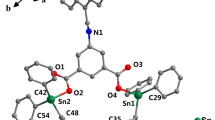
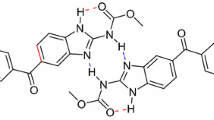
A new quinoline–thiosemicarbazone derivative, 5,7-dichloro-8-hydroxyquinoline-2-carboxaldehyde-4-phenyl-3-thiosemicarbazone (QPS) and seven complexes of QPS with d-transition metals (Mn, Fe, Co, Ni, Cu, Zn, Cd) were successfully synthesized. Spectrometry methods such as IR, ESI-MS, and 1H NMR were utilized to determine the structure of complexes that almost all complexes form the metal:ligand ratio of 1:1, except for FeQPS, which forms a 1:2 ratio. The center metals bond to QPS through O, Nquinoline and Nthiosemicarbazone. Four tested complexes (MnQPS, CoQPS, NiQPS, CuQPS) show medium inhibition to KB and Hep-G2 cancer cell lines. ZnQPS has the high activity against KB and Hep-G2 (IC50 = 13.51 and 10.28 μg/mL) and a high impact against the LU cancer cell line (IC50 = 2.0 μg/mL). CdQPS complexes showed excellent activity on both KB and Hep-G2 with IC50 very low, IC50 = 3.12 and 3.06 μg/mL, which is much lower than cisplatin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. INTRODUCTION
Nowadays, cancer is one of the most frequent causes of death in many countries of the world. The complexes of platinum(II), including cisplatin, carboplatin, oxaliplatin, nedaplatin, and lobaplatin, are used as anticancer drugs in chemotherapy regimens [1]. However, more and more types of cancer are discovered, and patients can suffer numerous side effects including anaphylaxis, cytopenia, hepatotoxicity, ototoxicity, cardiotoxicity, nausea or vomiting, diarrhea, mucositis, stomatitis, pain, alopecia, anorexia, cachexia, asthenia, and other dose-limiting side effects while using these drugs [2]. Finding new platinum complexes that have high antiproliferative activity and low toxicity is necessary [3]. In addition, the synthesis of other metal complexes with high antiproliferative activity and low costs, such as complexes of Co(II), Cu(II), Zn(II), etc., also requires attention [4].
In some research, the complexes of 8-hydroxylquinoline (8-HOQ) with metal ions such as platinum(II) show the excellent inhibition to cancer cells [5, 6]. Owing to the high inhibitory effect on cancer cells, the complexes of transition metals, namely Cu(II), Co(II), Zn(II), or Ni(II), with 8-HOQ derivatives, are attracting the attention of scientists [7, 8]. Furthermore, the cytotoxicity of some complexes is even higher than that of cisplatin on several lines. For example, some complexes of Co(II) containing 5,7-dihalo-8-quinolinol and 2,2′-bipyridine mixed ligand showed high antiproliferative activity on a HeLa cancer cell line, with IC50 = 0.8 μM ÷ 11.88 μM [9]. In addition, the complexes of these metal ions with thiosemicarbazone showed the abundance of antiproliferative activity, even a two-fold increase in correlation with the standard drug [10]. Recently, the complexes with novel tetradentate or tridentate ligands containing both quinoline and thiosemicarbazone components have attracted more scientists to research, which illustrates the effective impact in treating cancer, when the substituents are varied, the effectiveness of complexes could change considerably [11,12,13]. Some of them, Cu(II) complexes with tridentate ligand, which coordinate with the central metals through O, N, and S atoms, exhibited high selectivity toward cancerous cell lines with respect to normal cell lines [14].
In this work, we present the synthesis, structure, and antiproliferative activities of complexes of transition metals (Mn, Fe, Co, Ni, Cu, Zn, Cd) with 5,7-dichloro-8-hydroxyquinoline-2-carboxaldehyde-4-phenyl-3-thiosemicarbazone.
2. EXPERIMENTAL
2.1. Materials and Methods
Selene dioxide, 5,7-dichloro-8-hydroxy-2-methylquinoline, 4-phenyl-3-thiosemicarbazide were purchased from Sigma–Aldrich Chemicals; Ni(OAc)2∙4H2O,Zn(OAc)2∙2H2O, Cu(OAc)2∙H2O, Co(OAc)2∙4H2O, Mn(OAc)2∙2H2O, Cd(OAc)2∙2H2O, and FeCl3∙6H2O and common solvents: methanol, ethanol, acetone, 1,4-dioxane, CH2Cl2, CHCl3 were manufactured in China. All commercially available reagents and chemicals were of analytical grade purity and used without purification.
The IR spectrum was carried out by an IMPACK-410 NICOLET IR spectrometer using KBr pellets. NMR spectra were recorded by a Bruker XL-500 MHz spectrometer in DMSO. Mass spectra (ESI-MS) were measured by an LC-MSD-Trap-SL spectrometer in DMSO or CHCl3.
2.2. Chemistry
Procedure for the synthesis of the ligand. The synthesis of the ligand, 5,7-dichloro-8-hydroxyquinoline-2-carboxaldehyde from 5,7-dichloro-2-methylquinolin-8-ol, is described in Scheme 1.

Scheme 1
In the first step, 4.56 g 2-methyl-8-hydroxyquinoline (0.02 mol) and 2.55 g SeO2 (0.023 mol) were added to a flask containing 40 mL dioxane. The reaction was performed in argon flow at 80°C to remove the remaining air and maintain the inert condition. After 24 h, the mixture was filtered and washed by dichloromethane. The obtained solid was purified by column chromatography to gain the yellow crystal of ClQCHO. The yield of this process is approximately 65% [15].
In a second step, 5,7-dichloro-8-hydroxyquinoline-2-carboxaldehyde-4-phenyl-3-thiosemicarbazone (QPS) from the condensation reaction between 5,7-dichloro-8-hydroxyquinoline-2-carboxaldehyde (ClQCHO) and 4-phenyl-3-thiosemicarbazide (PTS). 0.145 g of ClQCHO (0.6 mmol) and 0,1 g PTS (0.6 mmol) were dissolved in 10 mL ethanol solution and stirred, the solution was refluxed in 5 h and at 75°C. The precipitate was separated and washed by hot ethanol; the obtained yellow solid was QPS. The yield of the process was about 90%. IR spectrum, vmax, cm-1: 3309, 3138 (O-H, N-H); 1594, 1547 (C=C, C=N); 1191 (C=S); 749 (C-S); 1327, 1254 (C-O, C-N).
1H NMR spectrum (DMSO-d6, δ, ppm): 12.26 (s,1H, NH); 10.8 (s, 1H, OH); 10.44 (s,1H, NH); 8.77 (d, J 9.0 Hz, 1H, ArH); 8.44 (d, J 9.0 Hz, 1H, ArH); 8.41 (s, 1H, CH=N); 7.82 (s, 1H, ArH); 7.56 (d, J 7.5 Hz, 2H, ArH); 7.41 (t, J 7.5 Hz, 2H, ArH); 7.26 (t, J 7.5 Hz, 1H, ArH).
General procedure for the synthesis of complexes. The synthesis of complexes was shown in Scheme 2. 78.2 mg QPS (0.2 mmol) was dissolved in 20 mL ethanol, and then 60 μL triethylamine (0.4 mmol) was dropped in to form an orange suspended solution. To the mixture was added 0.22 mmol salt as M(OAc)2∙nH2O (M: Mn, Co, Ni, Cu, Zn, Cd) or FeCl3. 6H2O dissolved in 10 mL ethanol, the mixture changed color, and then the precipitate appeared. After stirring for 12 h, the precipitate was washed by ethanol. The obtained solid dissolved in DMSO, and was insoluble in acetone, ethanol, methanol, and water. The yields were about 70 – 80%.

Scheme 2
For MnQPS. Red-brown solid.
IR spectrum, vmax, cm-1: 3659, 3332 (O-H, N-H); 1562, 1512, 1496 (C=C, C=N); 1338, 1312 (C-O, C-N); 1119 (C=S); 743 (C-S); 649, 592 (M-S, M-O, M-N).
Mass spectrum, m/z (%): +MS, 444 (100%), [Mn(QPS-2H)+ H]+; –MS, 480 (100%), [Mn(QPS-2H)+Cl]–.
For FeQPS. Red–orange solid.
IR spectrum, vmax, cm-1: 3197 (O-H, N-H); 1551 (C=C, C=N); 1345 (C-O, C-N); 1066 (C=S); 750 (C-S); 691, 497 (M-S, M-O, M-N).
Mass spectrum, m/z (%): +MS, 832 (100%), [Fe(QPS-2H) (QPS-1H) + H]+.
For CoQPS. Violet–black solid.
IR spectrum, vmax, cm-1: 3650 (O-H, N-H); 1550, 1497 (C=C, C=N); 1348 (C-O, C-N); 1135 (C=S); 748 (C-S); 690, 661 (M-S, M-O, M-N).
Mass spectrum (ESI MS), m/z (%): +MS, 449 (100%), [Co(QPS-2H) + H]+; –MS, 482 (100%), [Co(QPS-2H)+Cl]–.
For NiQPS. Brown–red solid.
IR spectrum, vmax, cm-1: 3232, 3186 (O-H, N-H); 1559, 1496 (C=C, C=N); 1338 (C-O, C-N); 1134 (C=S); 749 (C-S); 675, 582 (M-S, M-O, M-N).
Mass spectrum (ESI MS), m/z (%): +MS, 449 (100%), [Ni(QPS-2H)+ H]+; –MS, 447 (100%), [Ni(QPS-2H)-H]–.
For CuQPS. Brown–black solid.
IR spectrum, vmax, cm-1: 3229, 3194 (O-H, N-H); 1559, 1555, 1496 (C=C, C=N); 1340 (C-O, C-N); 1136 (C=S); 747 (C-S); 663, 583 (M-S, M-O, M-N).
Mass spectrum (ESI MS), m/z (%): +MS, 454 (80%), [Cu(QPS-2H) +H]+; –MS, 450 (50%), [Cu(QPS-2H) -H]–.
For ZnQPS. Orange–red solid.
IR spectrum, vmax, cm-1: 3332 (O-H, N-H); 1564, 1496 (C=C, C=N); 1346; 1238 (C-O, C-N); 1133 (C=S); 732 (C-S); 690, 548 (M-S, M-O, M-N).
1H NMR spectrum (DMSO-d6, δ, ppm): 10.00 (s, 1H, NH); 8.57 (d, J 8.5 Hz, 1H, ArH); 8.46 (s, 1H, CH=N); 7.89 (d, J 8.5 Hz, 1H, ArH); 7.79 (d, J 7.5 Hz, 2H, ArH); 7.61 (s, 1H, ArH); 7.30 (d, J 7.5 Hz, 2H, ArH); 7.02 (t, J 7.5 Hz, 1H, ArH).
Mass spectrum (ESI MS), m/z (%): +MS, 455 (100%), [Zn(QPS-2H) + H]+; –MS, 485 (100%), [Zn(QPS-2H)+Cl]–.
For CdQPS. Brown–red solid.
IR spectrum, vmax, cm-1: 3500 – 3100 (O-H, N-H); 1560, 1522, 1495 (C=C, C=N); 1357, 1248 (C-O, C-N); 1119 (C=S); 739 (C-S); 687; 552 (M-S, M-O, M-N).
1H NMR spectrum (DMSO-d6, δ, ppm): 9.98 (s, 1H, NH); 9.18 (s, 1H, CH=N); 9.16 (d, J 8.0 Hz, 1H, ArH); 8.55 (d, J 8.0 Hz, 1H, ArH); 8.44 (d, J 8.0 Hz, 1H, ArH); 8.29 (s, 1H, ArH); 8.04 (d, J 8.0 Hz, 1H, ArH); 7.74 (t, J 7.5 Hz, 2H, ArH).
Mass spectrum (ESI MS), m/z (%): +MS, 503 (100%), [Cd(QPS-2H)+ H]+; -MS, 537 (100%), [Cd(QPS-2H)+Cl]–.
2.3. Biology
Cell lines and culture. Cell lines are originally from the American Type Culture Collection (ATCC), include KB (CCL –17TM), Hep-G2 (HB –8065TM), LU (HTB –57TM) and MCF-7 (HTB –22TM). Cells were cultured in Dulbecco’s Modified Eagle Medium or Minimum Essential Medium with Eagle salt supplemented with 7 – 10% Fetal Bovine Serum and other necessary agents. The cells were incubated under standard conditions (% CO2, humidity 98%, temperature 37°C, absolutely sterile) before the toxicity test.
Antiproliferative activity. The colorimetric MTT [3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H tetrazolium bromide] assay was used to assess the antiproliferative activities [16, 17]. Testing samples were dissolved in DMSO and diluted to a serial concentration by adding culture medium and then adding them to 96-well microtiter plates. Trypsinization was used to separate the cells in a counting chamber. The cells were adjusted to about 1 – 3 × 104 cell/mL depending on the cell lines. To each well were added 10 μL of samples and 190 μL of cell solution. After 72 h of being incubated under standard conditions, samples were further incubated with 10 μL MTT (5 mg/mL) for 4 h, and then the culture medium was removed. The formed formazan crystal was dissolved in 100 μL DMSO 100%. The results were determined by OD value with excited wavelength at 540 nm in a BioTek spectrophotometer. The inhibition ratio was achieved from the calculation of three replicated tests.
3. RESULTS AND DISCUSSION
3.1. Chemistry
The composition and structure of new compounds were determined by several spectroscopies: Electrospray Ionization Mass Spectroscopy (ESI-MS), Infrared Spectroscopy (IR spectrum), Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR spectrum).
Infrared Spectrometry (IR spectrum). In the IR spectra of ligand and complexes, the absorption bands of all functional groups were observed and presented in Fig. 1. In the IR spectra of complexes, all signals of specific groups appeared with considerable change compared with the ligand. In the band at 3600–3400 cm-1, the absorption line of O-H vibration disappeared, which proves the deprotonization. In some cases, the absorption signal of N-H is covered by a broad signal of water in humid compounds.
The ligand spectrum displayed the strong absorption of double bond C=C in aromatic rings, C=N in quinoline scaffold in 1594 – 1547 cm-1 bands. This band decreased to 1560 – 1550 cm-1 and 1520 – 1496 cm-1 in almost all complexes, that is, evidence for the formation of the bonding between N, O in the quinoline ring with metal ions. The vibrational frequence of the C=S bond declines from 1190 cm-1 to 1130 – 1120 cm-1 in complexes and the appearance of peaks in 750 – 690 cm-1 area confirmed that the bonding between metal ions with S was formed. In conclusion, the IR spectra data show that the complexes were formed between metal ions with QPS.
Electrospray Ionization Mass Spectrometry (ESI-MS). In ESI-MS of complexes, the molecular peaks were assigned. Most of the molecular peaks reached the intensity at 100%, which was equivalent to [M+H]+ pieces in positive mode. Except for the signal of [M-H]– fragments in the case of NiQPS and CuQPS, [M+Cl]– ions, which are usually formed in the injection system as chloride-attachment ions, displayed a peak with intensity at 100% in negative mode. Although FeQPS was the only complex with a ratio between metal and ligand of 1:2, other complexes showed a ratio of 1:1.
Proton Nuclear Resonance Magnetic Spectrometry (1H NMR spectrum). The 1H NMR spectra are applied for diamagnetic compounds, so that in this work, only ligand, and ZnQPS and CdQPS used the 1H NMR method to support the structures. All proton signals are assigned and the spectra of ligand and Cd(II) complexes are displayed in Figure 2.
In 1H NMR spectra, all protons of the ligand are displayed as resonance signals similar to the predicted structure. The signal at 7.26 – 7.56 ppm was assigned as the proton of benzene rings and the single peak at 8.41, 10.44, and 12.36 ppm were equivalent to the proton directly connecting with the nitrogen atom and unsaturated carbon out of the aromatic rings. The result showed that thiosemicarbazide and quinoline rings were successfully condensed to form QPS.
With regard to the spectra of complexes, almost all protons show the signal as the ligand. The disappearance of OH phenol and NH (H13) signals in complex spectra was evidence for the deprotonation and formation of coordinated bonds through O and N atoms. The chemical shift of H11 increased, especially in CdQPS, owing to the center-metal coordinate to N imine that decreased the electron density in nitrogen and the chemical shift of proton H11 moved to the downfield area. Besides, the change of chemical shift to the upfield area of H15 showed that the resonance effect of nitrogen and aromatic rings decreased because of the chelation in complexes.
In the 1H NMR spectrum of NiQPS, the signal of all protons appeared similar in the ligand and changed slightly, but most of the signals were broadened. The reason could be the coordination of the solvent, DMSO, which changes the complex of Ni(II) from a diamagnetic square planar complex to the paramagnetic octahedral complex.
The combined results of ESI-MS spectra showed that except for the Fe(III) complex formed in the ratio of 1:2; in other complexes, the ratio between metal and ligand was 1:1. The data from IR and 1H NMR spectra confirmed the formation of a coordinated bond between the metal ions and ligand via N and O atoms of the quinoline ring and N, S atoms in the thiosemicarbazone part.
3.2. Biology
The bioactivity test results are shown in Table 1. The results showed that the antiproliferative activity depended on both center ions and tested cancer cells. In KB and Hep-G2 cancer cells, the complexes had medium effects. Complex CoQPS and MnQPS exhibited the lowest effects on two types of cancer cells with IC 50 values over 50 μg/mL and the two complexes affected the KB cell line slightly better than Hep-G2.
The NiQPS complex inhibited both KB and Hep-G2 cell lines, but the effect was better to KB (IC50 = 29 μg/mL); this value to Hep-G2 was 62.32 μg/mL. On the contrary, the complex of Cu(II) showed the better inhibition to Hep-G2 cell line (IC50 = 17.20 μg/mL), about two-fold lower than the IC50 value to KB.
ZnQPS had high activity against KB and Hep-G2 (IC50 = 13.51 and 10.28 μg/mL) and an excellent impact against the LU cancer cell line (IC50 = 2.0 μg/mL), which was much lower than that of cisplatin and some other platinum(II) complexes [6]. CdQPS complexes showed the best activity on both KB and Hep-G2 with IC50 very low, IC50 = 3.12, and 3.06μg/mL.
4. CONCLUSION
In conclusion, a new quinoline–thiosemicarbazone derivative, 5,7-dichloro-8-hydroxyquinoline-2-carboxaldehyde-4-phenyl-3-thiosemicarbazone (QPS) and seven complexes of QPS with d-transition metal (Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Cd(II)) were successfully synthesized. The spectrometry methods such as IR, ESI-MS and 1H NMR were utilized to determine the structure of complexes that almost formed the metal:ligand ratio of 1:1, except for FeQPS with a 1:2 ratio. The ligand coordinated with center metals through O, N in the quinoline ring and S, N of the thiosemicarbazone part.
The in vitro cytotoxicity of the complexes (MnQPS, CoQPS) showed the medium inhibition against KB and Hep-G2 cancer cells with IC50 = 51 ÷ 72 μg/mL. The NiQPS complex inhibited KB better with an IC50 value of about 29 μg/mL, whereas CuQPS showed a good effect on Hep-G2 (IC50 = 17.20 μg/mL). In comparison, the ZnQPS complex exhibited high activity on KB and Hep-G2 (IC50 = 13.51 and 10.28 μg/mL) and the highest anticancer activity with the LU cancer cell line (IC50 = 2.0 μg/mL). CdQPS complexes showed excellent activity on both KB and Hep-G2 with very low IC50, IC50 = 3.12 and 3.06 μg/mL. This value is much lower than that of cisplatin, a cancer treatment drug that has been used.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
V. Cepeda, M. A. Fuertes, J. Castilla, et al., Anti-Canc. Agents Med. Chem., 7(1), 3 – 18 (2007).
R. Oun, Y. E. Moussa and N. J. Wheate, Dalton Trans., 47, 6645 – 6653 (2018).
T. C. Johnstone, K. Suntharalingam, S. J. Lippard., Chem. Rev., 116(5), 3436 – 3486 (2016).
C. Molinaro, A. Martoriati, L. Pelinski, and K. Cailliau, Cancers, 12(10), 2863 (2020)
L. T. H. Hai, N. T. N. Vinh, L. T. Tuyen, et al., J. Coord. Chem., 72(10), 1637 – 1651 (2019).
N. T. Chi, T. T. C. Mai, P. V. Thong, et al., Acta Cryst., Sec. C, 73(11), 1030 – 1037 (2017).
H. R. Zhang, Y. C. Liu, Z. F. Chen, et al., Russ. J. Coord. Chem., 44(5), 322 – 333, (2018).
Q. Y. Yang, Q. Q. Cao, Q. P. Qin, et al., Int. J. Mol. Sci., 19(7), 1874 (2018).
T. Meng, Q. P. Qin, H. H. Zou, et al., ACS Med. Chem. Lett., 10, 1603 – 1608 (2019).
K. Avinash, K. Vinaya, N. Krishna, et al., Bio-org. Chem., 112, 104962 (2021).
C. Santini, M. Pellei, V. Gandin, et al., Chem. Rev., 114(1), 815 – 862 (2014).
D. Rogolino, A. Cavazzoni, A. Gatti, et al., Eur. J. Med. Chem., 128, 140 – 153 (2017).
F. Bisceglie, A. Musiari, S. Pinelli, R. Alinovi, et al., J. Inorg. Biochem., 152, 10 – 19 (2015).
E. Ramachandran, V. Gandin, R. Bertani, et al., Molecules, 25, 1868 (2020).
S. H. Chan, C. H. Chui, S. W. Chan, ACS Med. Chem. Lett., 4(2), 170 – 174 (2013).
M. Tim, M., J. Imm. Meth., 65, 55 – 63 (1983).
D. A. Scudiero, R. H. Shoemaker, K. D. Paull, et al., Canc. Res., 48, 4827 – 4833 (1988).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Linh, P.T.H., Chi, M.P., Thao, L.P. et al. Synthesis, Structure, and Antiproliferative Activities of Complexes of Some Transition Metals with 5,7-Dichloro-8-Hydroxyquinoline-2-Carboxaldehyde-4-Phenyl-3-Thiosemicarbazone. Pharm Chem J 57, 1593–1598 (2024). https://doi.org/10.1007/s11094-024-03053-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03053-w