Abstract
Background
Lumbar spinal canal stenosis (LSCS) is one of the most common spinal disorders in the elderly, and ligamentum flavum (LF) hypertrophy is an important cause of LSCS. Matrix metalloproteinase 13 (MMP13) can degrade fibrillar collagens and elastic microfibrils, and is involved in inflammation and fibrosis. The purpose of this study was to compare the expression of MMP13 in the LF from LSCS patients with diabetes mellitus [DM (+)] with that in the LF from patients without DM [DM (−)] and to analyze the relationship among DM, MMP13 expression, and LF hypertrophy.
Methods
LFs from 11 DM (+) and 24 DM (−) LSCS patients were analyzed in this study. Histology analysis using hematoxylin and eosin and Masson’s trichrome stain was performed for each LF. The expression of MMP13 was analyzed by quantitative real-time PCR. The thickness of LF was measured by CT.
Results
In the LF from DM (+) LSCS patients, the elastic fibers were more disorganized and had lower volumes than in the LF from DM (−) LSCS patients, while more fibrotic tissue was observed in the LF from DM (+) than from DM (−) LSCS patients. MMP13 expression was significantly higher in the LF from DM (+) LSCS patients (0.46 ± 0.61 vs. 0.05 ± 0.09, P = 0.002). The LF from the DM (+) LSCS patients was significantly thicker than that from the DM (−) LSCS patients (5.0 ± 0.9 vs. 3.1 ± 0.8 mm, P < 0.01), and the thickness was correlated with the expression of MMP13 (correlation coefficient = 0.43, P = 0.01, Pearson's correlation test).
Conclusion
DM-related MMP13 expression can be one of the factors contributing to fibrosis and hypertrophy of the LF. Further research on the mechanism of this process may lead to new therapies for LF hypertrophy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lumbar spinal canal stenosis (LSCS) is one of the most common spinal disorders in the elderly. The causes of LSCS include ligamentum flavum (LF) hypertrophy, hypertrophy of the facet joints, bulging of the intervertebral discs, and vertebral endplate osteophytosis. Among them, LF hypertrophy plays a dominant role in the narrowing of the lumbar spinal canal [1]. In the lower lumbar spine, the LF is composed of thick elastic fibers that are densely arranged with interspersed collagen fibers [2]. Histological changes in the hypertrophied LF from LSCS patients include fibrosis, degradation of elastic fibers with an increase in collagen fibers, granulation tissue proliferation, chondroid metaplasia, and calcification [3–6]. Fibrosis is considered to be the main cause of LF hypertrophy, and transforming growth factor (TGF)-β released by endothelial cells may stimulate the fibrosis, especially during the early phase of hypertrophy [3]. However, the pathomechanism of LF hypertrophy remains unclear.
The matrix metalloproteinases (MMPs) include over 20 zinc-dependent enzymes that degrade or modify extracellular matrix molecules, such as elastin, collagen, and proteoglycans [7, 8]. Several recent studies have demonstrated a role of MMP3 [9] or MMP inhibitors in the pathology of LF [10].
Of the MMPS, MMP13 can degrade fibrillar collagens, including type I, II, and III collagens, into gelatin. It can also degrade the elastic microfibrils that are involved in extracellular matrix remodeling [11]. In addition, MMP13 plays a role in inflammatory and fibrotic processes [12–14]. MMP13 was reported to be more highly expressed in the LF of LSCS patients than in that of disc herniation patients, and is expressed in LF fibroblasts [15]. Moreover, high plasma glucose increases the expression of MMP13 in vessels [16] and the cornea [17].
We hypothesize that diabetes mellitus (DM) can cause an increased expression of MMP13 in the LF, which may lead to fibrosis and extracellular matrix remodeling and finally cause LF hypertrophy. The purpose of this study was to compare the expression of MMP13 in the LF of LSCS patients with DM [DM (+)] with that in the LF of patients without DM [DM (−)] and to analyze the relationship among DM, MMP13 expression, and LF hypertrophy.
Materials and methods
Thirty-five LF samples were obtained from 35 LSCS patients who underwent decompressive laminectomy for neurogenic claudication. The demographic data of the patients are shown in Table 1. All patients gave informed consent to participate in this study, and the study was approved by the Institutional Review Board of our institute. The mean age was 76.7 ± 7.6 years in the DM (+) group and 72.6 ± 7.4 years in the DM (−) group. There was no difference in age or gender between the two groups (P > 0.05). CT images of the lumbar spine of 1 mm thickness were made for all the patients before surgery.
Histologic analysis with hematoxylin-eosin staining and Masson’s trichrome staining
During surgery, the LF was removed en-bloc, and the epidural fat was detached. Half of each sample was immediately stored in a −80°C freezer for subsequent quantitative real-time PCR analysis; the other half was fixed in 4% neutral formalin and decalcified in 20% ethylenediaminetetraacetic acid (EDTA) for 4 weeks, then embedded in paraffin for histologic analyses. Two consecutive sections (4 μm thick) were cut on a microtome and subjected to hematoxylin-eosin (H&E) and Masson’s trichrome staining, respectively. H&E staining was used to analyze the degradation of the elastic fibers, and Masson’s trichrome staining was used to determine the degree of fibrosis [18]. Masson’s trichrome staining exhibited elastic fibers as pink and collagen fibers as blue in color.
Paraffin sections of yellow ligament were stained with rabbit anti-MMP13 antibody (Abcam, no. ab39012) followed by Alexa488-conjugated goat anti-rabbit antibody (molecular probe) and TOTO3 (molecular probe) as a nuclear stain, and then observed under a confocal microscope (FV1000, Olympus).
Quantitative real-time PCR
The total RNAs were isolated from LF samples by TRIzol reagent (Invitrogen Corp.), and the concentration and quality were determined with an ND-1000 spectrophotometer (NanoDrop). The first-strand cDNAs were synthesized using an Advantage RT-for-PCR kit (Clontech Laboratories Inc.), then subjected to real-time PCR analysis using SYBR Premix ExTaq II (Takara Bio Inc.) according to the manufacturer’s instructions. The MMP13 mRNA was normalized to the β-actin mRNA in each sample. PCR amplification was carried out on a Thermal Cycler Dice Real-Time System (Takara Bio Inc.), and gene expression was quantified using the delta-delta Ct method [19]. Nucleotide sequences of the primers were as follows: MMP13 forward: 5′-GCCAGAACTTCCCAACCAT-3′, MMP13 reverse: 5′-GGGCCCAGAATTTTCTCC-3′, β-actin forward: 5′-TGAGCGCGGCTACAGCTT-3′, and β-actin reverse: 5′-TCCTTAATGTCACGCACGATTT-3′.
Measurement of LF thickness
The LF thickness was measured using the preoperative axial CT images. The thickness of the LF on both sides was measured at its midpoints at the level of the decompressed intervertebral disc, following the method proposed by Fukuyama et al. [20], using image-analysis software (Real INTAGE, Tokyo, Japan) that allowed digital measurements with a precision of 0.1 mm.
The measurements were repeated five times by the first author (G.C.), and the results were averaged. To assess the intra- and inter-observer reliability of measurements, CT images of LF from 20 patients were chosen in a random fashion and were measured again by the first author 2 weeks after his first measurement and by the second author (K.W.). The intra- and inter-observer reliability were statistically tested using an intraclass correlation coefficient. The data presented in the results section are based on the measurements by the first author.
Real-time PCR for mmp13
To evaluate the influence of glucose on mmp13 expression in fibroblasts, which are the main component of the LF, NIH3T3 fibroblastic cells from mice were cultured in serum-free Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich) for 24 h. Then cells were treated with or without 3 mg/ml glucose for 19 h. Subsequently, total RNA was isolated from NIH3T3 cells using an RNeasy mini kit (Qiagen, Hilden, Germany). Single-stranded complementary DNAs (cDNAs) were synthesized with reverse transcriptase (Clontech Laboratories, Palo Alto, CA). Real-time PCR was performed using SYBR Premix ExTaq II (Takara Bio Inc., Otsu, Shiga, Japan) with a DICE Thermal Cycler (Takara Bio Inc.), according to the manufacturer’s instructions. β-Actin expression served as an internal control. Primer sequences were as follows:
- mmp13-forward::
-
5′-AACCTGGACAAGCAGTTCCAAAG-3′
- mmp13-reverse::
-
5′-GAAATGGCTTTTGCCAGTGTAGG-3′
- β-actin-forward::
-
5′-TGAGAGGGAAATCGTGCGTGAC-3′
- β-actin-reverse::
-
5′-AAGAAGGAAGGCTGGAAAAGAG-3′
Statistical analysis
All values were reported as mean ± standard deviation. All data were analyzed with the SPSS system (version 13.0). Comparisons between the two groups were made by the Mann-Whitney U test. The relationship between MMP13 expression and LF thickness was analyzed by Pearson’s test. P < 0.05 indicated statistical significance.
Results
Hematoxylin-eosin staining
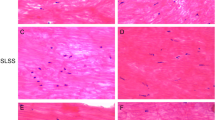
In the LF from DM (+) LSCS patients, the elastic fibers were fragmented, disorganized and focally lost, accompanied by a proliferation of collagen fibers (Fig. 1a, b), while, in the LF from DM (−) LSCS patients, rich elastic fibers were arrayed in parallel order (Fig. 1c, d).
a In the LF from DM (+) LSCS patients, elastic fibers were disorganized and focally lost, accompanied by a proliferation of collagen fibers. The elastic fibers also had low volumes and uneven diameters. H&E staining ×200. b LF from DM (+) LSCS patients. H&E staining ×40. c In the LF from DM (−) LSCS patients, a large area was stained pink with a regular arrangement, indicating a normal, non-fibrotic condition. H&E staining ×200. d LF from DM (−) LSCS patients. H&E staining ×40. e In the LF from DM (+) LSCS patients, a large area was stained blue, indicating the presence of massive fibrosis. Masson’s trichrome staining ×200. f LF from DM (+) LSCS patients. Masson’s trichrome staining ×40. g In the LF from DM (−) LSCS patients, rich elastic fibers were regularly arrayed, and the diameters of the elastic fibers varied only slightly. Masson’s trichrome staining ×200. h LF from DM (−) LSCS patients. Masson’s trichrome staining ×40
Masson’s trichrome staining
In the Masson’s trichrome staining of the LF from DM (+) patients, a large area was stained blue, indicating the presence of massive fibrosis (Fig. 1e, f), whereas in the LF from DM (−) patients, a large area was stained pink and showed a regular arrangement, indicating a normal, non-fibrotic condition (Fig. 1g, h).
The immunostaining of MMP13 using the rabbit anti-MMP13 antibody exhibited enhanced expression of MMP13 in the LF from DM (+) patients compared with DM (−) patients (Fig. 2).
MMP13 expression measured by quantitative real-time PCR
The ratio of MMP13 to β-actin mRNA was 0.46 ± 0.61 (range 0.0009–1.5801) for the DM (+) group and 0.05 ± 0.09 (range 0.000001–0.403321) for the DM (−) group (Fig. 3). The MMP13 mRNA expression was significantly higher in the LF from the DM (+) patients than in that from the DM (−) patients (P = 0.002).
Thickness of the LF
The mean thickness of the LF measured on CT images was 5.0 ± 0.9 mm (range 3.5–6.7 mm) in the DM (+) group and 3.1 ± 0.8 (range 1.9–4.7 mm) in the DM (−) group (Fig. 4). The LF from DM (+) patients was significantly thicker than that from DM (−) patients (P < 0.01). For intra-observer reliability of measurements, the intra-class correlation coefficient was 0.991 (95% confidence interval 0.984–0.996). For the inter-observer reliability, it was 0.970 (0.944–984). Thus, both intra- and inter-observer reliability were acceptably high.
Correlation between MMP13 expression and LF thickness
A positive correlation was observed between the MMP13 expression and thickness of the LF both in DM (+) LSCS patients (correlation coefficient = 0.646, P = 0.032, Pearson’s correlation test) and in DM (−) LSCS patients (correlation coefficient = 0.542, P = 0.006, Pearson’s correlation test). Thus, the correlation coefficient was higher in DM (+) patients than in DM (−) patients.
mmp13 expression in NIH3T3 cells
mmp13 expression in NIH3T3 cells analyzed by real-time PCR was 1.50 ± 0.06 times higher in the cells cultured with glucose than in those cultured without (P < 0.001) (Fig. 5).
Discussion
In this study, we found an increased expression of MMP13 in the LF from DM (+) LSCS patients compared with the LF from DM (−) LSCS patients. In addition, elastin degradation and fibrosis of the LF were more severe in the DM (+) LSCS patients than in the DM (−) patients, and the LF of the DM (+) LSCS patients was significantly thicker. Our results suggest that an increased expression of MMP13, which may be related to DM, can be one of the factors contributing to fibrosis and hypertrophy of the LF, resulting in the progression of stenosis of the lumbar spinal canal.
Previous studies showed that the LF in the lumbar region is rich in elastic fibers, whose principal components are elastin and fibrillin [2]. Fibrillin can be degraded by MMP13, which is an important process in connective tissue remodeling [9]. MMP13 has been described as a trigger for the activation of a positive MMP2 and MMP9 feedback loop in asbestos-induced pulmonary fibrosis in mice [12]. The importance of MMP13 in fibrosis has also been shown in reports on bleomycin-induced pulmonary fibrosis in rats [21] and systemic sclerosis in humans [22]. In vessels and cornea, high plasma glucose increases the expression of MMP13 [14, 15]. In this study, the expression of MMP13 in the LF was higher in DM (+) than in DM (−) LSCS patients, and the expression of MMP13 was correlated with the thickness of the LF. Expression of mmp13 was upregulated by the presence of glucose in mice fibroblastic-like cells. MMP13 degrades both collagen fibers and elastic fibers, and is highly involved in extracellular remodeling [7, 9]. Thus, high plasma glucose levels may increase the expression of MMP13 in the LF and cause fibrosis. However, the exact mechanisms of the upregulation of MMP13 in DM patients should be elucidated by further studies.
In conclusion, we found a higher expression of MMP13 in the LF from DM (+) LSCS patients than in the LF from DM (−) LSCS patients. In addition, the elastin degradation and fibrosis of the LF was more severe in DM (+) patients than in DM (−) patients. These results suggest that the increased expression of MMP13 associated with DM can be one of the factors contributing to LF fibrosis and hypertrophy.
References
Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18:679–86.
Nihei A, Hagiwara K, Kikuchi M, Yashiro T, Hoshino Y. Histological investigation of rabbit ligamentum flavum with special reference to differences in spinal levels. Anat Sci Int. 2003;78:162–7.
Sairyo K, Biyani A, Goel V, Leaman D, Booth R Jr, Thomas J, Gehling D, Vishnubhotla L, Long R, Ebraheim N. Pathomechanism of ligamentum flavum hypertrophy: a multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine. 2005;30:2649–56.
Okuda T, Baba I, Fujimoto Y, Tanaka N, Sumida T, Manabe H, Hayashi Y, Ochi M. The pathology of ligamentum flavum in degenerative lumbar disease. Spine. 2004;29:1689–97.
Schrader PK, Grob D, Rahn BA, Cordey J, Dvorak J. Histology of the ligamentum flavum in patients with degenerative lumbar spinal stenosis. Eur Spine J. 1999;8:323–8.
Postacchini F, Gumina S, Cinotti G, Perugia D, DeMartino C. Ligamenta flava in lumbar disc herniation and spinal stenosis: light and electron microscopic morphology. Spine. 1994;19:917–22.
Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39.
Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4.
Oh IS, Ha KY. Matrix metalloproteinase-3 on ligamentum flavum in degenerative lumbar spondylolisthesis. Spine. 2009;34:E552–7.
Park JB, Lee JK, Park SJ, Riew KD. Hypertrophy of ligamentum flavum in lumbar spinal stenosis associated with increased proteinase inhibitor concentration. J Bone Joint Surg Am. 2005;87:2750–7.
Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodeling. Biochem J. 1999;340(Pt 1):171–81.
Flechsig P, Hartenstein B, Teurich S, Dadrich M, Hauser K, Abdollahi A, Gröne HJ, Angel P, Huber PE. Loss of matrix metalloproteinase-13 attenuates murine radiation-induced pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 2010;77:582–90.
Uchinami H, Seki E, Brenner DA, D’Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44:420–9.
Tan RJ, Fattman CL, Niehouse LM, Tobolewski JM, Hanford LE, Li Q, Monzon FA, Parks WC, Oury TD. Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice. Am J Respir Cell Mol Biol. 2006;35:289–97.
Park JB, Kong CG, Suhl KH, Chang ED, Riew KD. The increased expression of matrix metalloproteinases associated with elastin degradation and fibrosis of the ligamentum flavum in patients with lumbar spinal stenosis. Clin Orthop Sur. 2009;1:81–9.
Sachidanandam K, Hutchinson JR, Elgebaly MM, Mezzetti EM, Dorrance AM, Motamed K, Ergul A. Glycemic control prevents microvascular remodeling and increased tone in type 2 diabetes: link to endothelin-1. Am J Physiol Regul Integr Comp Physiol. 2009;296:R952–9.
Takahashi H, Akiba K, Noguchi T, Ohmura T, Takahashi R, Ezure Y, Ohara K, Zieske JD. Matrix metalloproteinase activity is enhanced during corneal wound repair in high glucose condition. Curr Eye Res. 2000;21:608–15.
Leeson CR, Leeson S, Paparo AA. In: Atlas of histology. 2nd ed. Philadelphia: Saunders; 1985. pp. 249–66.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8.
Fukuyama S, Nakamura T, Ikeda T, Takagi K. The effect of mechanical stress on hypertrophy of the lumbar ligamentum flavum. J Spinal Disord. 1995;8:126–30.
Tian XL, Yao W, Guo ZJ, Gu L, Zhu YJ. Low dose pirfenidone suppresses transforming growth factor beta-1 and tissue inhibitor of metalloproteinase-1, and protects rats from lung fibrosis induced by bleomycina. Chin Med Sci J. 2006;21:145–51.
Asano Y, Ihn H, Kubo M, Jinnin M, Mimura Y, Ashida R, Tamaki K. Clinical significance of serum levels of matrix metalloproteinase-13 in patients with systemic sclerosis. Rheumatology (Oxford). 2006;45:303–7.
Conflict of interest
This study was partly supported by a grant from Ono Pharmaceutical Co., Tokyo, Japan. All authors had no other financial relation regarding the study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cui, G., Watanabe, K., Miyauchi, Y. et al. Matrix metalloproteinase 13 in the ligamentum flavum from lumbar spinal canal stenosis patients with and without diabetes mellitus. J Orthop Sci 16, 785–790 (2011). https://doi.org/10.1007/s00776-011-0135-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-011-0135-2