Abstract
Load and activity changes of the spine typically cause symptoms of nerve root compression in subjects with spinal stenosis. Protrusion of the intervertebral disc has been regarded as the main cause of the compression. The objective was to determine the changes in the size of the lumbar spinal canal and especially those caused by the ligamentum flavum and the disc during loaded MRI. For this purpose an interventional clinical study on consecutive patients was made. The lumbar spines in 24 supine patients were examined with MRI: first without any external load and then with an axial load corresponding to half the body weight. The effect of the load was determined through the cross-sectional areas of the spinal canal and the ligamentum flavum, the thickness of ligamentum flavum, the posterior bulge of the disc and the intervertebral angle. External load decreased the size of the spinal canal. Bulging of the ligamentum flavum contributed to between 50 and 85% of the spinal canal narrowing. It was concluded that the ligamentum flavum, not the disc had a dominating role for the load induced narrowing of the lumbar spinal canal, a finding that can improve the understanding of the patho-physiology in spinal stenosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The characteristic symptoms in spinal stenosis, neurogenic claudication, are believed to be caused by an encroachment of the nerve roots of the cauda equina. The symptoms typically develop or are worsened during walking or in certain postures both assumed to initiate or aggravate the nerve root constriction. Since the diagnostic MRI or CT examinations of the spine, in suspected spinal stenosis, usually are performed in the supine patient, the loading conditions differ from those known to elicit the symptoms in the stenotic spine. To achieve more realistic loading conditions during imaging of the spine special devices have been developed [1, 2, 16, 17]. By applying an axial spinal load corresponding to 50% of the body weight during the MRI or CT examinations a further constriction of the cauda was noted in quite many patients with suspected spinal stenosis [1, 16]. The contribution of different structures, e.g. the disc or the ligamentum flavum to the load induced constriction of the spinal canal and the dural sac was never determined [2, 16, 17].
The degenerated intervertebral disc has usually been regarded as the structure contributing mostly to load induced, dynamic constriction of the cauda and in such away also to the typical load and activity-related symptoms of spinal stenosis [1, 12, 17].
Recent in vitro findings have indicated, however, that ventral bulging of the ligamentum flavum is the most common deformation within the spinal canal creating nerve root compression or causing intrusion in the lumbar as well as the cervical spinal canal [3, 5].
To better understand the patho-physiology in central spinal stenosis the purpose of this study was to quantify in vivo, the contribution of the disc and the ligamentum flavum to the load and position induced narrowing of the lumbar spinal canal during loaded MRI examinations.
Materials and methods
Twenty-four subjects, 9 women with a mean age of 46.0 (SD 9.7) years and 15 men with a mean age of 44.1 (SD 7.7) years were included in the study. All of them were referred to the Department of Radiology Sahlgrenska University Hospital Mölndal due to long-lasting, average 9 (SD 8.3) years, non specific low back pain with or without radiating pain down one or both legs (Table 1). None of the examined patients or levels had any spondylo- or retrolisthesis.
The MRI was performed on a 1.5-T system using a surface coil. All the subjects were first examined in a supine psoas-relaxed position with slight flexion in the hips and knees and without any external loads. In this position, sagittal T1-weighted as well as axial T1- and T2-weighted turbo spin-echo sequences were performed. The slice thickness was 4 mm. The box for transverse slices was placed as parallel as possible to the disc examined.
Fifty disc levels from L2/3 to L4/5 were examined unloaded and loaded in the 24 subjects.
Axial loading of the spine
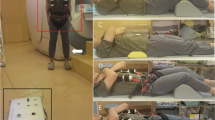
Axial loading of the spine was performed using a non magnetic compression device and a harness (DynaWell, DynaMed AB, Stockholm, Sweden) [17]. When loaded the patient was lying supine with extended hips and knees. To prevent flexion of the spine during compression, a small cushion was placed beneath the lumbar spine. The feet were positioned against a footplate on the compression device. The harness worn by the patient was attached to the compression device using side straps, which were tightened for axial loading of the lumbar spine. The load regulated by tightening or loosening adjustment knobs on the compression device, was registered on special indicators.
The external load was calculated to approximately 50% of the subject’s body weight, with equal load distribution on both legs. The load was applied for at least 5 min, after which axial T1- and T2-weighted sequences were performed.
Measures determined
All the radiological assessments depended on both an experienced spine surgeon (A) and an experienced radiologist (B).
The actual measures were determined using a work station specially designed for such purposes (Fig. 1). On every disc level measurements were performed on transverse sections located between the middle of the disc and the cranial endplate. The same region was used when measures were determined from sagittal sections.
All measures included in the study were determined at three separate occasions. The average of these three measurements was used in the study. The following measures were determined and used in the analyses:
Spinal canal cross-sectional area (SCcsa)
When outlining the area of the canal we started at the medial edge of one of the facet joints. Following the posterior border of the disc, we turned down to reach the opposite side’s facet joint edge. From here we followed the border of the ligamentum flavum back to the starting point.
Ligamentum flavum thickness (LFthi)
A line was drawn along the laminar side of the ligament curve and along the side of the ligament facing the spinal canal. The thickness was determined at the thickest point.
The same outlining was used for the determination of the ligamentum flavum cross-sectional area, LFcsa as for the LFthi (Fig. 2).
A schematic drawing of the measures determined from the transverse projections. 3 is the thickness of the ligamentum flavum, LFthi, 4 is the cross-sectional area of the ligamentum flavum on the right side LFcsa, and 5 is the cross-sectional area of the spinal canal SCcsa. 1 and 2 mark the cross-sectional area of the dural sac and its sagittal diameter but are not included in the analysis but shown to clarify the difference from the SCcsa
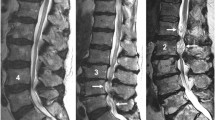
Sagittal disc bulge (Dbul)
The bulge was measured from the sagittal projections and from the line connecting the cranial and caudal posterior edges of the two adjoining vertebral bodies to the most bulging point of disc [6] (Fig. 3).
Intervertebral angle (IA)
The angle between the two lines parallel to the cranial and caudal endplates of the two adjacent vertebral bodies (Fig. 3).
Intra and inter observer variations
To determine the intra and inter observer variations five repeat measurements were performed for all the included measures in five different patients and by the two investigators independently and at separate occasions (Table 2).
Intra observer variation was expressed as a coefficient of variation, CV. It was calculated for each subject as the standard deviation of each observer’s five measurements divided by the mean of the five measurements multiplied by 100 to give a relative value, CV%. Standard deviation, SD was also calculated for each studied subject as the standard deviation of the observer’s five measurements. The mean value of the 24 subjects was used as a summary measure for each variable. The intra class correlation coefficient, ICC was also calculated for each variable. The average inter-observer variation, CV% was 26.5.
Statistics
In the final analysis only measurements from one of the observers (A) were used.
Parametric tests, Student’s t-test and Paired t-test were used for normally distributed variables. When normal distribution not was present or assumed non parametric test, Wilcoxon Signed Rank test was used. Regression analyses were used to determine relations between different parameters. P < 0.05 was regarded as statistically significant difference. The statistical software used was SPSS 15.0 for Windows.
Results
Of the 50 disc levels examined in the 24 subjects unloaded and loaded, respectively, three were at L2/L3, 23 at L3/L4 and 24 at L4/L5.
The lumbar lordosis in the unloaded and loaded spine
When unloaded the lordosis between T12/L1 and L5/S1 ranged from 17° to 65° in the 24 subjects. Loading of the spine increased the lordosis in all the subjects to range from 28° to 81° with an average increase of 12.9° (SE 0.6).
The thickness of the ligamentum flavum with and without external load
The average thickness of the unloaded flavum at its thickest point on the right and left sides was 4.3 (SE 0.3) and 4.9 (SE 0.2) mm, respectively, on the L3/L4 level and 4.8 (SE 0.2) and 5.3 (SE 0.3) mm on the L4/L5 level. When loaded the corresponding mean values were 4.7 (SE 0.3) and 5.2 (SE 0.2) mm on the L3/L4 level and 5.2 (SE 0.3) and 5.9 (SE 0.4) mm on the L4/L5 level. The increase in thickness during loading was statistically significant on both the levels and on both left and right side (Table 3).
The cross-sectional area of ligamentum flavum (LFcsa) with and without external spinal load
The average LFcsa on the right and left sides in the unloaded spine was 64.9 (SE 3.9) and 73.4 (SE 4.5) mm2, respectively, on the L3/L4 level. After loading of the spine those values increased to 73.8 (SE 4.5) and 76.1 (SE 3.2) mm2, respectively. The relative increase in area was almost 14% on the right side but on the left side just above 3% only. The increase in LFcsa during external loading of the spine was statistically significant at both the right and the left sides (P < 0.000).
At the L4/L5 level the average LFcsa on the right and left side in the unloaded spine was 73.9 (SE 4.0) and 80.2 (SE 5.0) mm2, respectively. In the loaded spine the LFcsa on the right side was at an average 86.8 (SE 4.9) mm2 and at the left side it was 81.8 (SE 5.6) mm2. The relative average increase on the right side was 17 and 2% on the left side and the increase at both sides was statistically significant (P < 0.000).
The total average increase in LFcsa including both the right and left sides at the L3/L4 level was 11.6 and 14.4 mm2 at the L4/L5 level (P < 0.000). The differences between LFcsa in the supine, unloaded subjects and when externally loaded can be seen in Table 4.
Relations between the thickness (LFthi) and cross-sectional area of the ligamentum flavum (LFcsa)
Statistically significant correlations (P < 0.01) were found between the average thickness (LFthi) and the cross-sectional area (LFcsa) on both the L3/L4 and L4/L5 levels and that in both the unloaded and loaded spines suggesting a similar behaviour at both the disc levels when loaded. The correlation coefficients ranged from 0.79 to 0.90. No correlations were found, however, between the changes in thickness and changes in CSA between loaded and unloaded conditions. It suggested a non symmetric increase of, e.g. the area of the ligament.
The effect of loading on the posterior sagittal disc bulge
The average posterior disc bulge (Fig. 3) for all the 50 studied levels was 3.1 (SE 0.4) mm in the unloaded supine spine and 3.3 (SE 0.4) mm when loaded with 50% of the body weight. It meant that the load induced bulging or protrusion of the disc was 0.2 mm (P < 0.002) at an average.
The average protrusion at the L3/L4 in the unloaded and the loaded disc was 1.6 SE 0.4 and 1.8 SE 0.5 mm, respectively. The difference was 0.2 (SE 0.1) mm (P < 0.026). At the L4/L5 level the average unloaded disc protrusion was 4.3 (SE 0.4) mm and the protrusion during loading 4.4 (SE 0.5) mm with an average difference between unloaded and loaded of only 0.1 (SE 0.1) mm (P > 0.05).
Load induced changes of the spinal canal’s cross-sectional area (SCcsa)
The average cross-sectional area of the spinal canal, SCcsa (Fig. 1) in the unloaded spines decreased with an average of 23 mm2 at the L3/L4 level when exposed to axial loading. The corresponding decrease at the L4/L5 level was 17 mm2 (Table 5).
Ligamentum flavum’s contribution to the load induced changes in the cross-sectional area, SCcsa of the spinal canal
Loading of spine reduced the available space inside the spinal canal at the L3/L4 level with 23 mm2 and at the L4/L5 level with 17 mm2. The ligamentum flavum’s share of this reduction of the available space inside the canal was 8.9 + 2.7 = 11.6 mm2 for the right and left side at the L3/L4 level. At the L4/L5 level the corresponding figures were 12.9 + 1.6 = 14.5 mm2 (Table 4). Consequently, the yellow ligament was responsible for 50% (11.6/23 × 100) of the reduction of available space within the spinal canal at the L3/L4 level and as much as 85% (14.5/17 × 100) at the L4/L5 level.
Changes of the intervertebral angle without and with external load
When the unloaded spines were loaded the intervertebral angle at the 50 examined levels (Fig. 2) increased with 2.2° (P < 0.001) at an average. At the L3/L4 level the increase was 3.0° (P < 0.000) while it was 1.3° (P < 0.001) at the L4/L5 level.
Intervertebral angle, IA and its relation to LFcsa and LFthi with and without external spinal loading
No correlations were found between the intervertebral angle, IA and the LFcsa and LFthi at the L3/L4 or L4/L5 levels when tested separately. When all 50 levels were tested together statistically significant relationships were found between the IA and LFcsa and LFthi, respectively (P < 0.01).
Discussion
This study confirmed what several other studies already have shown, that the available space within the lumbar spinal canal varies depending on the loading conditions of the spine [2, 12, 17]. By quantifying separately each structure’s contribution to the reduction of the size of the spinal canal in the unloaded and loaded spines (Fig. 1), it was evident that the yellow ligament’s share in that process has been underestimated. The finding that ligamentum flavum contributed to between 50 and 85% of the load induced narrowing of the spinal canal made it the most significant and “dynamic structure” determining most of the load induced changes of the available space within the spinal canal. Consequently, load induced symptoms due to cauda equina encroachment seemed more likely to be caused by bulging of the ligamentum flavum than protrusion of the disc. The load induced bulging of the ligamentum flavum was reflected both by the measures of its thickness and cross-sectional area. The changes were closely related but for the purpose of determining the intrusion of the ligament into the spinal canal only the area measure was appropriate.
So far protrusion of the disc when walking or in certain postures has been regarded as the dynamic factor with the greatest potential to compress the cauda equina and in such a way elicit symptoms of compression, e.g. neurogenic claudication [12].
With the ligamentum flavum not the disc as the structure with greatest dynamic constricting potentials some of the present concepts of spinal stenosis might need a re-evaluation. Since the yellow ligament contributes to as much as 85% of the dynamic size changes of the spinal canal it is easier to understand why laminotomy or laminectomy, including resection of ligamentum flavum, many times is a successful surgical procedure in central spinal stenosis. The promising but preliminary results after so called micro laminectomy with removal of less bone but still all the flavum might also indicate the importance of flavum in at least the moderately stenotic spinal canal.
Typically claudication symptoms are relieved by forward bending or stooping. It seems easier to explain this relief of symptoms as caused by stretching of the ligamentum flavum rather than an increased protrusion of the disc. Recent findings revealing that the disc in the lumbar spine is bulging more in the forward bent spine than in the upright indirectly support an important dynamic role of the flavum [6]. The findings in the current study might also explain the reportedly quite positive effects in spinal stenosis of devices meant to separate the inter spinous processes, i.e. relieving load and flex the intervertebral joint and in such a way elongate an otherwise bulging and encroaching ligamentum flavum [19].
Experimentally, a thinning of the ligament is directly depending on the distraction applied and can depending on the distraction force exceed 2 mm [14].
Ligamentum flavum has some unique properties. It is among others more elastic than any other ligament in the body [15]. In spinal stenosis, however, the ligament degenerates and loses elasticity [8, 18]. This loss of elasticity will increase the bulging of the ligament when the distance between the laminae is narrowed and in such a way reduce the available space in the spinal canal [8].
The results in the present study revealed that loading of the spine increased extension of the entire lumbar spine as well as at the three levels analysed. Extension seemed to have two direct noticeable effects on the size of the lumbar spinal canal. It narrowed the proximal and distal insertions of the flavum thus allowing this elastic ligament to bulge or crumble and it also increased the backward protrusion of the disc. In an already constricted spinal canal extension will worsen the constriction by increasing the bulging of especially the flavum but also the disc.
So far an increased loading of the spine due to walking, extension of the spine or both have been regarded as typical activities triggering the symptoms in spinal stenosis. It is likely that loading and extension are coupled but hard to separate with the present set up.
The validity of the results in this study was supported by both cadaver and open gantry MRI studies. Such studies have shown that extension of the spine from a flexed position and the change of the spinal loads between a supine and a sitting or a standing position (real or simulated) both decrease the size of the spinal canal and increase the thickness of the flavum ligament [9, 12].
In a “normally wide” spinal the decrease of the available space of the magnitudes found in the present study will be of no clinical significance. In an already constricted spinal canal, however, further constriction, e.g. through a bulging ligamentum flavum will start to encroach upon the cauda and eventually generate symptoms of nerve compression, i.e. neurogenic claudication.
Experimental in vitro findings in humans and recent clinical results suggest that clinically significant encroachment in many subjects with central spinal stenosis occurs when the available space for the neural structures (the dura and its content = cross-sectional area of dura) is approaching 75 mm2 [4, 10, 11].
Limitations
By selecting subjects with problems of the lumbar spine but without a specific diagnosis a wide age spectra and a wide range of differently degenerated as well as normal levels were included. There was, however, only one of all the 24 subjects that got a spinal stenosis diagnosis (the cross-sectional area of the dural sac was less than 75 mm2) after the MRI examinations. For this reason it cannot be taken for granted that the ligamentum flavum changes revealed in the study parallel those in subjects with “manifest” spinal stenosis.
The relatively few subjects and levels studied is at least a relative limitation. The loading situation is static although in different positions. Loading of the spine meant not only an increased load but also a somewhat changed lordosis angle of the spine. It could not be determined with the present set up if the loading alone or the loading in combination with the degree of hip flexion caused the increase of the lordosis.
Strengths
The introduction of the area measure made it possible to determine both absolute and relative changes of the different “compartments” influencing upon the size of the spinal canal. The intra and inter personal variations of the measurements were similar to those reported by others [13]. The consistent finding of an increased thickness and area of the yellow ligament in every loaded subject supported the validity of the results.
Conclusions
Ligamentum flavum caused more of the narrowing of the lumbar spinal canal than the disc in axially loaded spines. The bulging ligament represented from 50 to 85% of the reduction in size of the spinal canal when in the supine patient the load was changed to simulate sitting or upright standing. For that reason a load induced bulging of the ligamentum flavum seemed more likely to cause also the classical load and motion-related symptoms due to cauda equina encroachment than a protruding disc.
References
Danielson B, Willen J (2001) Axially loaded magnetic resonance image of the lumbar spine in asymptomatic individuals. Spine 26:2601–2606. doi:10.1097/00007632-200112010-00015
Danielson BI, Willen J, Gaulitz A, Niklason T, Hansson TH (1998) Axial loading of the spine during CT and MR in patients with suspected lumbar spinal stenosis. Acta Radiol 39:604–611
Gu R, Zhu Q, Lin Y, Yang X, Gao Z, Tanaka Y (2006) Dynamic canal encroachment of ligamentum flavum: an in vitro study of cadaveric specimens. J Spinal Disord Tech 19:187–190. doi:10.1097/01.bsd.0000190816.28682.83
Ogikubo O, Forsberg L, Hansson T (2007) The relationship between the cross-sectional area of the cauda equina and the preoperative symptoms in central lumbar spinal stenosis. Spine 32:1423–1428. doi:10.1097/BRS.0b013e318060a5f5 discussion 1429
Okuda T, Fujimoto Y, Tanaka N, Ishida O, Baba I, Ochi M (2005) Morphological changes of the ligamentum flavum as a cause of nerve root compression. Eur Spine J 14:277–286. doi:10.1007/s00586-004-0782-5
Parent EC, Videman T, Battie MC (2006) The effect of lumbar flexion and extension on disc contour abnormality measured quantitatively on magnetic resonance imaging. Spine 31:2836–2842. doi:10.1097/01.brs.0000245834.30646.aa
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26:1873–1878. doi:10.1097/00007632-200109010-00011
Postacchini F, Gumina S, Cinotti G, Perugia D, DeMartino C (1994) Ligamenta flava in lumbar disc herniation and spinal stenosis. Light and electron microscopic morphology. Spine 19:917–922. doi:10.1097/00007632-199404150-00009
Schmid MR, Stucki G, Duewell S, Wildermuth S, Romanowski B, Hodler J (1999) Changes in cross-sectional measurements of the spinal canal and intervertebral foramina as a function of body position: in vivo studies on an open-configuration MR system. AJR Am J Roentgenol 172:1095–1102
Schonstrom N, Bolender NF, Spengler DM, Hansson TH (1984) Pressure changes within the cauda equina following constriction of the dural sac. An in vitro experimental study. Spine 9:604–607. doi:10.1097/00007632-198409000-00011
Schonstrom N, Hansson T (1988) Pressure changes following constriction of the cauda equina. An experimental study in situ. Spine 13:385–388. doi:10.1097/00007632-198804000-00001
Schonstrom N, Lindahl S, Willen J, Hansson T (1989) Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res 7:115–121. doi:10.1002/jor.1100070116
Schulte TL, Heidenreich JO, Schilling AM, Stendel R, Pietila TA, Hopfenmuller W, Brock M, Wolf KJ (2004) Comparison of metric analysis of spinal structures, exemplarily of the ligamentum flavum, obtained with CT and MRI. Eur J Radiol 52:224–228. doi:10.1016/j.ejrad.2003.11.022
Schönström N, Hansson T (1991) The thickness of the ligamentum flavum as a function of load. An invitro experimental study. Clin Biomech (Bristol, Avon) 6:19–24. doi:10.1016/0268-0033(91)90037-Q
Viejo-Fuertes D, Liguoro D, Rivel J, Midy D, Guerin J (1998) Morphologic and histologic study of the ligamentum flavum in the thoraco-lumbar region. Surg Radiol Anat 20:171–176. doi:10.1007/BF01628891
Willen J, Danielson B (2001) The diagnostic effect from axial loading of the lumbar spine during computed tomography and magnetic resonance imaging in patients with degenerative disorders. Spine 26:2607–2614. doi:10.1097/00007632-200112010-00016
Willen J, Danielson B, Gaulitz A, Niklason T, Schonstrom N, Hansson T (1997) Dynamic effects on the lumbar spinal canal: axially loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine 22:2968–2976. doi:10.1097/00007632-199712150-00021
Yoshida M, Shima K, Taniguchi Y, Tamaki T, Tanaka T (1992) Hypertrophied ligamentum flavum in lumbar spinal canal stenosis. Pathogenesis and morphologic and immunohistochemical observation. Spine 17:1353–1360
Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, Johnson DR 2nd, Skidmore GA, Vessa PP, Dwyer JW, Puccio ST, Cauthen JC, Ozuna RM (2005) A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine 30:1351–1358. doi:10.1097/01.brs.0000166618.42749.d1
Acknowledgments
The study was supported by grants from The Swedish Council for Working Life and Social Research, AFA Insurance, Sahlgrenska Academy at Gothenburg University. The study was made possible through the cooperation with the Department of Orthopaedic Surgery, Nagoya City University, Nagoya Japan and its chairman professor Takanobu Otsuka. The study was approved by the Regional Ethical Committee in Gothenburg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hansson, T., Suzuki, N., Hebelka, H. et al. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J 18, 679–686 (2009). https://doi.org/10.1007/s00586-009-0919-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-009-0919-7