore, in the present study, each patient served as his own control, with the advantage that the preconditions for control and affected sites were mainly identical.
Fe, Zn, and Cu were found in the control biopsies. Using inductively coupled plasma-mass spectrometry to analyze metal components of human gingiva, Rechmann [16, 17] also found Cu and Zn in controls. He concluded that the presence of these metals cannot be used as a reliable indicator for metal exposure through dental alloys. Zn and Cu might play a role as endogenous elements, e.g., being part of metal-binding metallothioneins, which probably represent detoxification procedures [3, 13], or of metal-binding enzymes (metalloproteinases), which play an important role in periodontal health status [19]. Fe may be released from hemoglobin and thus can also be regarded as endogenous [18]. Furthermore, data for the ubiquitous metal Zn must be interpreted cautiously because of possible contamination (e.g., through air) despite thorough hygiene.
In the affected sites, Ag, Au, Cu, and Pd prevailed, in contrast to the control sites. These data reflect—except for Pt—the frequency distribution of single metals in the adjacent alloys. In this context, previous adjacent amalgam fillings must be considered as an additional source of metal exposure, too. In most cases (84%), at least one of the metals was an alloy component and also detected in the adjacent tissue. This is in agreement with data from Rechmann [16, 17], who performed laser microprobe mass spectrometry and inductively coupled plasma-mass spectrometry on 26 nondiscolored necropsies or biopsies taken from human gingiva adjacent to dental alloys. In his study, 73% of the samples contained metallic compounds, mainly Ag, Pd, Au, Pt, Cu, and Zn, being typical components of dental cast alloys. For all gold-reduced and Pd-based alloys, at least one metal component was also detected in adjacent tissue. This was in contrast to high-gold and Cr-Co alloys, with some cases in which no component was found in adjacent tissue. Although the number of cases is low, these observations may reflect the better corrosion resistance of high-gold and Cr-Co alloys than gold-reduced and Pd-based alloys [9, 12]. Kratzenstein et al. [12] reported on eight patients wearing prosthodontic restorations in whom gold-reduced alloys also showed higher corrosion rates than high-gold alloys. For all alloy groups, components such as Au, Ag, Cu, Pd, Ni, and Cr were found in adjacent tissues. The accumulation of alloy components was also confirmed in animal experiments. Bergenholtz et al. [1] found in 42 dog gingiva samples with class V gold inlays that Au, Zn, and Cu penetrated the adjacent tissue, but not the controls. However, there is no information about the composition of the gold alloys used.
Interpretation of the metal content of biopsies is hampered by inherent limitations in the AAS: no differentiation between oxidation levels of the metal content was possible. However, the valence of a metal affects its biologic activity, e.g., being mutagenic, hexavalent Cr crossed the cell membrane in contrast to trivalent Cr during in-vitro studies [10, 20]. Additionally, no differentiation between small particles of metals and metals as ionic corrosion products was possible in the present study. Using light microscopy, Rechmann [16, 17] showed that in 42.3% of the samples, metallic particles were found implanted in the tissue, most probably mechanically, e.g., through polishing or scaling.
A statistically significant difference was observed in total metal content between the control and affected sites. From the study data, it would have been desirable to extract content limits for each single metal below which no adverse effect occurs. However, for Ag, Au, Cr, Cu, Fe, Ga, In, Ni, and Pd, the maximum values for the controls were higher than the minimum values of the affected sites, giving no indication on the magnitude of a hypothetical limit. Because of the lack of information on limit values, at present, metal analysis of oral tissues does not seem to add relevant information for routine diagnosis and treatment of patients claiming adverse effects from dental alloys.
Conclusion
Within the limitations of this study, it can be concluded that metal components from almost all dental cast alloys can be detected in adjacent tissue. High-gold and Cr-Co alloys seem to show better corrosion resistance than gold-reduced and Pd-based alloys.
References
Bergenholtz A, Hedegård B, Söremark R (1965) Studies of the transport of metal ions from gold inlays into environmental tissues. Acta Odont Scand 23:135–146
Bundesärztekammer (Bundesverband der deutschen Zahnärztekammern) und Kassenzahnärztliche Bundesvereinigung (1995) Das Dental Vademekum. Deutscher Ärzte-Verlag, Köln München
Cherian M, Goyer R (1978) Metallothioneins and their role in the metabolism and toxicity of metals. Life Sci 23:1–9
Datenbank für Dentale Metallegierungen (1997) Von Praktikern für Praktiker; für (fast) alle Legierungen, die Zahnärzte in Deutschland einsetzen können. Edn 1.0. Spitta, Balingen
European Committee for Standardization (1995) EN ISO 1562: dental casting alloys. Brussels
European Committee for Standardization (1996) EN ISO 6871–1: dental base metal casting alloys. Part 1: cobalt-based alloys. Brussels
European Committee for Standardization (2000) EN ISO 8891: Dental casting alloys with noble metal content of at least 25% but less than 75%. Brussels
Garhammer P, Schmalz G, Hiller KA, Reitinger T (2001) Patients with local adverse effects from dental cast alloys: frequency, complaints, symptoms, allergy. Clin Oral Invest 5:240–249
Geurtsen W (2002) Biocompatibility of dental casting alloys. Crit Rev Oral Biol Med 13:71–84
Gray SJ, Stirling K (1950) Tagging of red cells and plasma proteins with radioactive chromium. J Clin Invest 29:1604–1613
Hensten-Pettersen A (1992) Casting alloys: side effects. Adv Dent Res 6:38–43
Kratzenstein B, Sauer KH, Weber H, Geis-Gerstofer J (1986) In-vivo-Korrosionsuntersuchungen goldhaltiger Legierungen. Dtsch Zahnärztl Z 41:1272–1276
Lau JC, Jackson-Boeters L, Daley TD, Wysocki GP, Cherian MG (2001) Metallothionein in human gingival amalgam tattoos. Arch Oral Biol 46:1015–1020
Marcusson JA (1996) Contact allergies to nickel sulfate, gold sodium thiosulfate and palladium chloride in patients claiming side effects from dental alloy components. Contact Dermatitis 34:320–323
Nau H (1997) Toxikokinetik. In: Marquard H, Schäfer SG (eds) Lehrbuch der Toxikologie. Spektrum, Heidelberg
Rechmann P (1992) LAMMS and ICP-MS detection of dental metallic compounds in not-discolored human gingiva. J Dent Res 71:599
Rechmann P (1993) Nachweis metallischer Restaurationsmaterialien in klinisch unauffälliger Gingiva. Dsch Zahnärztl Z 48:270–275
Rechmann P (1995) Aufnahme von Metallen in die Mundschleimhaut. Dtsch Z Mund Kiefer GesichtsChir 19:107–114
Reynolds JJ, Meikle MC (1997) Mechanisms of connective tissue matrix destruction in periodontitis. Periodontology 2000 14:144–157
Schäfer SG, Hannover B, Elsenhaus B, Forth W, Schümann K (1997) Metalle. In: Marquard H, Schäfer SG: Lehrbuch der Toxikologie. Spektrum, Heidelberg
Schmalz G, Garhammer P (2002) Biologic interactions of dental cast alloys with oral tissues. Dent Mat 18:396–406
Taylor DT, Morton TH Jr (1991) Ulcerative lesions of the palate associated with removable partial denture castings. J Prosthet Dent 66:213–221
Wataha JC (2000) Biocompatibility of dental casting alloys: A review. J Prosthet Dent 83:223–234
Wirz J, Schmidli F (1988) Korrosionsverhalten von Co-Basislegierungen für Kronen- und Brückenarbeiten. Quintessenz 11:1997–2008
Wirz J, Schmidli F, Jäger K (1992) Splittertest. Quintessenz 43:1017–1023
Acknowledgements
This study was supported by the German Ministry of Health. We thank Peter Geisenberger for EDX analysis of the cast alloys.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garhammer, P., Schmalz, G., Hiller, KA. et al. Metal content of biopsies adjacent to dental cast alloys. Clin Oral Invest 7, 92–97 (2003). https://doi.org/10.1007/s00784-003-0204-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-003-0204-9