Abstract
Two new copper thiosemicarbazone complexes with an ONNS quadridentate system were synthesized and evaluated for anticancer activity on cisplatin-resistant neuroblastoma cells. Among these two copper complexes, the substituted 8-hydroxyquinoline-2-carboxaldehyde–4,4-dimethyl-3-thiosemicarbazide (CuHQDMTS) exhibited stronger cell growth inhibition activity than the unsubstituted copper 8-hydroxyquinoline-2-carboxaldehyde thiosemicarbazide complex (CuHQTS). Both CuHQTS and CuHQDMTS showed dose-dependent cell growth inhibition, cell cycle arrest and apoptosis induction activities on the SK-N-DZ neuroblastoma cells. Increased expression of p53 protein molecules was detected in the SK-N-DZ cells treated with CuHQTS. The data obtained in this study suggest that CuHQDMTS and CuHQTS hold potential as new, effective drugs for treatment of refractory neuroblastoma in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma (NB) is a malignant tumor originating from the peripheral sympathetic nervous system in children [1]. Prognosis of children diagnosed with advanced NB with high risk factors, such as amplification of N-myc oncogene and deletion of chromosome 1p36, remains poor [2, 3]. Rapid development of acquired resistance to conventional chemotherapeutic drugs, such as cisplatin and carboplatin, is one of the major causes of treatment failure [4, 5]. New drugs are urgently needed for treatment of refractory NB in children [6].
Thiosemicarbazone and their derivatives such as triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) have been extensively studied for their anticancer activities [7–10]. Transition metal complexes of thiosemicarbazone and their derivatives attracted considerable interest for their potent anticancer activities [11–17]. Many efforts were devoted to study of the structure–activity relationship of metal thiosemicarbazone complexes [18–21]. It was reported that anticancer activities of the thiosemicarbazones were closely related to the parent aldehyde or ketone group, metal chelation ability and terminal amino substitution [22–24]. Of them, the parent aldehyde or ketone group was considered critical for the anticancer activity of thiosemicarbazones. Heterocyclic thiosemicarbazones showed higher activity compared with aromatic thiosemicarbazones [18]. On the other hand, metal chelation was also found to have a remarkable effect on the potency of anticancer activity of the thiosemicarbazones. Complexation of thiosemicarbazones with metal ion often led to enhanced anticancer activity by the thiosemicarbazones [21, 22]. Indeed, enhanced anticancer activities were observed following introduction of the tridentate system NNS [15, 17, 25] and ONS [19, 26, 27] into the thiosemicarbazones. The terminal amino substitution of thiosemicarbazones was found to have different effects on anticancer activity through changing the hydrophilic–lipophilic character and stereochemistry of the thiosemicarbazones. Some substituted thiosemicabazone derivatives showed reduced anticancer activity compared with those that were unsubstituted [19], while other substituted derivatives showed enhanced anticancer activity compared with those that were unsubstituted [28]. To enhance anticancer activity of the thiosemicabazone derivatives, it is critical to introduce substitution groups compatible with the whole scaffold of the thiosemicarbazones [18].
Among the various transition metal thiosemicarbazone complexes, anticancer copper thiosemicarbazone complexes and their derivatives are especially attractive because copper ions are physiological trace elements in human bodies. On the other hand, biodistribution and targeted delivery of the anticancer copper complexes in vivo may be monitored noninvasively with positron emission tomography imaging. Accordingly, we synthesized two new copper thiosemicarbazone complexes with a new ONNS quadridentate system. In this new ONNS quadridentate system, an O atom and a N atom are from 8-hydroxylquinoline quadridentate copper ion, together with an azomethine N atom and a S atom from thiosemicarbazone molecule. The new copper thiosemicarbazone complexes were tested for anticancer activity against cisplatin-resistant human NB cells.
Materials and methods
Chemistry
8-Hydroxyquinoline-2-carboxaldehyde, thiosemicarbazide and 4,4-dimethyl-3-thiosemicarbazide were commercially available from Acros, and triethylamine and CuCl2·2H2O were purchased from Sigma-Aldrich and used without further purification. IR spectra were recorded from 4,000 to 400 cm−1 as KBr pellets with a Tensor 27 Fourier transform IR spectrophotometer. UV–vis spectra were recorded from 190 to 900 nm using a Cary 50 spectrometer. Electrospray ionization (ESI) mass spectra were measured in a triple quadrupole Micromass QuattroLC spectrometer with an electroscopy/atmospheric pressure chemical ionization source. Elemental analyses were performed by Midwest Microlab (Indianapolis, IN, USA).
8-Hydroxyquinoline-2-carboxaldehyde thiosemicarbazide
8-Hydroxyquinoline-2-carboxaldehyde thiosemicarbazide (HQTS) was synthesized by condensation of thiosemicarbazide with 8-hydroxyquinoline-2-carboxaldehyde. An ethanolic solution (10 mL) of thiosemicarbazide (1 mmol, 0.091 g) was added dropwise to the solution of 8-hydroxyquinoline-2-carboxaldehyde (1 mmol, 0.173 g) in ethanol (50 mL). The resulting mixture was stirred for 1 h and refluxed for 3 h. The volume of solvent was reduced to 10 mL and the yellow precipitate was collected with filtration, washed with diethyl ether and dried in a vacuum. IR [KBr, ν(cm−1)]: 3,400 (br) (OH), 3,384 (m) and 3,156 (m) (NH2), 3,124 (m) (NH), 1,605 (s) (C=N), 1,538 (s) (CSC), 842 (s) (C=S). UV–vis [λ(nm)/ε(M−1 cm−1), dimethyl sulfoxide (DMSO)]: 271 (10,120), 346 (14,430). ESI–mass spectrometry (MS) (methanol, m/z): 269 (M + Na+). Anal. calcd. (%): C, 53.6; H, 4.1; N, 22.7; S, 13.0. Found (%): C, 53.2; H, 4.1; N, 22.5; S, 12.9.
8-Hydroxyquinoline-2-carboxaldehyde–4,4-dimethyl-3-thiosemicarbazide
8-Hydroxyquinoline-2-carboxaldehyde–4,4-dimethyl-3-thiosemicarbazide (HQDMTS) was prepared by a similar procedure to that for HQTS except thiosemicarbazide was replaced by 4,4-dimethyl-3-thiosemicarbazide (1 mmol, 0.119 g). IR [KBr, ν(cm−1)]: 3,380 (br) (OH), 3,280, 3,187 (m) (NH), 1,618 (s) (C=N), 1,551 (s) (CSC), 815 (s) (C=S). UV–vis [λ(nm)/ε(M−1 cm−1), DMSO]: 289 (10,110), 346 (12,410). ESI–MS (methanol, m/z): 275 (M + H+). Anal. calcd. (%): C, 56.9; H, 5.1; N, 20.4; S, 11.7. Found (%): C, 56.4; H, 5.1; N, 20.1; S, 11.7.
Copper 8-hydroxyquinoline-2-carboxaldehyde thiosemicarbazide
HQTS (0.73 mmol, 0.182 g) and triethylamine (1.46 mmol, 0.20 mL) were mixed in ethanol (100 mL), and a solution of CuCl2·2H2O (0.73 mmol, 0.099 g) in ethanol (10 mL) was added to this mixture under nitrogen. The resulting solution was stirred overnight at room temperature. The volume of solvent was reduced to 10 mL and a brown precipitate was formed. The precipitate was collected with filtration and washed with diethyl ether. Recrystallization with acetone (10 mL) yielded brown microcrystals of copper 8-hydroxyquinoline-2-carboxaldehyde thiosemicarbazide (CuHQTS) which were filtered off and dried in a vacuum. IR [KBr, ν(cm−1)]: 3,376 (m), 3,256 (m) (NH2), 1,562 (s) (C=N), 1,545 (s) (CSC), 838 (s) (C=S), 512 (w) (Cu–O), 442 (w) (Cu–N). UV–vis [λ(nm)/ε(M−1 cm−1), DMSO]: 287 (10,010), 338 (12,410), 408 (5,260), 431 (5,830). ESI–MS (methanol, m/z): 308 (M + H+). Anal. calcd. (%): C, 42.9; H, 2.6; N, 18.2; S, 10.4. Found (%): C, 42.6; H, 2.8; N, 17.9; S, 10.3.
Copper 8-hydroxyquinoline-2-carboxaldehyde–4,4-dimethyl-3-thiosemicarbazide
Copper 8-hydroxyquinoline-2-carboxaldehyde–4,4-dimethyl-3-thiosemicarbazide (CuHQDTMS) was prepared by a similar procedure to that for CuHQTS with replacement of HQTS by HQDMTS (0.73 mmol, 0.200 g). IR [KBr, ν(cm−1)]: 1,589 (s) (C=N), 1,061 (m), 906 (m) (N–N), 808 (m) (C=S), 515 (w) (Cu–O), 435 (w) (Cu–N). UV–vis [λ(nm)/ε(M−1 cm−1), DMSO]: 262 (10,120), 355 (11,520), 410 (5,430), 434 (5,740). ESI–MS (methanol, m/z): 336 (M + H+). Anal. calcd. (%): C, 46.4; H, 3.6; N, 16.6; S, 9.5. Found (%): C, 46.2; H, 3.9; N, 16.5; S, 9.2.
3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide assay
3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate growth inhibition activity of the copper complexes using a MTT assay kit from Chemicon (Temecula, CA, USA), as described previously [29]. Briefly, SK-N-DZ cells, a cisplatin-resistant human NB cell line from ATCC (Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Biosource International, Camarillo, CA, USA) supplemented with fetal bovine serum (10%), penicillin (100 U mL−1), streptomycin (100 mg mL−1) and glutamine (100 mg mL−1), at 37 °C in an atmosphere of 5% CO2. The cells were seeded in a 96-well plate (1 × 104/0.1 mL per well) and were treated with the copper complexes at noted concentration for 12 h. The cells in control groups were treated with HQTS ligand, HQDMTS ligand, or copper chlorides, respectively, at the same concentration. Upon the end of drug treatment, MTT solution was added and the optical density (OD) was measured at 570 nm with a reference wavelength of 630 nm. The results of the MTT assay were recorded as inhibition of cell proliferation (percent) calculated from the formula [(OD of the cells in treatment group/OD of the cells in negative control group) × 100%]. Each experiment was repeated at least three times and each point was determined in triplicate.
Microscopic examination of cytopathological effects
To evaluate cytopathological effects, the SK-N-DZ cells seeded in a 24-well plate were treated with the copper complexes or ligands dissolved in medium containing 0.01% DMSO at noted concentrations for 24 h. The cells of the negative control were treated with medium solvent containing 0.01% DMSO. Upon completion of the treatment, the cells were examined under a microscope and cytopathological effects were recorded using a phase-contrast microscope equipped with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA).
Cell cycle analysis
DNA contents and cell cycle progression for cells were determined by flow cytometry as described previously [15]. The SK-N-DZ cells seeded in a six-well plate (1.6 × 105 cells per well) were treated with the CuHQTS complex, CuHQDMTS complex, HQTS ligand or HQDMTS ligand dissolved in medium containing 0.01% DMSO at noted concentration for 12 or 24 h. The cells of the negative control were treated with solvent medium containing 0.01% DMSO for 12 and 24 h. At the end of the treatment, the cells were harvested and washed with ice-cold phosphate-buffered saline (PBS) and fixed with 70% ethanol. Subsequently, 100 μL of RNase A (1 mg mL−1) and 400 μL of propidium iodide (PI; 50 μg mL−1) were added to the cell pellet, which was resuspended and incubated at 37 °C for 30 min. Analysis was performed with a FACScan flow cytometer (Beckman Coulter, Miami, FL, USA). Ten thousand events were analyzed for each analysis.
Apoptosis induction assay
Proapoptotic activity of the copper complexes was evaluated by annexin V–fluorescein isothiocyanate (FITC)/PI staining and fluorescence activated cell sorter (FACS) analysis as described previously [29]. For flow cytometry analysis, the SK-N-DZ cells seeded in a six-well plate and treated (1.6 × 105 cells per well) were treated with the complexes and ligands at noted concentrations for 12 and 24 h. At the end of the treatment, the cells were harvested, stained with annexin V–FITC/PI and subjected to flow cytometry analyses using a FACScan flow cytometer (Beckman Coulter, Miami, FL, USA). The cells treated with solvent medium containing 0.01% DMSO for 12 and 24 h were used as a negative control. Hoechst 33258 nuclear staining was performed as a confirmatory test of the data from annexin V–FITC/PI flow cytometry analysis as described previously [30]. Briefly, the SK-N-DZ cells (1.6 × 105 cells) were seeded onto a glass plate in a Petri dish, followed by treatment of the cells with the copper complexes at noted concentrations for 12 h. At the end of the incubation, the cells attached to the glass plates were washed twice with ice-cold PBS, and then were fixed in ethanol and acetic acid (3:1) for 10 min. A solution of Hoechst 33258 (20 μg mL−1) was added to stain the cells for 10 min in the dark at 37 °C. Subsequently, the stained cells were covered with a mounting solution (0.1 M citric acid–0.2 M disodium phosphate–glycerol, 1:1:2) and examined for condensed nucleus under a fluorescence microscope, and were recorded with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA).
Immunoblot assay
Immunoblot assay was performed to evaluate the effects of the copper complexes on expression of p53 protein molecules in the SK-N-DZ NB cells in a method modified from that described previously [31]. Briefly, the SK-N-DZ cells (1.6 × 105 cells) seeded in a Petri dish were treated with the copper complexes of noted concentrations for 12 or 24 h. Subsequently, the cells were harvested and the cellular proteins extracted from the cells were quantified with a protein quantification assay kit from Bio-Rad (Hercules, CA, USA). The cellular protein samples were separated by sodium dodecyl sulfate polyacryamide gel electrophoresis and transferred onto a poly(vinylidene fluoride) (PVDF) membrane. After blocking with a tris(hydroxymethyl)aminomethane-buffered saline (TBS) solution containing 5% nonfat milk and 0.1% Tween 20, we reacted the PVDF blots with a monoclonal antibody specific for human p53 protein (1:3,000, R&D Systems, Minneapolis, MN, USA) for 1 h at room temperature. After washing with TBS ×3, we visualized the immunoreactivity of p53 protein on the membrane by chemoluminescence using horseradish peroxidase (HRP) conjugated rabbit-anti-mouse secondary antibody and an enhanced Immun-Star™ HRP chemoluminescence kit from Bio-Rad. Equal loads of the sample were verified by equal immunoreactivity of human β-actin bands on the membrane reacted with anti-human β-actin monoclonal antibody (1:1,000, Novus Biologicals, Littleton, CO, USA).
Statistical analysis
A series of two-factor analysis of variance models were employed to examine differences in mean cell values between study groups by concentration types and between the hour variable category (12 or 24 h). Assumptions of normality and homogeneity of variance were checked and verified. Post hoc pairwise tests were conducted using the Sidak test to control for multiple tests of hypotheses and to balance type I (false-positive) error and power. Statistically significant differences were considered achieved at P ≤ 0.05, two-tailed. All analyses were conducted using SPSS version 15.
Results and discussion
Chemistry
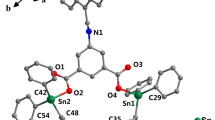
Two new thiosemicarbazone ligands, HQTS (unsubstituted) and HQDMTS (dimethyl-substituted), were synthesized by simple condensation of thiosemicarbazide or 4,4-dimethyl-3-thiosemicarbazide with 8-hydroxyquinoline-2-carboxaldehyde (Fig. 1). Corresponding copper complexes (CuHQTS and CuHQDMTS) were obtained as precipitates through directly reacting copper chloride with HQTS or HQDMTS in ethanolic solution (Fig. 1). Different from most of thiosemicarbazones, the sulfur atoms in these two new thiosemicarbazones were found to be very active and the reaction of these two new ligands with copper ions under regular air would lead to loss of the sulfur atoms in the complex molecules. Copper chelation reaction of these new thiosemicarbazones had to be conducted under an inert gas, a phenomenon reported in the reaction of other thiosemicarbazones with copper ions [27].
These two thiosemicarbazones and their copper complexes have well-defined molecular vibrations in IR region, which is helpful for determining the mode of coordination of the new copper thiosemicarbazone complexes. In the 3,500–3,000-cm−1 region, in addition to the asymmetric and symmetric stretching frequencies of terminal NH2, the IR spectra of HQTS and HQDMTS exhibit an intermolecular hydrogen bond ν(OH) vibration at approximately 3,400 cm−1 [32], which disappeared upon coordination with copper(II) ions, indicating the coordination from hydroxyquinoline oxygen to copper(II) ion, which was further confirmed by the appearance of a band at 500–515 cm−1 due to a ν(Cu–O) stretch in the spectra of CuHQTS and CuHQDMTS [27]. The bands around 1,600–1,620 cm−1 in the spectra of HQTS and HQDMTS were assigned to hydroxyquinoline nitrogen ν(C=N) and azomethine nitrogen ν(C=N), which underwent shifts towards the lower-energy side by approximately 40 cm−1 upon coordinating to copper(II) ions. The bonding of the nitrogen as the coordinating site in CuHQTS and CuHQDMTS was further confirmed with the presence of bands around 440 cm−1 assigned to ν(Cu–N) hydroxyquinoline nitrogen and ν(Cu–N) azomethine nitrogen vibration [33]. Although the Schiff-base HQTS and HQDMTS, in principle, can exhibit thione–thiol tautomerism owing to the presence of a thioamide –NH–C=S functionality (Scheme 1), the absence of the ν(S–H) band near 2,600–2,500 cm−1 and the presence of a ν(N–H) stretching frequency at approximately 3,140 cm−1 indicated that they existed as the thione form [34]. The ν(NH) bands were subsequently found to be absent in the spectra of CuHQTS and CuHQDMTS, indicating the existence of the thiol form of thiosemicarbazone which enabled the sulfur atom to coordinate with the copper ion through the thiolate functionality [35]. The strong bands at 842–815 cm−1 in the spectra of HQTS and HQDMTS assigned to the ν(C=S) stretch were shifted towards lower energy upon copper complexation [36], supporting the sulfur coordination.
The UV–vis spectra of HQTS and HQDMTS showed similar bands in the regions 270–290 and 300–400 nm assigned to (n → π*)quinoline and (n → π*)thiosemicarbazone, respectively [37]. After complexation with copper(II) ions, the bands at 300–400 nm decreased and new bands appeared at 360–495 nm and were attributed to S → Cu(II) and O → Cu(II) charge transfer bands [38, 39].
The results of IR and UV–vis spectra indicated the involvement of Ohydroxyquinoline, Nhydroxyquinoline, Nazomethine and Sthiolate in the coordination of copper(II) ions in CuHQTS and CuHQDMTS. The results of elemental analysis indicated that CuHQTS and CuHQDMTS existed as a 1:1 chelating mode (copper-to-ligand ratio), and no chloride ions or water molecules were appended to the final copper complexes, which was further confirmed by ESI–MS with m/z peaks at 308 (M + H+) for CuHQTS and 336 (M + H+) for CuHQDMTS. The data from IR, UV–vis, ESI–MS and elemental analysis confirmed that the oxygen atom and the nitrogen atom from 8-hydroxylquinoline together with the sulfur atom and the azomethine nitrogen atom from thiosemicarbazone were contributing to the coordination of copper(II) ion in which oxygen and sulfur atoms deprotonized to chelate copper(II) ion, affording an uncharged compound as shown in Fig. 1. The majority of the previously reported anticancer copper thiosemicarbazone complexes have NNS or ONS tridentate systems. The two new thiosemicarbazone ligands we report here have a unique ONNS quadridentate system, which should have enhanced anticancer activity according to their high-affinity binding with copper ions.
Inhibition of cell proliferation and cytotoxicity
Proliferation of the SK-N-DZ NB cells was inhibited by treatment of the cells with CuHQTS and CuHQDMTS in a dose-dependent manner (Fig. 2a). The IC50 values were determined at 0.13 ± 0.03 μM for CuHQDMTS and 0.64 ± 0.03 μM for CuHQTS. Statistical analysis revealed a significant difference of the mean cell growth inhibition activity between CuHQDMTS and CuHQTS by concentration type (P < 0.001). In comparison, free HQTS and HQDMTS ligands showed no significant growth inhibition activities at a concentration as high as 100 μM, which indicated that the chelation of HQDMTS and HQTS ligands with copper ions was essential for anticancer activities of these two new copper complexes. Because proliferation of the SK-N-DZ cells was not inhibited by incubation of the cells with CuCl2 at a concentration up to 100 μM, cell growth inhibition activity by CuHQDMTS and CuHQTS must be derived from biological activities of these two copper complexes, not simply from free copper chaperoned into the cells by thiosemicarbazone. It also was suggested that the terminal amino-substituted CuHQDMTS complexes are more potent than the unsubstituted CuHQTS complexes to inhibit proliferation of the SK-N-DZ cells. In addition to their growth inhibition activities, marked cytopathological effects, e.g., shrinkage, spherical morphology and detachment of the cells, were observed on the SK-N-DZ cells treated with the CuHQTS or CuHQDMTS complexes at a concentration of 0.6 μM (approximately IC50 value of CuHQTS) for 24 h (Fig. 2b). No significant cytopathological effects were observed in the cells treated with free copper(II) ions, HQTS, HQDMTS or solvent medium containing 0.01% DMSO at the same concentration (0.6 μM). These results are consistent with those in most of the previous reports that alkylation on the terminal amino of thiosemicarbazones enhances their anticancer activities [28], but are different from the results in a previous report that substitution on the terminal amino of thiosemicarbazone leads to ineffective or lower antiproliferative activities of unsubsituted thiosemicarbazones [19].
Cell growth inhibition and cytopathological effects of the SK-N-DZ cell treated with CuHQTS or CuHQDMTS. a Proliferation of SK-N-DZ cells was inhibited after treatment of the cells with CuHQTS, CuHQDMTS, HQTS, HQDMTS and cisplatin for 24 h. The results are expressed as the mean ± SD. b Marked cytopathological effects were observed on the SK-N-DZ cells treated with CuHQTS or CuHQDMTS at 0.6 μM for 24 h; no or minimal effects were observed on the cells treated with the same concentrations of HQTS, HQDMTS, CuCl2 or solvent medium containing 0.01% dimethyl sulfoxide (DMSO). Magnification ×100
Cell cycle arrest
Effects of the CuHQTS and CuHQDMTS on cell cycle progression of the SK-N-DZ cells were analyzed by PI staining and FACS analysis. After treatment of the SK-N-DZ cells with CuHQTS or CuHQDMTS at a concentration of 0.1 μM (approximately IC50 value of CuHQDMTS) for 12 h, accumulations of cell population in S phase was observed with simultaneous decrease of cells in G0/G1 phase (Fig. 3). Prolonged incubation of the SK-N-DZ cells with CuHQTS or CuHQDMTS up to 24 h led to accumulation of more cells in S phase and G2/M phase and a dramatic reduction of cell population in G0/G1 phase (Fig. 3). The mean difference of the study groups (CuHQDMTS, CuHQTS, HQDMTS, HQTS and control) was statistically significant at P < 0.001. The S phase and the G2/M phase cell cycle arrest observed in the cells treated with the CuHQTS and the CuHQDMTS complexes suggest that these two new copper complexes inhibit DNA synthesis of the SK-N-DZ cells. It has been reported that thiosemicarbazones and their derivatives inhibited cancer cell proliferation through various mechanisms, such as inhibiting ribonucleotide reductases [40, 41] or the RNA-dependent DNA polymerase [42], inducing oxygen active species [43], or reacting with cell thiols [25]. Mechanisms of S-phase cell cycle arrest by CuHQTS or CuHQDMTS in NB cells remain to be elucidated. Because HQTS and HQDMTS ligands themselves caused minimum cell cycle arrest even at a much higher concentration of 100 μM, binding of the copper ions is essential for enhanced anticancer activities of the copper complexes of these two ligands.
Cell cycle arrest of the SK-N-DZ cells by flow cytometry. Accumulation of the cells at S phase was evident after treatment of the cells with CuHQTS or CuHQDMTS at a concentration of 0.1 μM (approximately IC50 value of CuHQDMTS) for 12 and 24 h, respectively. No cell cycle arrest was observed on the negative control cells treated with solvent (cell culture medium containing 0.01% DMSO). The results are presented as the mean ± SD
Apoptosis induction
The CuHQTS and CuHQDMTS complexes induced apoptosis and necrosis of the SK-N-DZ cells, as shown in Fig. 4a. After treatment of the SK-N-DZ cells with CuHQDMTS at a concentration of 0.1 μM (approximately IC50 value of CuHQDMTS) for 12 h, 7.3% of the cells stained positive for annexin V–FITC and negative for PI (early apoptosis) and 17.1% of the cells stained positive for both annexin V–FITC and PI (late apoptosis or necrosis). Proapoptotic activity of the CuHQTS complex was weaker than that of the CuHQDMTS complex. After treatment of the cells with 0.1 μM CuHQTS, 3.9% cells stained positive for annexin V–FITC and negative for PI (early apoptosis) and 7.8% of the cells stained positive for both annexin V–FITC and PI (late apoptosis or necrosis). After treatment of the cells with the CuHQDMTS complex for a longer time of 24 h, 31.3% of the cells were found to be late apoptotic or necrotic, compared with 18.3% with the CuHQTS complex. In contrast, the HQDMTS and HQTS ligands showed no significant proapoptotic activity on the SK-N-DZ cells. After treatment of the cells with the HQDMTS or HQTS ligands for 12 h, 1.3% of the cells were found to be early apoptotic and 0.20% of the cells were late apoptotic or necrotic. On treatment of the cells with these free ligands for a longer time of 24 h, only 3.3 or 0.84% of the cells were found to be early apoptotic or late apoptotic, respectively. Statistical analysis revealed a significant difference between groups of CuHQDMTS, CuHQTS, HQDMTS and HQTS (P < 0.001).
Apoptosis of the SK-N-DZ cells induced by CuHQTS or CuHQDMTS. a Apoptotic cells stained positive for annexin V–fluorescein isothiocyanate (FITC) (early apoptosis) or positive for both annexin V–FITC and propidium iodide (late apoptosis or necrosis) were detected by flow cytometry analysis after treatment of the cells with CuHQTS or CuHQDMTS at a concentration of 0.1 μM (approximately IC50 value of CuHQDMTS) for 12 or 24 h. Negative control cells were treated with solvent only (cell culture medium containing 0.01% DMSO). b Intense Hoechst 33258 nuclear staining was present in the SK-N-DZ cells treated with CuHQTS or CuHQDMTS at a concentration of 0.1 μM for 12 h
The results of the FACS analysis were further confirmed by strong DNA fluorochrome Hoechst 33258 staining of the condensed, apoptotic nuclei of the SK-N-DZ cells treated with the copper complexes (Fig. 4b). The stronger proapoptotic activities of the CuHQDMTS complexes further support the suggestion that alkylation on the terminal amino of the HQTS ligand enhances anticancer activity of this copper-binding ligand.
Increased expression of p53
As the initial experiment to investigate the molecular mechanism of apoptosis induction by the CuHQTS complexes, immunoblot assays were performed to evaluate the effects of these two new copper complexes on expression of p53 protein molecules in the SK-N-DZ cells. Dramatically increased expression of p53 protein molecules was detected in the cells treated with the CuHQTS complexes at various noted concentrations for 12 h (Fig. 5). In contrast, less and less p53 protein molecules were detected in the cells with increased concentration of the CuHQTS complexes or incubation time prolonged to 24 h (Fig. 5). The results are consistent with previous reports that p53 protein expression was elevated in response to DNA damage [44]. Because the same concentration of free copper(II) ion did not cause increased expression of p53 protein molecules in the SK-N-DZ cells, it is likely that the HQTS ligand plays a role in transporting copper ions to specifically targeted organelles such as the nucleus, in order to cause DNA damage, as suggested from the previous report on apoptosis induction activity of the Schiff-base copper(II) complexes on the NB cells [45]. Fewer or none of p53 protein molecules were detected in the cells treated with higher concentration of the CuHQTS complex, which most likely was related to a decreased number of viable tumor cells.
Elevated expression of p53 protein in the SK-N-DZ neuroblastoma cells treated with CuHQTS. Elevated expression of p53 protein molecules was detected in the SK-N-DZ cells treated with CuHQTS at noted concentrations for 12 h. In contrast, low or no expression of p53 protein molecules was detected in the cells treated with the CuHQTS for 24 h
Conclusions
Two new thiosemicarbazone ligands (HQTS and HQDMTS) were synthesized which chelate copper ions through a unique ONNS quadridentate system, affording uncharged copper compounds (CuHQTS and CuHQDMTS) without the contribution of anions and water. The CuHQTS and CuHQDMTS complexes inhibited proliferation of cisplatin-resistant SK-N-DZ NB cells, and caused S-phase cell cycle arrest, cytopathologic effects and apoptosis of the SK-N-DZ cells. Increased expression of p53 protein molecules was detected in the SK-N-DZ cells treated with the CuHQTS complex. Furthermore, the terminal amino-substituted complex, CuHQDMTS, showed stronger anticancer activity than that of the unsubstituted complex, CuHQTS. In comparison, free HQDMTS and HQTS ligands showed no significant growth inhibition activity on the SK-N-DZ cells. The data from this present study suggest that the CuHQTS and CuHQDMTS complexes merit further investigation as new drugs for treatment of NB refractory to cisplatin chemotherapy.
References
Seeger RC, Reynolds CP (1993) In: Holland JF, Frei E, Bast RC, Kufe DW, Morton DL, Weichselbaum RR (eds) Cancer medicine. Lea & Febiger, Philadelphia, pp 2172–2184
Olshan AF, Bunin GR (2000) In: Brodeur GM, Sawada T, Tsuchida Y, Voute PA (eds) Neuroblastoma. Elsevier, New York, pp 33–37
Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK (2003) J Natl Cancer Inst 95:1276–1299
Hartmann O, Pinckerton CR, Philip T, Zucker JM, Breatnach F (1988) J Clin Oncol 6:44–50
Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, Fojo T (1996) Biochem Pharmacol 52:1855–1865
Niethammer D, Handgretinger R (1995) Eur J Cancer 31:568–571
Finch RA, Liu MC, Cory AH, Cory JG, Sartorelli AC (1999) Adv Enzyme Regul 39:3–12
Rappa G, Lorico A, Liu MC, Kruh GD, Cory AH, Cory JG, Sartorelli AC (1997) Biochem Pharmacol 54:649–655
Liu MC, Lin TS, Cory JG, Cory AH, Sartorelli AC (1996) J Med Chem 39:2586–2593
Cory JG, Cory AH, Rappa G, Lorico A, Liu MC, Lin TS, Sartorelli AC (1994) Biochem Pharmacol 48:335–344
Kolocouris A, Dimas K, Pannecouque C, Witvrouw M, Foscolos G, Stamatiou G, Fytas G, Zoidis G, Kolocouris N, Andrei G, Snoeck R (2002) Bioorg Med Chem 12:723–727
Afrasiabi Z, Sinn E, Padhye S, Dutta S, Newton C, Anson CE, Powell AK (2003) J Inorg Biochem 95:306–314
Belicchi-Ferrari M, Bisceglie F, Casoli C, Duro S, Morgenstern-Badarau I, Pelosi G, Pilotti E, Pinelli S, Tarasconi P (2005) J Med Chem 48:1671–1675
Richardson DR, Sharpe PP, Lovejoy DB, Senaratne D, Kalinowski DS, Islam M, Bernhardt PV (2006) J Med Chem 49:6510–6521
Easmon J, Purstinger G, Heinisch G, Roth T, Fiebig HH, Holzer W, Jager W, Jenny M, Hofmann J (2001) J Med Chem 44:2164–2171
Baldini M, Belicchi-Ferrari M, Bisceglie F, Pelosi G, Pinelli S, Tarasconi P (2004) Inorg Chem 43:7170–7179
Aguirre MC, Borras J, Castineiras A, Garcia-Monteagudo JM, Garcia-Santos I, Niclos J, West DX (2006) Eur J Inorg Chem 6:1231–1244
Hu W, Zhou W, Xia C, Wen X (2006) Bioorg Med Chem Lett 16:2213–2218
Baldini M, Belicchi-Ferrari M, Bisceglie F, Pelosi G, Pinelli S, Tarasconi P (2003) Inorg Chem 42:2049–2055
Padhye S, Kauffman GB (1985) Coord Chem Rev 63:127–160
Saryan LA, Ankel E, Krishnamuri C, Petering DH, Elford H (1979) J Med Chem 22:1218–1221
Scovill JP, Klayman DL, Franchino CF (1982) J Med Chem 25:1261–1264
Brlicchi-Ferrari M, Bisceglie F, Pelosi G, Tarasconi P, Albertini R, Pinelli S (2001) J Inorg Biochem 87:137–147
Miller MC, Stineman CN, Vance JR, West DX, Hall IH (1998) Anticancer Res 18:4131–4139
Garcia-Tojal J, Garcia-Orad A, Diaz AA, Serra JL, Urtiaga MK, Arriortua MI, Rojo T (2001) J Inorg Biochem 84:271–278
Afrasiabi Z, Sinn E, Kulkarni PP, Ambike V, Padhye S, Deobagakar D, Heron M, Gabbutt C, Anson CE, Powell AK (2005) Inorg Chim Acta 358:2023–2030
Chikate RC, Belapure AR, Padhye SB, West DX (2005) Polyhedron 24:889–899
Kowol CR, Berger R, Eichinger R, Roller A, Jakupee MA, Schmidt PP, Arion VB, Keppler BK (2007) J Med Chem 50:1254–1265
Shakya R, Peng F, Liu J, Heeg MJ, Verani CN (2006) Inorg Chem 45:6263–6268
Chen D, Peng F, Cui QZ, Daniel KG, Orlu S, Liu J, Dou QP (2005) Front Biosci 10:2932–2939
Burnette WN (1981) Anal Biochem 112:195–203
Garge PL, Chikate RC, Padhye SB, Savariault JM, Loth P, Tuchagues JP (1990) Inorg Chem 29:3315–3320
Labisbal E, Haslow KD, Pedrares AS, Martines JV, Ortega SH, West DX (2003) Polyhedron 22:2831–2837
Wang M, Wang L, Li YZ, Li QX, Xu ZD, Qu DM (2001) Transition Met Chem 26:307–310
Jouad EM, Riou A, Allain M, Khan MA, Bouct GM (2001) Polyhedron 20:67–74
Ferrari MB, Bonardi A, Fava GG, Pelizzi C, Tarasconi P (1994) Inorg Chem Acta 223:77–86
West DX, Lockwood MA, Albert JN, Liberta AE (1991) Spectrochim Acta A 49:1809–1816
West DX, Salberg MM, Bain GA, Liberta AE, Valdesmartinez J, Fermandez-Ortega SJ (1996) Transition Met Chem 21:206–212
West DX, Yang Y, Klein TL, Goldberg KI, Liberta AE, Valdes-Martinez J, Toscano RA (1995) Polyhedron 14:1681–1693
Moore EC, Zedeck MS, Agrawal KC, Sartorelli AC (1970) Biochemistry 9:4492–4498
Thelander L, Graslund A (1983) J Biol Chem 258:4063–4066
Kaska W, Carrano C, Michalowski J, Jackson J, Levinson W (1978) Bioinorg Chem 8:225–236
Bymes RW, Mohan M, Antholine WE, Xu RX, Petering DH (1990) Biochemistry 29:7046–7053
Bennet MR (1999) Biochem Pharmcol 58:1089–1095
Filomeni G, Cerchiaro G, Ferreira C, Martino A, Pedersen JZ, Rotilio G, Ciriolo MR (2007) J Biol Chem 282:12010–12021
Acknowledgements
We thank Claudio Verani for help in chemical characterization of the complexes and Jiu-sheng Wu for technical assistance. This project was partly funded by a faculty research development award from the Carman and Ann Adams Foundation and a new investigator award from Fighting Children’s Cancer Foundation to Fangyu Peng.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Thomas, R., Oupicky, D. et al. Synthesis and characterization of new copper thiosemicarbazone complexes with an ONNS quadridentate system: cell growth inhibition, S-phase cell cycle arrest and proapoptotic activities on cisplatin-resistant neuroblastoma cells. J Biol Inorg Chem 13, 47–55 (2008). https://doi.org/10.1007/s00775-007-0299-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-007-0299-6