Abstract
Physical and chemical hypoxia have been widely used in the study of hypoxic injury; however, both of these hypoxia models have their own limitations. Physical hypoxia is usually difficult to control and maintain. Chemical hypoxia, which is usually induced by chemical hypoxia-mimicking agents, such as CoCl2, may result in heavy metal toxicity or impose security threats. To develop a more suitable hypoxia model, we focused on sodium sulfite (Na2SO3) and evaluated its ability to remove dissolved oxygen in aqueous solutions. Our results showed that sodium sulfite successfully induced hypoxic conditions. The degree of hypoxia and the guarantee period of the sodium sulfite solution could be easily controlled by the concentration of soluble sodium sulfite. In addition, we used sodium sulfite to create a hypoxia model in Caenorhabditis elegans. Similar to physical hypoxia, the sodium sulfite solutions induced hypoxia-related death in the worms and led to morphologic cell defects and C. elegans hypoxia inducible factor 1 stabilization. Taken together, our data show that sodium sulfite is a potential hypoxia inducer that mimics hypoxic stress in C. elegans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxia occurs under a large number of physiological and pathophysiological processes, such as embryonic development, adaptation to high altitudes, wound healing, inflammation, cancer, and ischemic diseases [1]. To study the human diseases associated with hypoxia, a variety of hypoxia models have been developed, such as physical and chemical hypoxia models. Although physical hypoxia models simulate the hypoxic environment and cause hypoxia-related insults to animals and cells, the establishment of these models requires a protocol that is not easy to control. Gas cylinders, which usually contain a desired gas mixture of 95% nitrogen and 5% carbon dioxide, and hypoxia chambers are generally needed to produce and maintain the conditions of physical hypoxia [2]. Oxygen analyzers are also needed to precisely control the oxygen concentration. For the establishment of chemical hypoxia models, hypoxia-mimicking agents, such as cobalt chloride, deferoxamine, and sodium hyposulfite, are usually used [3–5]. Although the mechanism by which cobalt and the iron chelator deferoxamine mimic the hypoxic response is based on competition with iron (a key factor of the oxygen sensing mechanism) [6], cobalt is a heavy metal that leads to tissue and cellular toxicity during exposure [7]. Despite all of these facts, cobalt only mimics some of the hypoxic insults induced by physical hypoxia. Another hypoxia model uses sodium hyposulfite to simulate the hypoxic response by depriving aqueous solutions of oxygen, similar to physical hypoxia [3]. However, sodium hyposulfite spontaneously combusts, and its reaction with water is exothermic and accompanied by the generation of sodium sulfite (SS; Na2SO3) and sulfur dioxide (SO2) [8].
During recent years, we have used both the physical and the chemical hypoxia models in our studies [9] and found that it is important to develop new hypoxia models to illustrate the underlying mechanism of hypoxic insults. Analysis of the literature indicates that SS has been widely used as an oxygen scavenger to remove the last trace of oxygen from boiler feedwater in industrial practices [10]. In addition, SS has also been used as a leading food preservative because of its ability to induce anaerobic conditions that effectively inhibit the growth of aerobic microorganisms [11]. Nair and Elmore [12] showed that SS had a negative effect in mutagenicity studies and was not irritating in clinical tests, although other evidence has indicated that SS directly induces reactive oxygen species production and inflammation [13]. Taken together these findings indicate that, as an oxygen scavenger used in biological research, SS has more advantages than sodium hyposulfite. The reaction of SS with water does not result in spontaneous combustion and does not generate sulfur dioxide. Therefore, we examined the possibility of establishing a hypoxia model using SS in aqueous solutions.
In the present study, Caenorhabditis elegans was used to establish the hypoxia model. In 1963, Brenner [14] introduced C. elegans as a model to study development and neurobiology. Currently, this nematode is widely used to study a variety of biological processes, including apoptosis, cell signaling, cell cycle, hypoxia, gene expression and regulation, metabolism, aging, and sex determination [15, 16]. Here, we examine a series of hypoxia-related factors [including death rate, cellular morphology, and C. elegans hypoxia inducible factor 1 (CeHIF-1) expression] in C. elegans exposed to SS solutions. Our data show that SS is a potential hypoxia inducer that mimics hypoxic stress in C. elegans.
Materials and methods
Measurements of dissolved oxygen in aqueous SS solutions
A film-electrode dissolved oxygen (DO) meter (JPB-607, precision 0.1 mg/L) was used to determine the level of DO in aqueous SS solutions. The DO in M9 buffer (22 mM KH2PO4, 42 mM Na2HPO4, 85 mM NaCl, 1 mM MgSO4) and different concentrations of SS (0.1, 0.2, 0.5, 1.0, 2.0, and 4.0 g/L) dissolved in M9 buffer were monitored at different times at 26 °C under atmospheric pressure.
Additionally, the recovery curve of DO in M9 buffer was monitored after physical hypoxia. At first, the DO in the M9 buffer was excluded by the pure nitrogen at 26 °C. Immediately after that, we used a film-electrode DO meter to monitor the level of DO in the M9 buffer in real time and continuously record the time required for the level of DO to increase by 0.1 mg/L.
C. elegans strains and growth conditions
The wild-type strain of C. elegans N2 Bristol was used in this study [14]. The strain daf-2(e1370), which carries a reduction-of-function mutation in the homolog of the human insulin/insulin-like growth factor receptor [17], was used because it is markedly hypoxia-resistant compared with the wild-type N2 strain. Another strain, daf-16(mu86), which carries a reduction-of-function mutation in an HNF-3/forkhead family member and completely suppresses the hypoxia-resistant phenotype compared with N2, was also used [18]. All three of these strains were kindly provided by the Caenorhabditis Genetics Center (University of Minnesota, USA). To visualize hypoxic injury in neuronal cells, the plasmid Punc-119::EGFP and the marker plasmid Pmyo-2::dsRed were co-injected into the gonads of young adult hermaphrodites of the N2 strain to ultimately create the integrant strain IS2, which expresses enhanced green fluorescent protein (EGFP) in pan-neuronal cells. Worms were fed Escherichia coli OP50 and grown at 20 °C on nematode growth medium (NGM) agar plates [14].
RNA interference experiments
The E. coli feeding RNA interference (RNAi) library was obtained from GeneService (Cambridge, UK). RNAi experiments were performed by the RNAi feeding method as described previously [19]. Eggs of the nematodes were plated onto NGM agar plates (containing 1 mM isopropyl-d-thiogalactoside and 50 μg/mL Carbenicillin) that had been seeded with E. coli HT115 containing an RNAi colony and were maintained on the plates for 72 h at 20 °C. Then, the adult animals could be used for hypoxia analysis. The identity of the RNAi colony was confirmed by sequencing plasmid of DNA isolated from the E. coli feeding RNAi library. Control RNAi was performed by feeding worms with E. coli HT115 that were transformed with an empty vector, L4440.
Incubation with SS
Synchronized populations of L1 worms were obtained by incubating embryos from hypochlorite-treated adults for 16 h in M9 buffer at 20 °C [20]. The worms were then spread on fresh NGM agar plates with E. coli OP50 and incubated for 3 days at 20 °C. To establish the hypoxia model, SS solutions of three different concentrations (0.5, 1.0, and 2.0 g/L) were prepared in M9 buffer. Because SS exposed to air or solution is eventually oxidized to sodium sulfate (Na2SO4), we used sodium sulfate as a control in this analysis using the N2 strain. Synchronized worms (day 3) were washed three times with M9 buffer. The worms were then transferred to 1.5-mL centrifuge tubes in 1 mL of fresh incubation solution and incubated for 10, 12, 14, and 16 h at 26 °C. The worms were further transferred to NGM plates to allow recovery for 24 h at 20 °C.
Physical hypoxia
Synchronized worms (day 3) were washed three times with M9 buffer. Worms in 1 mL of M9 buffer were incubated in the hypoxia chamber (Coy Laboratory Products, USA) at 26 °C. The oxygen concentration in the hypoxia chamber was monitored by an oxygen meter and electrode (Coy Laboratory Products, USA) and kept at less than 0.2% during the experiment.
Death rate of the worms and cellular morphology analysis
After a 24-h recovery period, the death rates of these worms were scored. Worms that lacked pharyngeal pumping or did not respond to striking with a platinum pick were scored as dead [21]. To determine if the hypoxia model was established, we examined the cellular morphology changes of the Bristol N2 strain. We then observed the changes in the axons of the transgenic strain IS2, which expresses EGFP in its neuronal cells, after incubation. About five worms were picked with a platinum pick, mounted on a 3% agar pad (0.5-mm thick) containing 10 mM sodium azide on a glass slide, and covered with a coverslip. We used a microscope (Nikon, Japan) to obtain differential interference contrast images and fluorescence images.
Western blot
After incubation of the SS solution, worms were washed three times with M9 buffer as soon as possible. For immunoblotting analysis, the sedimented worms were suspended in an equal amount (vol/vol) of lysis buffer [160 mM tris(hydroxymethyl)aminomethane–HCl, 4% sodium dodecyl sulfate, 30% glycerol] and lysed by ultrasonication with three 30-s pulses. Then, the homogenates were centrifuged at 12,000 rpm for 30 min, and the supernatants were collected. The protein concentration was determined using a micro bicinchoninic acid protein assay kit with bovine serum albumin as a standard. The samples were boiled with equal amounts (vol/vol) of 2× sample buffer at 96 °C for 4 min. Samples containing equal amounts of protein (50 μg per lane) were loaded onto a 12.5% sodium dodecyl sulfate–polyacrylamide gel, electrophoresed, and subsequently transferred onto a nitrocellulose membrane (Millipore, USA). The membrane was blocked in 5% nonfat milk and probed with antibodies specific for CeHIF-1 (polyclonal 1:4,000, provided by Peter J. Ratcliffe) or glyceraldehyde 3-phosphate dehydrogenase (1:1,000, Sigma, USA). Detection was achieved with horseradish peroxidase conjugated anti-rabbit IgG secondary antibodies (Santa Cruz, USA). The specific reaction was visualized by the chemiluminescent substrate luminol (GE Healthcare, UK) and autoradiography.
Statistical analysis
All data are presented as the mean ± the standard deviation. The results were statistically evaluated for significance using Student’s t test. P < 0.05 was considered a statistically significant difference.
Results and discussion
Oxygen deprivation effect and hypoxia-maintenance ability of SS in solution
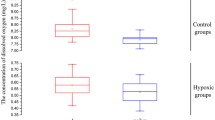
To illustrate the oxygen deprivation effect and hypoxia-maintenance ability of aqueous SS solutions, we examined the concentration of SS-suppressed oxygen dissolved in M9 buffer for 60 h at 26 °C. In distilled water, DO was maintained at 7.35 ± 0.07 mg/L, whereas in M9 buffer, DO was maintained at 7.15 ± 0.06 mg/L (Fig. 1a). As expected, the reduction of the level of DO was largely related to the increased salt concentration, which has been shown to lead to the difference between distilled water and M9 buffer [22]. In SS/M9 buffer, DO could be suppressed to create hypoxic conditions for a period of time. The levels and duration of the hypoxic conditions were dependent on the concentration of SS in the solutions. For example, a solution of 0.2 g/L SS suppressed and maintained the DO at 0.4 mg/L for 10 h, 0.5 g/L SS maintained the DO at 0.1–0.2 mg/L for at least 24 h, 1.0 g/L SS maintained the DO at 0.1 mg/L for at least 40 h, and 2.0 g/L SS maintained the DO at nearly 0 g/L for at least 48 h (Fig. 1a). These data strongly suggest that SS is a potential and valuable chemical agent that can be used to mimic hypoxia in aqueous solutions.
The changing pattern of dissolved oxygen (DO) in aqueous solutions. a Oxygen deprivation effect and hypoxia-maintenance ability of sodium sulfite (SS) in the M9 buffer solutions. b The recovery curve of DO in the M9 buffer after physical hypoxia treatment. All data were measured at 26 °C under atmospheric pressure
According to the recovery curve of DO in M9 buffer after physical hypoxia, the level of DO in M9 buffer needed about 3 h (188.7 min) to increase from 0 mg/L to the normal concentration of 6.9 mg/L (Fig. 1b). Inversely, nearly 40 h was needed in 0.2 g/L SS/M9 buffer to raise the DO level from 0.6 mg/L (20 h) to the normal 6.7 mg/L (60 h) (Fig. 1a), which could be used to explain why SS can maintain the hypoxic condition for a period of time. On the basis of a theoretical analysis, 0.058 g SS was needed to remove DO in 1 L distilled water completely [23]. Actually, a low concentration of SS could not eliminate the total DO in aqueous solutions, because the atmospheric oxygen could gradually redissolve in aqueous solutions. Taking an example of 0.2 g/L SS/M9 buffer (1 L), except for the 0.058 g SS which reacted with DO, the remaining 0.142 g (0.2–0.058) SS was still able to suppress the level of DO to maintain the stabilization of the hypoxic condition for a period of time.
SS-induced hypoxia increases the death rate of C. elegans in a time- and dose-dependent manner
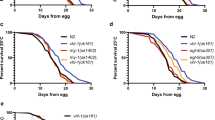
After incubation with the aqueous SS solutions, the death rates of the N2 strain were carefully examined. As expected, the death rate of the N2 strain showed a time- and dose-dependent pattern after incubation with the aqueous SS solutions (Fig. 2a–c). Most importantly, our data clearly show that the sodium sulfate solutions, as a control, had nearly no effect on the death rate of C. elegans (Fig. 2d). Because one molecule of SS can produce the same amount of sodium sulfate, these data strongly suggest that SS has little or even no toxicity compared with its hypoxia-inducing effect on C. elegans. Interestingly, the death rates of C. elegans showed a time- and dose-dependent manner (Fig. 2e), which is similar to that associated with physical hypoxia [21]. To further conform that incubation with the SS solutions could mimic a hypoxic condition, we studied the hypoxia-resistant phenotype of daf-2(e1370) during SS-induced hypoxia. It is reported that daf-2(e1370) is hypoxia-resistant on exposure to physical hypoxia, and daf-2(e1370) requires the daf-16 gene to produce hypoxia resistance [21]. Our results showed that daf-2(e1370) was markedly resistant to SS-induced hypoxia compared with the wild-type N2 strain. To show that daf-2 acts via daf-16 to protect animals during SS-induced hypoxia, we also examined the death rates of daf-2(e1370);daf-16(RNAi), and daf-2(e1370);control(RNAi). Our data showed that daf-16 RNAi suppressed the hypoxia-resistant phenotype of daf-2(e1370) during SS-induced hypoxia. These results were consistent with those of physical hypoxia (Fig. 2e). Therefore, the results further supported our conclusion that SS incubation could mimic hypoxic stress.
Lethal effects of SS, sodium sulfate, and physical hypoxia on Caenorhabditis elegans. Incubation with 0.5 g/L SS (a), 1.0 g/L SS (b), 2.0 g/L SS (c), sodium sulfate (d), and physical hypoxia (e) in M9 buffer. All animals were scored 24 h after recovery from incubation in the SS solutions (a–c). Data are shown as means ± the standard deviation. Asterisk versus wild type, number sign versus daf-2(e1370). Single asterisk or number sign: P < 0.05, double asterisks or number signs: P < 0.01
SS-induced hypoxia causes morphologic cell defects in C. elegans
Morphologic defects of the C. elegans cells are usually observed as a result of hypoxia-induced insults [21]. Worms need continuous food intake, and an abundant supply of oxygen is vital to maintain the activity of their pharyngeal muscle cells; therefore, swollen necrotic pharyngeal cells are observed when oxygen is scarce [24]. According to our data, the death rate of C. elegans treated with SS (2.0 g/L) is consistent with physical hypoxia (0.2% O2) treatment. We further examined the cell morphology of C. elegans treated with SS (2.0 g/L) to investigate whether SS can also cause morphologic changes to C. elegans cells. Compared with the normoxic condition (M9 buffer, 16 h) (Fig. 3a), standard hypoxia-exposed worms contained multiple, strikingly swollen, necrotic-looking pharyngeal cells (Fig. 3b), and SS-exposed (2.0 g/L, 16 h) worms also contained many swollen, necrotic-looking cells, which were seen among multiple cell types and organs, including the pharynx and body wall muscle (Fig. 3c). Additionally, we divided the injury severity of C. elegans into three categories: (1) minor injury (swollen necrotic cells are observed mainly in and around the corpus); (2) moderate injury (swollen necrotic cells also appeared in the terminal bulb in addition to the corpus); and (3) severe injury (both the pharynx and surrounding tissues have a large area swollen necrotic cells). Furthermore, we compared the injury severity of the wild-type N2 strain treated with SS (2.0 g/L) and physical hypoxia (0.2% O2) separately. We randomly counted 57 C. elegans worms suffering from physical hypoxia (0.2% O2) and 52 worms treated with SS (2.0 g/L). Our results clearly showed that the proportion of the three different injury severities caused by the physical hypoxia was 40% (23/57) minor injury, 44% (25/57) moderate injury, and 16% (9/57) severe injury, which was consistent with the proportion for SS-treated worms [40% (21/52), 39% (20/52), and 21% (11/52), respectively]. The χ 2 test demonstrated that there was no significant difference of injury severity of C. elegans between SS (2.0 g/L) and physical hypoxia (0.2% O2) treatment.
SS incubation induced morphologic cell defects. a Normoxia (M9 buffer, 16 h) of the wild-type N2 strain as a control. b Hypoxia-treated (0.2% or less, 16 h) N2 strain with swollen necrotic pharyngeal cells (arrowheads). c SS-incubated (2.0 g/L, 16 h) N2 strain with swollen necrotic pharyngeal cells (arrowheads). d Normoxia (M9 buffer, 16 h) of the wild-type N2 strain with normal morphologic neuronal cells. Hypoxia (0.2% or less, 16 h) and SS incubation (2.0 g/L, 16 h) induced nerve cell defects (arrowheads) in the transgenic strain IS2 (Punc-119::EGFP). Scale bars 50 μm
Hypoxia can also lead to axonal beading in C. elegans [21]. By using the transgenic strain IS2 (unc-119::EGFP), which expresses EGFP in its neuronal cells, we examined the fluorescence stability of EGFP in the IS2 stain with a fluorescence microscope. As expected, the normoxia-exposed (M9 buffer, 16 h) worms showed nearly no morphologic defects in their neuronal cells. Compared with these normoxia-exposed worms, the SS-exposed (2.0 g/L, 16 h) worms showed striking axonal fragmentation in their neuronal cells, completely in accord with physical hypoxic insults (Fig. 3d). These results further indicate that SS-induced hypoxic stress could induce cytomorphological changes that are similar to those resulting from physical hypoxia.
Hypoxia inducible factor 1 is stabilized in C. elegans treated with SS
We then wanted to know whether hypoxia-related genes were affected by SS exposure. To address this issue, we used western blot to determine the expression level of hypoxia inducible factor 1 (HIF-1). HIF-1 is a transcriptional complex that plays a central role in mammalian oxygen homeostasis and was identified by Semenza and Wang [25, 26] in 1992. In addition, Jiang et al. [27] demonstrated the existence of an HIF-1 homolog in C. elegans. Although HIF-1 can be activated by heavy metals, heat stress, and hyperoxia, it is the most critical gene for the hypoxic response [28–30]. The antibody against CeHIF-1 recognizes the two HIF-1 isoforms in C. elegans: HIF-1(F38A6.3a) and HIF-1(F38A6.3c). Our results clearly show that SS incubation induced stabilization of both HIF-1 isoforms in the N2 strain as compared with normoxic subjects (Fig. 4).
Expression of the C. elegans hypoxia inducible factor (CeHIF-1) protein in the wild-type N2 strain of C. elegans treated with different concentrations of SS and incubated for 16 h at 26 °C. Compared with normoxic subjects, aqueous SS solution treatment induced stabilization of both CeHIF-1 isoforms. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control
From the aforementioned results, we infer that the stabilization of HIF-1 resulted from hypoxia, although there was no significantly different expression in HIF-1 upon incubation with different concentrations of SS. According to Epstein et al. [31], the expression of CeHIF-1 is also stabilized similarly and is not dependent on the concentration of oxygen in hypoxic conditions. Thus, our western blot analysis can only show if CeHIF-1 was stabilized and does not reflect the trend of higher or lower expression. Recently, Millonig et al. [32] found that HIF-1 only responds to decrements but not absolute thresholds of oxygen pressure. The levels of HIF-1 were found to be associated with the reduction of oxygen pressure even though hypoxic conditions were not reached. Our results demonstrate that SS can lower the DO concentration of aqueous solutions and activate the HIF-1 regulatory pathway in C. elegans.
In conclusion, our data clearly show that SS is a candidate hypoxia inducer that mimics hypoxic stress in C. elegans and can also be used in the study of hypoxic preconditioning and in the development of antihypoxic agents. Furthermore, we expect that SS treatment can be introduced to cultured cells owing to its simplicity and reliability and can substitute for the currently established models of physical and chemical hypoxia.
Abbreviations
- CeHIF-1:
-
Caenorhabditis elegans hypoxia inducible factor 1
- DO:
-
Dissolved oxygen
- EGFP:
-
Enhanced green fluorescent protein
- HIF-1:
-
Hypoxia inducible factor 1
- NGM:
-
Nematode growth medium
- RNAi:
-
RNA interference
- SS:
-
Sodium sulfite
References
Brown J (2000) Exploiting the hypoxic cancer cell: mechanisms and therapeutic strategies. Mol Med Today 6:157–162
Koch C (1984) A thin-film culturing technique allowing rapid gas–liquid equilibration (6 sec) with no toxicity to mammalian cells. Radiat Res 97:434–442
Abudara V, Jiang R, Eyzaguirre C (2002) Behavior of junction channels between rat glomus cells during normoxia and hypoxia. J Neurophysiol 88:639–649
Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y (1996) Retardation of chemical hypoxia-induced necrotic cell death by Bcl-2 and ICE inhibitors: possible involvement of common mediators in apoptotic and necrotic signal transductions. Oncogene 12:2045–2050
Grasselli F, Basini G, Bussolati S, Bianco F (2005) Cobalt chloride, a hypoxia-mimicking agent, modulates redox status and functional parameters of cultured swine granulosa cells. Reprod Fertil Dev 17:715–720
Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye S, Kuliszewski M, Post M (2000) Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ 3. J Clin Invest 105:577–587
Vengellur A, LaPres J (2004) The role of hypoxia inducible factor 1 alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci 82:638–646
Green L (1976) Sulphur dioxide and food preservation––a review. Food Chem 1:103–124
Hang X, Li P, Li Z, Qu W, Yu Y, Li H, Shen Z, Zheng H, Gao Y, Wu Y (2009) Transcription and splicing regulation in human umbilical vein endothelial cells under hypoxic stress conditions by exon array. BMC Genomics 10:126
Shi Y, Zhan X, Lu T, Li L, Cao C, Shu X, Li C, Li L (2006) Determination of oxidation rate constants of antioxidants sodium sulfite, sodium bisulfite and sodium pyrosulfite. Acta Chim Sin 64:496–500
Schlatter J, Wuergler F, Kraenzlin R, Maier P, Holliger E, Graf U (1992) The potential genotoxicity of sorbates: effects on cell cycle in vitro in V79 cells and somatic mutations in Drosophila. Food Chem Toxicol 31:843–851
Nair B, Elmore A (2003) Cosmetic Ingredients Review Expert Panel. Final report on the safety assessment of sodium sulfite, potassium sulfite, ammonium sulfite, sodium bisulfite, ammonium bisulfite, sodium metabisulfite and potassium metabisulfite. Int J Toxicol 22:63–88
Ratthé C, Pelletier M, Roberge C, Girard D (2002) Activation of human neutrophils by the pollutant sodium sulfite: effect on cytokine production, chemotaxis, and cell surface expression of cell adhesion molecules. Clin Immunol 105:169–175
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Van Voorhies W, Ward S (2000) Broad oxygen tolerance in the nematode Caenorhabditis elegans. J Exp Biol 203:2467
Adachi H, Fujiwara Y, Ishii N (1998) Effects of oxygen on protein carbonyl and aging in Caenorhabditis elegans mutants with long (age-1) and short (mev-1) life spans. J Gerontol Ser A 53:B240
Kimura K, Tissenbaum H, Liu Y, Ruvkun G (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277:942–946
Lin K, Dorman J, Rodan A, Kenyon C (1997) daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278:1319–1322
Simmer F, Moorman C, Van Der Linden A, Kuijk E, Van Den Berghe P, Kamath R, Fraser A, Ahringer J, Plasterk R (2003) Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol 1:E12
Bianchi L, Driscoll M (2006) Heterologous expression of C. elegans ion channels in Xenopus oocytes. WormBook 1:1–16
Scott B, Avidan M, Crowder C (2002) Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 296:2388
Sherwood J, Stagnitti F, Kokkinn M, Williams W (1991) Dissolved oxygen concentrations in hypersaline waters. Limnol Oceanogr 36:235–250
Yan Y (2007) Application of Na2SO3 to chemical deoxidization. Ind Water Treat 27:88–90 (in Chinese)
de Bono M, Maricq A (2005) Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci 28:451–501
Semenza G (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24:97–106
Semenza G, Wang G (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447–5454
Jiang H, Guo R, Powell-Coffman J (2001) The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci USA 98:7916
Liu Z, Velazquez O (2008) Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal 10:1869–1882
Zagórska A, Dulak J (2004) HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol 51:563–586 (English edition)
Wang G, Semenza G (1993) Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood 82:3610–3615
Epstein A, Gleadle J, McNeill L, Hewitson K, O’Rourke J, Mole D, Mukherji M, Metzen E, Wilson M, Dhanda A (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54
Millonig G, Hegedüsch S, Becker L, Seitz H, Schuppan D, Mueller S (2009) Hypoxia-inducible factor 1α under rapid enzymatic hypoxia: cells sense decrements of oxygen but not hypoxia per se. Free Radic Biol Med 46:182–191
Acknowledgments
This work was supported by the National Basic Research Project (973 program) (2006CB504100), the National Key Technologies R&D Program for New Drugs (2009ZX09301-002, 2009ZX09503-002, 2009ZX09103-616), the General Program of the Chinese National Natural Science Foundation (30900862, 30900830, 30800196, 30771230) and the Major Program for Science and Technology Research of Beijing Municipal Bureau (7061004).
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Jiang, C. Ren, and Y. Li are co-first authors.
Rights and permissions
About this article
Cite this article
Jiang, B., Ren, C., Li, Y. et al. Sodium sulfite is a potential hypoxia inducer that mimics hypoxic stress in Caenorhabditis elegans . J Biol Inorg Chem 16, 267–274 (2011). https://doi.org/10.1007/s00775-010-0723-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-010-0723-1