Abstract
The in vivo effects of gavage administration of the synthetic, functional biomimetic cation [Cr3O(O2CCH2CH3)6(H2O)3]+ to healthy and type 2 diabetic model rats are described. After 24 weeks of treatment (0–1,000 μg Cr/kg body mass) of healthy Sprague Dawley rats, the cation results in a lowering (P<0.05) of fasting blood plasma low-density lipoprotein (LDL) cholesterol, total cholesterol, triglycerides, and insulin levels and of 2-h plasma insulin and glucose concentrations after a glucose challenge. Zucker obese rats (a model of the early stages of type 2 diabetes) and Zucker diabetic fatty rats (a model for type 2 diabetes) after supplementation (1,000 μg Cr/kg) have lower fasting plasma total, high-density lipoprotein, and LDL cholesterol, triglycerides, glycated hemoglobin, and insulin levels and lower 2-h plasma insulin levels in glucose tolerance tests. The lowering of plasma insulin concentrations with little effect on glucose concentrations suggests that the supplement increases insulin sensitivity. The cation after 12 and 22 or 24 weeks of administration lowers (P<0.05) fasting plasma glycated hemoglobin levels in the Zucker diabetic and Zucker obese rats, respectively, and thus can improve the glucose status of the diabetic models. The effects cannot be attributed to the propionate ligand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The element Cr in its trivalent oxidation state is generally considered to have a role in insulin-dependent carbohydrate and lipid metabolism [1–3]. Rats, for example, receiving a Cr deficient diet (approximately 30 μg Cr/kg diet) have elevated insulin areas (area under a plot of insulin concentration versus time) in glucose tolerance tests, suggesting the development of insulin resistance [4, 5]. In humans, the only potential cases of Cr deficiency arise from five subjects on total parenteral nutrition, before the intravenous fluid was supplemented with Cr [4]. The Food and Nutrition Board of the National Academy of Sciences (USA) in 2001 established that the daily adequate intake of Cr was 35 μg for men and 25 μg for women [6]. These recommendations were established by using data such as those of Anderson and Kozlovsky [7], who reported the Cr content of self-selected diets of American men and women. The average daily Cr intake for men was 33 μg; the average for women was 25 μg. Other studies [8, 9] have shown that humans consuming 35 μg of Cr daily are Cr-sufficient (i.e., the Cr quantity consumed in the diet greater than the Cr quantity lost in the stool and urine); thus, humans consuming reasonable diets are not likely to be Cr-deficient and would receive little if any benefit from supplementation of the diet with Cr. Recent meta-analyses [10, 11] and reviews of studies of Cr dietary supplementation [12] support this conclusion.
Chromium supplementation may have beneficial effects on individuals with altered carbohydrate and lipid metabolism; however, the results are not conclusive. Type 2 diabetes, for example, leads to increased urinary Cr loss [13]; this increased urinary Cr loss could potentially with time lead to a decrease in Cr status or even Cr depletion. A meta-analysis [14] of studies with diabetic subjects revealed that “A study of 155 diabetic subjects...” [15] “showed that chromium reduced glucose and insulin concentrations; the combined data from... the other studies did not” and indicated that the results on the studies with diabetic subjects were inconclusive. The positive placebo-controlled study performed by Anderson et al. [15] in China is the largest study of Cr supplementation of diabetic adults. Subjects received 0, 200, or 1,000 μg Cr as chromium picolinate daily for 4 months. Cr supplementation (1,000 μg/day) improved fasting serum glucose, insulin, glycated hemoglobin, and total cholesterol concentrations and insulin and glucose concentrations 2 h after a glucose challenge (P<0.05) compared with a placebo. At the 200-μg/day dosage, Cr supplementation improved fasting insulin and glycated hemoglobin concentrations and insulin concentrations 2 h after a glucose challenge (P<0.05), compared with a placebo. (The study results have been thoroughly reviewed by Hellerstein [16].) Thus, pharmacological, but not nutritional, levels of Cr may have beneficial effects on diabetic subjects. Anderson [17] has reviewed studies on the effects of Cr supplementation of type 2 diabetics and concluded that the amount of supplemental Cr was important with a threshold of more than 200 μg Cr daily postulated for beneficial effects.
The studies using large quantities of Cr (more than 200 μg/day) are supported by studies on rats. A trinuclear Cr(III) propionate complex, the cation [Cr3O(O2CCH2CH3)6(H2O)3]+, has beneficial effects [lower fasting plasma total and low-density lipoprotein (LDL) cholesterol, triglycerides, and insulin levels] on Zucker obese (ZKO) rats, models for early stages of type 2 diabetes [18, 19], while in this case significant effects were also noted in healthy rats. Rats received 5, 10, or 20 μg Cr intravenously as the cation per kilogram body mass daily for 12 or 24 weeks. Cefalu et al. [20] have observed beneficial effects on insulin sensitivity in type 2 diabetic model rats with cardiovascular disease, but not in healthy rats, from oral chromium picolinate administration (18 μg/kg body mass). Thus, two Cr(III) complexes in pharmacological doses have been shown to have beneficial effects on genetic rodent models of diabetes, although only the trinuclear cation has been shown to have an effect on healthy rats. Studies of healthy rats supplemented with chromium picolinate and CrCl3 (up to 100 mg Cr/kg diet for 24 weeks, a dose larger than any of the intravenous doses of the trinuclear cation, even after absorption is taken into consideration) found no effects of Cr on fasting glucose, cholesterol, or triglycerides levels [21].
Hence, only one Cr(III) complex to date has been shown to have reproducibly significant effects on blood plasma variables in healthy rats. The trinuclear chromium propionate cation has other unique properties that may explain these observations. The complex has been shown to mimic the effects of the naturally occurring, chromium-containing oligopeptide chromodulin in that it can stimulate the tyrosine kinase activity of insulin-activated insulin receptor [22] and is thus a functional biomimetic of chromodulin. When introduced intravenously, the complex can enter cells intact, i.e., in the form that can potentially increase insulin signaling. Additionally, the complex can be recrystallized from dilute mineral acid [23] and could potentially survive oral ingestion intact. The complex is absorbed very efficiently, with absorption ranging from greater than 60% at nutritionally relevant doses (approximately 4 μg Cr/kg) to 40% at pharmacologically relevant doses (approximately 4 mg/kg) [24]. However, the effects of oral administration of the complex on blood variables have not previously been examined.
Herein is reported the effects of oral administration of the biomimetic trinuclear cation to healthy and diabetic male rats for 24 weeks. ZKO rats and Zucker diabetic fatty (ZDF) rats were chosen to model type 2 diabetes. The ZKO rat is a genetic model for the early stages of type 2 diabetes, characterized by insulin insensitivity. The ZDF rat is a genetic model for type 2 diabetes and possesses elevated glucose levels and other diabetic symptoms. These studies indicate that the trinuclear cation has significant effects in healthy and type 2 diabetic rats on insulin, triglycerides, and total and LDL cholesterol levels and in diabetic rats on glycated hemoglobin levels, suggesting an increase in insulin sensitivity, and no acute toxic effects.
Materials and methods
The nitrate salt of the cation was prepared as described in the literature [25]. All operations were performed with doubly deionized water unless otherwise noted and were performed with plasticware whenever possible.
Animals
All rats were obtained from Charles River Laboratory. The 5-week-old male Sprague Dawley rats and ZKO rats were allowed to feed ad libitum on a commercial rat food (Harland Tekland Certified LM-485 Mouse/Rat Sterilizable Diet) and tap water. The ZDF rats were allowed to feed ad libitum on a high-fat commercial rat food (Purina 5008 Diet) and tap water; use of the high-fat food is necessary to guarantee diabetes. The high-fat diet contained approximately 0.3 g Cr/kg, while the normal diet contained approximately 0.4 g Cr/kg. Thus, the commercial foods provide Cr-adequate diets. Rats were raised in standard plastic and stainless steel cages on a 12-h light–dark cycle. Solid food intake and body mass were monitored at 4-day intervals. Thirty-two Sprague Dawley rats were divided randomly into four groups of eight. The first group was gavaged daily with an aqueous solution containing the biomimetic cation to give a total amount of chromium equivalent to 250 μg Cr/kg body weight. The second group received an aqueous solution of the cation to give 500 μg Cr/kg body mass; the third group received an aqueous solution of the cation to give 1,000 μg Cr/kg body mass. The last group was gavaged with an equal volume of doubly deionized water daily and served as the control. Sixteen ZKO and ZDF rats were each split into two groups of eight rats. For each, one group of eight received doubly deionized water and served as a control, while the other group received an aqueous solution of the cation to give 1,000 μg Cr/kg body mass. After 24 weeks, the Sprague Dawley and ZKO animals were sacrificed by CO2 asphyxiation; because of health concerns, the ZDF rats were sacrificed after 22 weeks. Liver, kidney, heart, spleen, pancreas, testes, and epididymal fat were quickly harvested and weighed on plastic weighboats. Part of the largest lobe of the liver and a kidney were placed into plastic screw-top containers and frozen and stored for metal analyses. The University of Alabama Institutional Animal Use and Care Committee approved all experiments involving rats.
Blood chemistry
Blood (approximately 1.5 mL) was collected from tail snips into polypropylene tubes after 4, 8, 12, 16, 20, and 24 (or 22 for ZDF) weeks of cation or H2O administration. Prior to blood collection, animals were fasted for 12–16 h. Immediately after blood removal, 0.5 mg/mL heparin and 10 mg/mL NaF were added to the blood, and a small aliquot (approximately 200 μL) of the blood was removed. The blood was next immediately centrifuged; the blood plasma was tested for glucose, total cholesterol, triglycerides, LDL cholesterol, and high-density lipoprotein (HDL) cholesterol using diagnostic kits from Sigma Chemical Co. (St. Louis, MO, USA) and for insulin using antibody-coated kits from ICN Biomedicals (Costa Mesa, CA, USA). The aliquot of whole blood was used for glycated hemoglobin assays; glycated hemoglobin was measured using a kit from Biotron Diagnostics (Hemet, CA, USA) on blood samples from weeks 4, 12, and 24 (or 22 for ZDF). UV–vis measurements were made with a Hewlett-Packard 8453 spectrophotometer. Gamma counting was performed with a Packard Cobra II auto-gamma counter.
Glucose tolerance tests
After 6, 10, 14, 18, and 22 weeks of cation or H2O administration, the rats were injected under the skin with aqueous solutions of glucose (1 mg/mL) such that each rat received 1.25 mg glucose/kg body mass [26]. After 2 h, blood (approximately 0.5 mL) was collected from tail snips and handled as described earlier. The plasma insulin and glucose concentrations were determined as described earlier.
Metal analyses
Samples of approximately equal mass from three randomly chosen livers and kidneys from each group were dried. All vessels used in the drying were acid-washed. Fe concentrations were determined by the method of Fish [27]. Cr concentrations were determined by graphite furnace atomic absorption spectroscopy using a PerkinElmer AAnalyst 80. Samples were prepared utilizing the method of Miller-Ihli [28]. Sample preparation blanks were analyzed, and all data were blank-corrected. Analytical accuracy was monitored through periodic analyses of certified reference materials from the National Institutes of Standards and Technology. The analysis wavelength was 357.9 nm.
Statistical analyses
Data were stratified by weeks of dietary Cr treatment into six groups (4, 8, 12, 16, 20, and 22 or 24 weeks). In each group of Sprague Dawley rats, analysis of variance was used to test the difference in mean concentrations of plasma variables: glucose, insulin, cholesterol, triglycerides, HDL, and LDL by four levels of dietary Cr; control or 0, 250, 500, and 1,000 μg Cr/kg body mass. Differences in mean organ mass by four dietary Cr levels were also tested by analysis of variance. A pooled Student’s t test was used in each group of ZKO and ZDF rats to evaluate the difference in mean concentrations of plasma variables between control and Cr-treated rats. Differences in the mean organ mass and Fe and Cr levels in the liver and kidney tissues were also analyzed by the pooled Student’s t test. The level of significance for all analyses was set at P≤0.05. Data was analyzed using SAS software (version 8.1).
Numerical values in the tables and text are presented as mean plus/minus the standard deviation unless otherwise indicated. The error bars in Figs. 1, 2, and 3 represent the standard error of the mean to keep overlaps to a minimum for presentation purposes.
Cr content of rat liver and kidney after 6 months of treatment with varying amounts of chromium complexes. a Cr content versus daily dose of Cr; b Cr content versus daily absorbed dose of Cr. Cr3—[Cr3O(O2CCH2CH3)6(H2O)3]+. Data for CrCl3 and Cr picolinate are adapted from Ref. [21].
Results
The daily food intake (not shown) and the percentage mass gain (\( {{\text{average mass gain}}} \mathord{\left/ {\vphantom {{{\text{average mass gain}}} {{\text{average mass on day 1}}}}} \right. \kern-\nulldelimiterspace} {{\text{average mass on day 1}}} \times {\text{100\% }} \)) of the control groups and the corresponding trinuclear-cation-administered groups (Figs. 1, 2) were generally statistically equivalent throughout the 24-week period. However, the more cation the healthy Sprague Dawley rats received the lower their percentage body mass gain tended to be. For the ZKO rats, the only statistically significant effects were observed at the very end of the study when rats receiving Cr had greater body mass gains; why these rats gain weight compared with controls is unclear. The general lack of a statistical difference in body mass with Cr-complex administration is consistent with numerous other studies [4, 21, 28–31]. All animals appeared normal throughout, and no visible differences were observed between the administered groups and their controls.
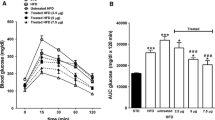
Blood plasma variables were determined for all eight groups after 4, 8, 12, 16, 20, and 24 weeks of administration (Tables 1, 2) (except the ZDF rats which were killed after 22 weeks). Some striking similarities are apparent between the healthy Sprague Dawley rats, the ZKO rats, and the ZDF rats receiving the Cr complex and their respective controls. For the Sprague Dawley rats, after 24 weeks of administration of trimer corresponding to 500 or 1,000 μg Cr daily, plasma total cholesterol, triglycerides, insulin, and LDL cholesterol levels are all significantly lower than those of the control. For the 250-μg Cr dose, total cholesterol, triglycerides, and insulin levels are all lower than for the controls. The reduction in insulin concentration is first observed for the higher two doses after just 4 weeks of administration and is also observed after 8 (1,000 μg only), 12, 16, and 20 weeks of administration. Reductions in insulin levels are also observed for the lowest dose of Cr starting at week 12. At week 20, the highest Cr dose results in a significantly lower insulin concentration than the other Cr doses. Plasma triglycerides levels become significantly lower after week 4 for the largest quantity of Cr and are significantly lower after each subsequent 4-week period for each dose of Cr. At week 8, the highest Cr dose results in a significantly lower triglycerides concentration than the other Cr doses. Plasma total cholesterol levels are not significantly lower for any dose at week 4 but with two exceptions are lower for all doses at all subsequent times. Plasma LDL cholesterol levels were significantly lower for at least two of the doses of the Cr complex each time they were examined. The highest two Cr doses generated lower LDL levels than the lowest Cr dose at week 20. Plasma HDL levels are essentially unchanged by cation administration. As HDL levels are unchanged and total cholesterol levels drop with cation treatment, the total cholesterol-to-HDL cholesterol ratio drops with treatment. Glucose concentrations for rats receiving the cation after week 4 tend to be lower than those of controls; however, the effects are not consistently significant. The effects on triglycerides and total and LDL cholesterol concentrations with the accompanying lowering of insulin levels with little effect on glucose concentrations suggests that the complex is significantly increasing insulin sensitivity in these rats. The dependence of the blood variables with time was also analyzed. At the 95% confidence limit, none of the blood variables displayed significant time dependence when compared with the levels of the control group.
Very large effects from Cr-complex administration are seen for the ZKO and ZDF rats. For the ZKO rats, plasma insulin was lower for the trimer groups after each 4-week period; the same pattern held for the ZDF rats except no significant difference in insulin levels was present at week 4. Triglycerides were lower for both diabetic models receiving Cr at each time point. LDL cholesterol was lower for the ZKO rats after weeks 8, 16, 20, and 24 and after weeks 12, 16, 20, and 22 for the ZDF rats. Total cholesterol was lower for the ZKO rats after weeks 8, 12, 16, 20, and 24 and after weeks 12, 16, 20, and 22 for the ZDF rats. At week 22 or 24, ZKO and ZDF rats receiving Cr had 38 and 21% lower total cholesterol, respectively. For HDL cholesterol, the ZKO rats treated with cation after weeks 8, 12, 16, 20, and 24 possessed lower levels; and levels were lower after weeks 8, 12, and 22 for the ZDF rats. This lowering of HDL cholesterol is actually a movement toward restoring HDL levels to normal, as the diabetic model rats have elevated HDL levels [32]. For the ZDF rats, the ratio of total to HDL cholesterol does not appreciably change compared with that of controls, while the ratio actually decreases about 15% in the ZKO rats. No consistent statistically significant effects were observed in the plasma glucose concentrations. Overall, the diabetic model rats seem to have significantly increased insulin sensitivity in response to treatment with the trimer in a fashion similar to that of the healthy Sprague Dawley rats. Notably, the ratio of plasma total cholesterol for the trimer-receiving rats to that of controls decreases significantly at the 95% confidence level as a function of time. None of the other blood variables display time dependence.
Similar trends are observed in the results from glucose tolerance tests (Tables 3, 4). For healthy Sprague Dawley rats, both glucose and insulin concentrations 2 h after administration of the biomimetic trimer are consistently lower than those of controls. The effects are most notable for insulin levels where a distinct dependence on Cr dosage is discernable. The ZKO and ZDF rats receiving trimer had lower plasma insulin concentrations in the tests from 10 to 22 weeks of treatment, while the ZKO rats receiving Cr also had lower 2-h insulin concentrations after 6 weeks. In each test period, the ZKO and ZDF rats receiving Cr had lower 2-h plasma glucose concentrations; however, the effect was never statistically significant. The lowering of plasma insulin and glucose displays no time dependence at the 95% confidence limit. Thus, in all groups receiving the Cr complex significant effects on 2-h insulin levels were observed, indicating less insulin is required to lower elevated glucose concentrations. Glucose concentrations also appear to drop faster at these reduced insulin levels. These studies are also consistent with the biomimetic increasing insulin sensitivity. In this regard, the decrease in triglycerides, LDL cholesterol, and total cholesterol notably is consistent with the effects of insulin on type 2 diabetic human patients [33]. These studies are also consistent with previous studies in which rats were administered the trinuclear cation intravenously for 12 or 24 weeks [18, 19]. However, some differences occur in the time dependence of the lowering of plasma variables between the oral and intravenous studies. For ZKO rats receiving the biomimetic trimer intravenously, total cholesterol dropped as a function of time. The difference based on the method of administration is not currently understood but may reflect that intravenous administration provides all of the cation to the bloodstream at essentially one time where oral administration allows the complex to be absorbed into the blood over a period of time as the complex passes through the gastrointestinal tract [24].
To examine whether the administration of the biomimetic cation was having any effect on the long-term glycemic control of the rats, fasting plasma glycated hemoglobin concentrations were determined after weeks 4, 12, and 22 or 24 (Table 5). Glycated hemoglobin is formed noncatalytically, and thus slowly, from hemoglobin and glucose; consequently, the percentage of hemoglobin that is glycated provides a “glycemic history” of the previous 120 days, the average erythrocyte life span. The concentrations of glycated hemoglobin were not affected in the healthy Sprague Dawley rats. This is not surprising as insulin levels but not glucose levels were affected by treatment with chromium. However, after 12 and 22 weeks the ZDF rats experienced a significant decrease in the percentage of glycated hemoglobin, reaching almost a 22% drop compared with controls by week 22. For the ZKO rats, the glycated hemoglobin percentage is only significantly lower at week 24 (27% lower than that of controls). This is consistent with the tendency of the fasting plasma glucose levels of the ZKO and ZDF rats receiving the cation to be lower than those of their controls; (glucose levels are lower for both types of rats at all but one time point each and are statistically lower for the ZDF rats receiving cation at week 16). Thus, the administration of the biomimetic cation is successfully treating the symptoms of the type 2 diabetes, although alone it is not restoring the altered glycated hemoglobin and other plasma variables to their healthy range. Further studies looking at the effects of the complex in concert with insulin therapy and other treatments will be needed to further examine the potential of the complex to aid in the treatment of type 2 diabetes.
Comparison of organ masses after 24 weeks of administration revealed some statistically significant variations from those of the controls (Tables 6, 7); however, no trends are apparent between groups. For example, for the healthy Sprague Dawley rats, rats receiving the largest amount of the trimer had less epididymal fat than the control group (32%). This loss of fat might be reflected in the trend toward body mass reduction in this group compared with controls. Yet, ZDF rats receiving the complex only had a smaller pancreas. No effects were observed in the ZKO rats. In this laboratory’s previous study with healthy Sprague Dawley rats receiving the trinuclear cation for 12 weeks, healthy rats receiving the trimer on average had a larger pancreas mass and a lower testes mass compared with those of controls [18]; in an earlier 24-week study, healthy rats had increased heart, testes, and spleen masses compared with those of controls, while ZKO rats had smaller hearts, liver, testes, and kidneys [19]. None of these effects were observed in this study. No organs were visibly different for any of the groups.
Cr has been suspected of potentially adversely affecting Fe metabolism [34]. Thus, the Fe and Cr levels of liver and kidney tissue from rats of each group were examined. No differences in the Fe content of the liver and kidney of the rats (Table 8) were observed in the ZDF rats receiving Cr, which had lower kidney Fe concentrations than their controls. Surprisingly, the Cr supplementation has no significant effect on tissue Cr levels (Table 8). Kidney Cr concentrations are generally greater than liver concentrations, as previously observed in the intravenous studies [19] and in studies in which other Cr complexes were given orally to rats [21].
As the biomimetic degrades in vivo, propionate is likely to be released. Propionate has been proposed to be able to lower plasma cholesterol and glucose concentrations; however, these results are quite controversial [36]. This laboratory has also given rats sodium propionate intravenously in amounts equivalent to the propionate contained in the largest quantity of trimer used in the intravenous studies [18, 19]. Blood plasma levels were measured after 4, 8, 12, 16, 20, and 24 weeks and 2-h glucose tolerance tests were performed after weeks 6, 10, 14, 18, and 22 (Tables S1, S2). The propionate had no significant effect on fasting plasma glucose, insulin, triglycerides, and total or HDL cholesterol levels; similarly, the propionate had no effect on plasma glucose or insulin levels in response to glucose administration and no effects on body mass (Fig. S1), food intake, and organ mass (Table S3). Thus, the effects were in stark contrast to those from the trimer.
Discussion
In only the last decade, the effects of Cr deficiency and the effects of administration of chromium to healthy rats have been established to an appreciable degree; the interpretation of studies before 1990 is complicated by methodological problems [1]. Effects from Cr supplements have been observed for otherwise healthy rats fed an apparently Cr-deficient diet; generation of Cr-deficiency is extremely difficult, requiring strict environmental control, such as the removal of any stainless steel objects [4, 5]. The only consistent effect of the apparent Cr deficiency appears to be higher plasma insulin levels in glucose tolerance tests [4, 5]. Rats on an apparently Cr-deficient and high fat diet may have higher plasma insulin levels and in glucose tolerance tests, higher triglycerides areas [37]. In previous studies with healthy rats on a normal diet, receiving an oral dose of Cr had no effect on the body composition or blood variables of the rats [4, 21, 28–31]. For example, in the largest such study, feeding rats a diet containing up to 100 mg Cr/kg diet as Cr picolinate or CrCl3 for 24 weeks had no effect on body mass and tissue masses nor on serum glucose, cholesterol, or triglycerides concentrations [21]. The rats receiving a 100 mg Cr/kg diet received approximately 15 mg Cr/kg body mass daily [21]. This quantity is 15 times the highest dose used in the present study. Hence, the two most common forms of Cr used in human nutritional supplements when given to rats for 6 months at a dose 15-fold higher than in this study had no effect on the body composition or serum glucose, cholesterol, or triglycerides concentrations. The difference in absorption should also be considered here. Oral CrCl3 and Cr picolinate are absorbed with an efficiency of approximately 0.5–2% [38, 39]. In contrast, the trinuclear Cr propionate cation is absorbed with 40–60% efficiency [24]. After correcting for absorption efficiency, the rats in this study and the earlier study with CrCl3 and Cr picolinate that received the maximum dose of Cr had approximately the same quantity of Cr entering their bloodstreams. While the trinuclear cation generated significant reductions in fasting plasma triglycerides, insulin, and LDL cholesterol starting after 4 weeks of treatment and an additional lowering of total cholesterol after 8 weeks, the two popular supplements had no effect after 6 months of treatment. Effects on blood variables were also observed for the smaller doses of Cr as the biomimetic complex. Thus, the increased insulin sensitivity in the healthy rats observed in this study would appear to arise from the trinuclear Cr propionate complex, not from Cr(III) itself. Insulin-resistance results in fat cells increasing their intracellular hydrolysis of triglycerides, releasing fatty acids into the bloodstream; the increased flux of fatty acids stimulates the liver to increase the formation and excretion of very low density lipoprotein (VLDL) cholesterol and triglycerides (generating hypertriglyceridemia) [40]. In the plasma collisions between VLDL and LDL in the presence of cholesteryl transfer protein result in exchange of VLDL triglycerides for LDL cholesterols, leading to the formation of small dense LDL particles. Thus, insulin resistance results in changes in plasma lipids and increases the level of triglycerides [40]. Increases in insulin sensitivity have the opposite effect. Hence, the changes in plasma lipid and cholesterol concentrations also reflect increases in insulin sensitivity.
This may be explained by the biomimetic cation’s ability to stimulate insulin receptor (in a fashion similar to the oligopeptide chromodulin); hence, the functional biomimetic could potentially trap insulin-stimulated insulin receptor in vivo in its active conformation beyond its normal levels, resulting in increased insulin signaling and subsequent cellular action (i.e., increased insulin sensitivity). For this to be significant, the trinuclear cation must remain intact in vivo for an appreciable period of time. Two hours after intravenous injection of rats with 51Cr-labeled trimer, approximately 90% of 51Cr in liver cells from labeled trimer is localized to the microsomes, indicating selective transport into these organelles [41]. The molecular weight of the species in the microsomes is similar to that of the trimer, suggesting it may reach these organelles intact. However, the complex has a lifetime of less than 24 h in vivo [36]. The effects seen in rats administered the biomimetic complex daily presumably then could arise from only the period of time in which the complex remains intact; however, this cannot be definitely stated as the unique ability of the complex to be absorbed from the gastrointestinal tract and then enter cells, such that higher intracellular Cr levels are possible, could also be responsible.
Such a case for a unique action for the trinuclear cation cannot be made in the case of the rat models of diabetes. Cefalu et al. [20], using JCR(LA)-cp rats (a genetic type 2 diabetes model with cardiovascular disease) administered chromium picolinate (aqueous solution in a water bottle) at a dose of 18 μg Cr/kg body mass, observed that Cr administration resulted in lower fasting plasma insulin and total cholesterol levels and lower plasma glucose and insulin levels in glucose tolerance tests, similar to the results of the current study. However, fasting HDL levels increased in the rats receiving Cr picolinate compared with those of controls, in contrast to the results with the biomimetic cation observed here and in earlier intravenous administration studies [18, 19]. The JCR(LA)-cp rats have greatly elevated plasma HDL cholesterol levels (in a similar fashion to the ZKO and ZDF rats). The disparity between the results on HDL levels using the two sources of Cr is difficult to explain at the current time; additionally, the health consequences of increasing elevated HDL levels in the JCR(LA)-cp rats with previously existing elevated HDL levels need to be ascertained.
The safety of Cr-containing nutritional supplements and potential therapeutic agents has been a matter of debate [12, 42, 43], although most of the attention has been focused on potential deleterious effects of Cr picolinate [44–52]. The complex has been shown to be a mutagen in cell culture [47] and in Drosophila melanogaster [51] and to give rise to oxidative damage in test tube studies [49], cell culture studies [46], and at high doses [52] and nutritional doses [50] in rats. In March 2003, the Expert Group on Vitamins and Minerals determined that Cr picolinate was a potential carcinogen and requested that the health supplement industry voluntarily withdraw the products containing the compound while consulting on a ban on the use and sale in Great Britain [53]. One study looking for oxidative damage in humans failed to observe any harmful effects [54]. The potentially deleterious effects appear to be unique to this complex compared with other supplements [42, 43]. For example, the biomimetic cation does not give rise to DNA damage in the test tube studies [55] and does not generate developmental delays and decreases in the number of successful progeny in Drosophila (D. Stallings, J. O’Donnell, and J.B. Vincent, unpublished results). The lack of accumulation of Cr from the cation in liver and kidney when given daily for approximately half a year in large oral doses minimizes the potential for deleterious effects; accumulation of Cr from Cr picolinate has been described as a potential health concern [56].
As shown in Fig. 3a, liver and kidney Cr levels for the Sprague Dawley rats remain constant within error in stark contrast to those when Cr picolinate and CrCl3 are given orally to rats [21]. The Cr intake for these two supplements was calculated assuming the food consumption rate from Ref. [21] (15 g food daily for a 100-g rat). However, Fig. 3a does not tell the entire story; the contrast is even more apparent when the differences in absorption are considered—2% absorption of Cr from Cr picolinate and CrCl3 (vide infra), and 40% absorption of the trinuclear cation [24]. For both Cr picolinate and CrCl3, Cr accumulates in the kidney and liver in proportion to the dose, with the greater amounts being in the kidney. For the chloride, the increase in liver Cr levels is small but statistically significant. The observation that the Cr complex that is absorbed to the greatest degree results in the least accumulation of Cr in the kidney and liver would at first appear to be contradictory. However, absorption and retention studies may explain this apparent problem. Anderson and Polansky [35] have given 51CrCl3 orally to rats and examined the distribution of 51Cr in the tissues and body fluids from 5 min to 24 h after administration. The concentration of 51Cr in the kidney and the liver (cpm 51Cr/g tissue) was significantly higher 24 h after administration than at any earlier time. In fact, the concentration was almost 10 times higher after 24 h than after 1 h for the kidney and almost 5 times higher for the liver [35]. For the biomimetic complex, the maximum concentrations of Cr in the liver and kidney after an oral dose comparable to the highest dose used in this work are reached rapidly (30 min after injection); the concentration in either organ is several fold lower 24 h after injection than after 30 min [24]. Consequently, while the biomimetic cation is absorbed to a greater extent, it is not retained. This difference in 24-h retention levels between the complexes if retention does not change over 6 months of daily administration could easily explain the different Cr accumulations between the complexes observed in Fig. 3.
Current drugs for treating diabetes (in addition to insulin treatment) fall into five categories: metformin (whose mechanism of action is uncertain), thiazolidines [which activate peroxisome proliferator-activated receptor gamma (PPARγ)], α-glucosidase inhibitors, and the last two sulfonylureas and non-sulfonylurea insulin secretagogues (which increase insulin secretion by the pancreas) [57]. None of their modes of action resemble those of the trinuclear Cr cation. Ross et al. [57] have recently reviewed the types of emerging therapeutic agents for treating type 2 diabetes. Two of these types of agents are small-molecule insulin receptor mimetics and inhibitors of protein tyrosine phosphatase 1B (PTP1B). The first insulin receptor mimic was developed at Merck Research Laboratories and was a small, nonpeptidyl fungal metabolite [58, 59]; this compound is active in the absence of added insulin but has toxic side effects. In contrast Telik has developed nonpetidyl small molecules that increase insulin receptor phosphorylation in the presence of insulin [59, 60]. This mode of action strongly resembles that proposed for the trinuclear Cr propionate cation. Complexes of another transition metal have been suggested as therapeutic agents for treating type 2 diabetes—vanadate complexes. Vanadate complexes fall into the category of PTP1B inhibitors. Despite the nature of the vanadate complex, the active species in phosphatase inhibition appears to be “naked” vanadate [61]. In contrast to the trinuclear Cr complex, vanadate increases the basal levels of phosphorylation of insulin receptor and insulin receptor substrate-1 and the activity of PI3-kinase, but these levels are not further stimulated by insulin [62]. The trinuclear Cr complex does not inhibit PTP1B activity or the activity of other phosphatases examined to date [63].
Conclusion
As demonstrated in Tables 1 and 2, the functional biomimetic has a striking effect on fasting plasma triglycerides, total cholesterol, LDL cholesterol, and insulin levels over 24 weeks of administration in healthy Sprague Dawley, ZKO, and ZDF rats. Insulin concentrations for all the rats and for the healthy rats’ glucose concentrations as well were lower in 2-h glucose tolerance tests for rats receiving the cation. The effects of the biomimetic compound on insulin, cholesterol, and triglycerides on the healthy rats suggest that the trinuclear complex serves not as simply a chromium source but possesses an intrinsic activity, in contrast to other sources of chromium previously examined. The complex also lowers blood plasma glycated hemoglobin levels in the rat models of diabetes, indicating the complex can be used to improve the status of the diabetic animals. Also no toxic effects were observed for supplementation with the trinuclear cation. These results suggest that the compound may have potential as a therapeutic agent.
Abbreviations
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- PTP 1B:
-
Protein tyrosine phosphatase 1B
- SD:
-
Standard deviation
- VLDL:
-
Very low density lipoprotein
- ZDF:
-
Zucker diabetic fatty
- ZKO:
-
Zucker obese
References
Vincent JB (2001) Polyhedron 20:1–26
Vincent JB (2000) Acc Chem Res 33:503–510
Lukaski HC (1999) Ann Rev Nutr 19:279–301
Striffler JS, Law JS, Polansky MM, Bhathena SJ, Anderson RA (1995) Metabolism 44:1314–1320
Striffler JS, Polansky MM, Anderson RA (1999) Metabolism 48: 1063–1068
Trumbo P, Yates AA, Schlicker S, Poos M (2001) J Am Diet Assoc 101:294–301
Anderson RA, Kozlovsky AS (1985) Am J Clin Nutr 41:1177–1183
Bunker VW, Lawson MS, Delues HT, Clayton BE (1984) Am J Clin Nutr 39:797–802
Offenbacher EG, Spencer H, Dowling HJ, Pi-Sunyer FX (1986) Am J Clin Nutr 44:77–82
Pittler MH, Stevinson C, Ernst E (2003) Int J Obesity 27:522–529
Nissen SL, Sharp RL (2003) J Appl Physiol 94:651–659
Vincent JB (2003) Sports Med 33:213–230
Morris BW, MacNeil S, Hardisty CA, Heller S, Burgin C, Gray TA (1999) J Trace Elem Med Biol 13:57–61
Althius MD, Jordan NE, Ludington EA, Wittes JT (2002) Am J Clin Nutr 76:148–155
Anderson RA, Cheng NC, Bryden NA, Polansky MM, Cheng N, Chi J, Feng J (1997) Diabetes 46:1786–1791
Hellerstein MK (1998) Nutr Rev 56:302–306
Anderson RA (1998) J Am Coll Nutr 17:548–555
Sun Y, Mallya K, Ramirez J, Vincent JB (1999) J Biol Inorg Chem 4:838–845
Sun Y, Clodfelder BJ, Shute AA, Irvin T, Vincent JB (2002) J Biol Inorg Chem 7:852–862
Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC (2002) J Nutr 132:1107–1114
Anderson RA, Bryden NA, Polansky MM (1997) J Am Coll Nutr 16:273–279
Davis CM, Royer AC, Vincent JB (1997) Inorg Chem 36:5316–5320
Johnson MK, Powell DB, Cannon RD (1981) Spectrochim Acta 37A: 995–1006
Clodfelder BJ, Chang C, Vincent JB (2004) Trace Elem Biol Res 98:159–170
Earnshaw A, Figgis BN, Lewis J (1966) J Chem Soc A 1656–1663
Schwarz K, Mertz W (1959) Arch Biochim Biophys 85:292–295
Fish WW (1988) Methods Enzymol 158:357–364
Miller-Ihli NJ (1996) J Food Comp Anal 9:290–300
Hasten DL, Hegsted M, Keenan MJ, Morris GS (1997) Nutr Res 17:283–294
Hasten DL, Hegsted M, Keenan MJ, Morris GS (1997) Nutr Res 17:1175–1186
Morris GS, Guidry KA, Hegsted M, Hasten DL (1995) Nutr Res 15: 1045–1052
Sparks JD, Shaw WN, Corsetti JP, Bolognino M, Pesek JF, Sparks CE (2000) Metabolism 49:1424–1430
Brunzell JD, Chait A (1990) Lipoprotein pathophysiology and treatment. In: Rifkin H, Porte D Jr (eds) Ellenberg and Rifkin’s diabetes mellitus: theory and practice. Elsevier, New York, pp 756–767
Lukaski HC, Bolonchuk W, Siders WA, Milne DB (1996) Am J Clin Nutr 63:954–965
Anderson RA, Polansky MM (1995) Biol Trace Elem Res 50:97–108
Shute AA, Chakov NE, Vincent JB (2001) Polyhedron 20:2241–2252
Striffler JS, Polansky MM, Anderson RA (1998) Metabolism 47: 396–400
Olin KL, Stearns DM, Armstrong WH, Keen CL (1994) Trace Elem Electrolytes 11:182–186
Anderson RA, Bryden NA, Polansky MM, Gautschi K (1996) J Trace Elem Exp Med 9:11–25
Ginsberg HN (2000) J Clin Invest 106:453–458
Shute AA, Vincent JB (2002) J Inorg Biochem 89:272–278
Vincent JB (2004) Biol Trace Elem Res 99:1–16
Vincent JB (2003) Sports Med 33:213–230
Stearns DM, Wise JP Sr, Patierno SR, Wetterhahn KE (1995) FASEB J9: 1643–1648
Bagchi D, Bagchi M, Balmoori J, Ye X, Stohs SJ (1997) Res Commun Mol Pathol Pharmacol 97:335–346
Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG (2002) Toxicology 180:5–22
Manygoats KR, Yazzie M, Stearns DM (2002) J Biol Inorg Chem 7:791–798
Stearns DM, Silveira SM, Wolf KK, Luke AM (2002) Mutat Res 513: 135–142
Speetjens JK, Collins RA, Vincent JB, Woski SA (1999) Chem Res Toxicol 12:483–487
Mahboob L, McNeil L, Toliver T, Odgen L (2000) Toxicol Sci 66(1-S): 32
Hepburn DDD, Xiao J, Bindom S, Vincent JB, O’Donnell (2003) Proc Natl Acad Sci USA 100:3766–3771
Hepburn DDD, Burney JM, Woski SA, Vincent JB (2003) Polyhedron 22: 455–463
http://www.foodstandards.gov.uk/news. newsarchive/safetyhighdosesvitsandmins accessed on 08 May 2003
Kato I, Vogelman JH, Dilman V, Karkoszka J, Frenkel K, Durr NP, Orentreich N, Toniolo P (1998) Eur J Epidemiol 14:621–626
Speetjens JK, Parand A, Crowder MW, Vincent JB, Woski SA (1999) Polyhedron 18:2617-2624
Stearns DM, Belbruno JJ, Wetterhahn KE (1995) FASEB J 9:1650–1657
Ross SA, Gulve EA, Wang M (2004) Chem Rev 104:1255–1282
Zhang B, Salituro G, Szalkowski D, Li Z, Zhang Y, Royo I, Vilella D, Diez MT, Pelaez F, Ruby C, Kendall RL, Mao X, Griffin P, Calaycay J, Zierath JR, Heck JV, Smith RG, Moller DE (1999) Science 284:974–977
Li M, Youngren JF, Manchem VP, Kozlowski M, Zhang BB, Maddux BA, Goldfine ID (2001) Diabetes 50:2323–2328
Pender C, Goldfine ID, Manchem VP, Evans JL, Spevak WR, Shi S, Rao S, Bajjalieh S, Maddux BA, Youngren JF (2002) J Biol Chem 277: 43565–43571
Peters KG, Davis MG, Howard BW, Pokross M, Rastogi V, Diven C, Greis KD, Eby- Wilkens E, Maier M, Evdokimov A, Soper S, Genbauffe F (2003) J Inorg Biochem 96:321–330
Goldfine AB, Patti ME, Zuberi L, Goldstein BJ, LeBlanc R, Landaker EJ, Jiang ZY, Willsky GR, Kahn CR (2000) Metabolism 49:400–410
Goldstein BJ, Zhu L, Hager R, Zilering A, Sun Y, Vincent JB (2001) J Trace Elem Exp Med 14:393–404
Acknowledgements
The authors wish to thank Christine Chang, Jelena Hamilton, Allison Pickering, and James A. Neville and the staff of The University of Alabama Animal Care Facility for assistance with the rat studies. Funding was provided by the National Institutes of Health (DK62094–01) (J.B.V.).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00775-005-0651-7
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Clodfelder, B.J., Gullick, B.M., Lukaski, H.C. et al. Oral administration of the biomimetic [Cr3O(O2CCH2CH3)6(H2O)3]+ increases insulin sensitivity and improves blood plasma variables in healthy and type 2 diabetic rats. J Biol Inorg Chem 10, 119–130 (2005). https://doi.org/10.1007/s00775-004-0618-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-004-0618-0