Abstract
Bone is a frequent site of metastasis for multiple types of solid tumors in organs such as prostate, breast, lung, etc., accounting for significant morbidities and mortalities of afflicted patients. One of the major problems of bone metastasis is lack of biomarkers for early diagnosis and for monitoring therapeutic responses. Medical imaging modalities such as computerized tomography, magnetic resonance imaging, and radioactive isotope-based bone scans are currently standard clinical practices, yet these imaging techniques are limited to detect early lesions or to accurately monitor the metastatic disease progression during standard and/or experimental therapies. Accordingly, development of novel blood biomarkers rationalizes extensive basic research and clinical development. This review article covers the up-to-date information on protein- and cell-based biomarkers of bone metastasis that are currently used in the clinical practices and also are under development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone metastasis is a multi-step process involving detachment from the primary tumor, intravasation, survival in the bloodstream, extravasation in the bone microenvironment, dormancy, and subsequent outgrowth and colonization in the unique microenvironment comprising hard tissue (i.e., calcified matrices) and soft tissue (i.e., bone marrow). Nearly all types of solid cancers metastasize to bone, but several types of cancer, most notably breast and prostate cancers, preferentially develop bone metastasis. From the clinical aspect, bone metastasis causes specific morbidities known as skeletal-related events (SREs) including pathologic bone fracture, spinal cord compression, hypercalcemia, and bone pain [1, 2]. Accordingly, bone metastasis remains a major cause of mortality of afflicted patients.
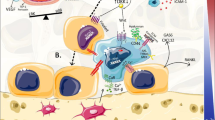
The matrix of bone tissue is densely calcified yet the internal hollow is filled with well-vascularized soft tissue (bone marrow) [3]. Disseminating metastatic cancer cells first locate adjacently to the endosteal surface, and interact with various types of bone and marrow cells to form micro-metastatic colonies termed as metastatic niche [4, 5]. Meanwhile, osteoblasts first become activated and produce diverse cytokines and growth factors essential to activate osteolysis and proliferation of cancer cells [6]. However, these micro-metastatic lesions are undetectable using current imaging technologies.
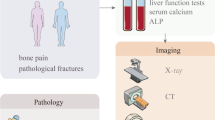
Current standard diagnostic approach for bone metastasis use imaging modalities, including whole-body bone scintigraphy (WBS) and magnetic resonance imaging (MRI). Since WBS is highly sensitive for detecting both osteolytic and osteoblastic lesions [7, 8], WBS is commonly utilized to screen patients for bone metastasis. On the other hand, flare phenomenon, an inadvertently increased uptake despite favorable treatment responses, often results in misdiagnoses and misguided changes in treatment plans [9]. Whole-body MRI is the most sensitive method for detection of cellular changes in bone, but MRI is unsuitable for routine follow-up examinations or screening large cohorts. Given these limitations of WBS and MRI, there is a substantial clinical demand for practically feasible and sensitive diagnostic modalities to sensitively detect micro-metastatic bone lesions.
Over the past decades, many studies have been conducted to develop alternatives to invasive biopsy for diagnosing cancer and monitoring treatment response. Classically, measurement of serum or urine protein biomarkers has been used, and more recently, liquid biopsy techniques have received much attention. Liquid biopsies involve isolating tumor-derived entities such as circulating tumor cells, circulating tumor DNA, and tumor extracellular vesicles, that are present in the body fluids of cancer patients [10]. Regarding bone metastasis, especially due to the characteristics of hard tissue, collecting and processing the biopsy specimen are difficult in cancer clinics, and thus there remains a strong clinical unmet need for non-invasive diagnostic tests. Here, we will review the protein- and cell-based biomarkers as diagnostics for bone metastasis.
Protein-based biomarkers of bone metastasis
Bone turnover markers (BTMs)
Bone metastasis alters normal bone homeostasis, creating a pro-tumorigenic bone marrow microenvironment, i.e., so-called vicious cycle of bone metastasis. At this time, activated osteoclasts and osteoblasts propel bone turnover. Therefore, numerous investigators first focused on BTMs as potential biomarkers of bone metastasis in the past few decades. BTMs can be broadly categorized into bone resorption and bone formation markers. Bone resorption markers include by-products of type-I collagen (the major type of collagen in bone matrix) degradation or osteoclastic enzymes/cytokines, whereas bone formation markers include mainly by-products of osteogenesis or osteoblastic enzymes released by active osteoblasts (Table 1).
Among bone resorption-related BTMs, urinary N-terminal cross-linked telopeptide of type-I collagen (NTX) is the most reported indicator for the risk of SRE and death in patients with bone metastasis and for monitoring response to anti-resorptive bisphosphonate [11,12,13,14,15,16,17]. Similar to NTX, C-telopeptide of type-I collagen (CTX), cross-linked carboxy-terminal telopeptide of type-I collagen (ICTP), and pyridinoline/deoxypyridinoline (PYD/DPD) bridging collagen molecules have showed positive correlations with bone metastasis [16,17,18,19,20,21,22]. Multiple reports have reported that cancer patients with elevated levels of these BTMs in serum or urine are at a high risk for bone-specific recurrence, but not for other (soft tissue) metastases [18]. In addition to collagen degradation by-products, tartrate-resistant acid phosphatase (TRAP), an enzyme of osteoclasts, is highly detected in the serum of bone metastasis patients, and the receptor activator of nuclear factor κB ligand and osteoprotegerin ratio (RANKL/OPG), an index of osteoclastogenesis-related cytokines used to measure degree of bone resorption, were also significantly detected in the serum of breast cancer patients with bone metastasis, showing clinically applicable sensitivity and specificity [17, 22,23,24,25,26].
Regarding bone formation-related BTMs, there are many reports that peptides cleaved from pro-collagen such as pro-collagen type 1 N- and C- terminal propeptide (P1NP and P1CP) can be used as biomarkers for bone metastasis in breast and prostate cancer [16,17,18, 21,22,23, 27, 28]. P1NP is considered an useful diagnostic and prognostic factor for bone metastasis, supported by correlation of high serum P1NP levels with shorter time to the development of bone metastases and lower overall survival in patients with stage I–III breast cancer [27]. Alkaline phosphatase (ALP), an enzyme and differentiation marker of osteoblasts, is a representative BTM that has been shown to associate with bone metastasis in prostate cancer and solid tumors [14,15,16, 23, 29,30,31]. Although serum levels of osteocalcin (OCN), another marker of bone formation, have been suggested as a marker for bone metastasis, the fact that OCN reflects the response to treatment through its hormonal effects and inconsistent results in the non-small cell lung cancer (NSCLC) patients with bone metastasis raise questions about accuracy [32,33,34,35]. Rather than serum OCN levels, OCN-positive circulating osteoblastic cells as a cell-based biomarker showed a potential to be used as a biomarker for bone metastasis and will be discussed later in this article [36].
Although BTMs are robust markers for bone metastasis, BTMs are not cancer type specific but are a general marker of bone metabolism, and the usefulness of BTMs is limited by cancer patients’ characteristics that can affect levels of BTMs, such as age, sex, underlying kidney and/or liver diseases, and hormonal therapy [11]. Indeed, most of the studies compared cancer patients with or without bone metastasis with healthy subjects, not with the benign metabolic bone disease or fracture patients who can have comparable changes of BTMs. Further study is needed to validate it. Furthermore, due to its wide range of sensitivity and specificity values, the use of BTM as a sole marker cannot yet allow the replacement of imaging techniques (sensitivity and specificity for each biomarker are summarized in Supplementary Table 1). However, with the advantage of liquid biopsy diagnosis that multiple biomarkers can be measured at once, it may be possible to overcome sensitivity and specificity problems with a combination of biomarkers and to make a personalized diagnosis.
Additional protein-based biomarkers
Several studies have indicated that Wnt signaling is closely related to bone metastasis. Therefore, proteins secreted by cancer cells that alter the activity of Wnt signaling are involved in bone metastasis. Sclerostin (SOST), a Wnt inhibitor, is highly secreted by metastatic breast and prostate cancer and promotes osteolysis by inhibiting osteoblast differentiation [37, 38]. A Wnt-antagonist dickkopf-1 (DKK1) is increased in bone metastatic breast cancer and prompts osteoblast apoptosis [39, 40]. Serum levels of DKK1 have also been identified elevated in NSCLC patients with bone metastasis [41].
High plasma osteopontin (OPN) level was associated with distant metastases and low survival rates in renal cell carcinoma (RCC) patients, and OPN alone or in combination with BTMs showed significant differences secondary to presence or absence of bone metastases or survival rates [42, 43]. In a retrospective study of serum levels of parathyroid hormone-related peptide (PTHrP) in hypercalcemic lung cancer patients, high PTHrP was found to associate with increased bone metastasis incidence and decreased median survival [44]. Furthermore, in breast cancer, PTHrP (amino acids 12–48) levels were significantly increased in the plasma of patients with bone metastasis than in patients without bone metastasis, and the clinical measurement of PTHrP (12–48) in combination with NTX improved the detection of bone metastasis [45]. Prostate-specific antigen (PSA) as a bone metastasis marker of prostate cancer is controversial. However, there is an opinion that bone scans should be considered because patients with high PSA (≥ 20 ng/mL), locally advanced disease, or a Gleason score of 8 or higher have a higher risk of bone metastasis [46]. Although PSA alone cannot be used as a marker for bone metastasis in prostate cancer, PSA may be useful in combination with other markers [47].
Additional circulating effectors of bone metastasis have been investigated, including prolactin. High expression of the prolactin receptor (PRLR) in primary breast cancer cells was found to correlate with a shorter time to relapse, including relapse in bone, which results from increased osteoclast differentiation followed by osteolysis and secretion of the growth factors [48]. Serum CCL20 levels were significantly increased in renal cancer patients with bone metastasis [49]. Lee et al. demonstrated that metastasis-free survival and overall survival were decreased in breast cancer patients with high CCL20 expression, and administration of anti-CCL20 antibodies inhibited osteolytic breast cancer bone metastasis in mice [50]. Stress-induced phosphoprotein-1 (STIP1) was highly expressed both intracellularly and extracellularly in bone metastatic tissue samples from RCC patients and was upregulated in bone-seeking cells. STIP1 promoted the proliferation and migration/invasion of RCC tumor cells, while secreted STIP1 increased the differentiation and activation of osteoclasts along with increased cathepsin K production [51]. In addition, Tiedemann et al. showed that L-plastin is released through exosomes in human breast cancer cell lines, and suggested that L-plastin and peroxiredoxin-4 (PRDX4) secreted from breast cancer cells, as mediators of osteoclastogenesis, can contribute to bone colonization in a number of osteotropic cancers such as breast cancer, prostate cancer, and kidney cancer [52]. The above-mentioned circulating protein-based biomarkers are summarized in Table 1.
Here, we have addressed circulating protein markers identified in cancer patients and several potential targets in ongoing studies. Although many basic studies suggest new candidates for bone metastasis cancer markers, intensive follow-up studies are needed to determine whether they are clinically meaningful.
Cell-based biomarkers of bone metastasis
Circulating tumor cells (CTCs)
It has long been known that CTCs are detectable in cancer patients’ bloodstream. Numerous studies have reported that CTCs are closely associated with prognosis, tumor growth, molecular subtypes, and aggressiveness. Regarding bone metastasis, multiple groups reported clinical correlation between bone metastasis and CTCs in prostate, breast, and lung cancers [53,54,55,56,57]. These studies used quantity (i.e., cell numbers) and specific molecular markers, such as TFF-1, RANK, and CXCR4, for the diagnostic value of CTCs. Notably, Zhu et al. collected bilateral bone marrow and blood samples from breast cancer patients without clinical bone metastasis before surgery and identified that all patients with CTC had bone micro-metastases. In contrast, there was no significant correlation between CTC and sentinel lymph node metastasis. The authors concluded that CTC serves as a predictive marker for bone micro-metastasis in breast cancer patients [58, 59]. More recently, Trapp et al. performed a prospective clinical trial (SUCCESS A trial, NCT02181101), and measured CTCs in metastatic breast cancer patients before and after chemotherapy. The authors found that patients with CTCs at both time points showed bone-only first metastatic lesions as well as multiple-site first metastatic lesions more frequently than patients without detectable CTCs [60]. The authors concluded that CTCs might serve as a liquid biopsy surveillance marker for risk stratification for further adjuvant add-on treatments. A more extensive review of CTCs was published by Iuliani et al. [61]
In contrast to significant amount of data and publications supporting the role of CTCs in cancer progression in the past two decades, as well as a few biotech companies with advanced detection technologies and U.S. Food and Drug Administration (FDA) approvals, CTCs have not yet entered oncology clinics as a standard technology. The most significant issue is that not all CTCs are the same in the disease progression. It is unclear whether CTCs enter the systemic circulation via an active biological mechanism or via shedding from the primary tumor mass or both. Extensive further investigation is required to characterize distinct molecular characteristics using multi-omics approaches [62]. One of the major hurdles for this approach is the expansion of isolated CTCs in vitro for further analysis. Microfluidic chips and biological capture technologies have been developed and are currently under optimization for CTCs [63,64,65].
Osteoblast-lineage cells in the circulation
In 2005, the Khosla group at Mayo Clinic first reported that osteoblast-lineage cells exist in the human blood circulation [66]. Subsequently, these circulating osteoblast-lineage cells (cOB) were further characterized to contain two distinct populations (i.e., CD34 positive and negative populations). CD34+ cOB have low granularity and a small cell phenotype, whereas CD34− cOB have a larger and more granular phenotype [67]. Numerous subsequent reports from the same group and others have been published afterward, but the biological function and clinical significance of cOB have yet to be more extensively investigated. In the original discovery paper, the Khosla group demonstrated that adolescents in the active bone growth period have higher levels of cOB compared with adults. cOB also positively correlate with the pathologic changes of bone turnover in fracture, hypoparathyroidism, hereditary heterotopic ossification, or diabetes [68,69,70,71,72], supporting that cOB reflect changes of bone turnover and/or bone micro-architecture in either physiologic or pathologic status.
We recently performed a clinical study and demonstrated that the level of cOB, defined by CD15−CD34−Ocn+ cells in the peripheral blood mononuclear cells (PBMCs), predicted bone metastasis progression significantly earlier than the standard image-based diagnostics (computed tomography or bone scans). Briefly, we measured the level of cOB by flow cytometry in 92 Korean breast cancer patients and followed them up for disease progression for 18 months. The patients who had higher levels of cOB (the cutoff value was 0.069% of total CD45+ PBMCs) among those who did not have clinical metastatic lesions developed de novo bone metastasis within 18 months. In addition, among those who had bone metastasis at enrollment, bone metastases in patients who had higher levels of cOB (the cutoff value was 0.045% of CD45+ cells) progressed during the 18-month follow-up by bone scan images. Additional in vivo murine pre-clinical studies confirmed that cOB increased at early time points when bone micro-metastases were evident only by histology but undetectable by bioluminescence imaging. In addition, we found that cOB increased in the early phase of bone metastasis and could predict the progression of bone metastatic lesions. Taken together, the study demonstrated the clinical utility of cOB in diagnosing and monitoring bone metastasis progression in breast cancer patients [36, 73]. More recently, the Faccio group at the University of Washington in St. Louis demonstrated, using murine tumor models, that osterix (Osx or Sp7)-positive cells from the bone marrow are the origin of cancer-associated fibroblasts (CAF) in primary breast cancer tissue (the American Society for Bone and Mineral Research Annual Meeting 2022 Abstract No. 1030), and these Osx + osteoblast-lineage cells are detectable in blood samples (~ 1.5% of CD45+ cells). However, the authors did not confirm the detectability of Osx+ cells in human patient blood samples.
The roles of osteoblast-lineage cells in breast and prostate cancer bone metastasis progression have long been postulated and investigated by multiple groups [6, 74,75,76,77,78]. For example, osteoblasts provide an endosteal niche for bone metastatic prostate and breast cancer cells during dormancy and subsequent metastatic outgrowth. In addition, osteoblast-derived cytokines and growth factors, most notably receptor activator of nuclear factor kappa-B ligand (RANKL), contribute to tumor growth, angiogenesis, and osteolysis in the metastatic bone microenvironment. In contrast, the roles of osteoblast-lineage cells in the circulation started to draw attention only recently.
Platelets, neutrophils, and other myeloid-lineage cells
Bone is a primary immune organ where majority of immune cells originate. In this sense, given that bone metastatic tumor cells interact with nearly all types of cells in the bone microenvironment, bone marrow cells—particularly mature and pre-mature immune cells—are speculated to play a role in the progression of bone metastasis. Notably, neutrophils, representing 50–70% of myeloid-lineage cells, have been shown to contribute to tumorigenesis, metastasis, and patient prognosis. The quantity of tumor-associated neutrophils (TAN) and neutrophil-to-lymphocyte ratio have been shown to correlate with poor prognosis by multiple investigators [79]. For bone metastasis, Thio et al. analyzed 1,012 patients in a retrospective cohort and found that both neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independently associated with survival in patients who are treated for bone metastasis [80]. In addition, Wang et al. reported that high neutrophil-to-lymphocyte ratio was associated with poor prognosis [81]. However, there are smaller clinical cohort data contradicting the correlation between neutrophils and bone metastasis [82].
In addition to neutrophils, platelets have been known for decades as an important contributing factor for metastasis. Platelet integrin, αIIbβ3, is known to involve in circulating tumor cell adhesion and invasion [83, 84]. In addition, β3 knockout mice showed 95% decrease in development of bone metastasis in an intra-cardiac injection bone metastasis mouse model [85] and also showed reduced osteolysis [86]. However, there are currently no evidence for the association between platelet counts and bone metastasis, and for diagnostic value of platelet counts in oncology clinic.
More recently, myeloid-derived suppressor cells (MDSC), immature bone marrow-derived cells in the circulation with strong suppressive activity for anti-tumoral T cells, emerge as a biomarker for bone metastasis. Park et al. demonstrated using a chemotherapy-induced bone marrow expansion mouse model that CD11+ immature myeloid cells contribute to bone metastasis [87]. More recently, by analyzing more than 100 breast cancer patient blood samples, the same group demonstrated that monocytic subtype of MDSC is closely associated with bone metastasis but not with other soft tissue metastasis [88]. However, more research is required to develop MDSC as a biomarker for bone metastasis.
Conclusion
Biomarkers for early diagnosis prior to the initiation of massive osteolysis and for monitoring disease progression and/or therapeutic responses remain major clinical unmet needs in oncology practices. Diverse diagnostic markers and technologies including liquid biopsy are under extensive research and development. This review article summarized the updated information on BTM and cell-based biomarkers for bone metastasis. In conclusion, BTMs and serum protein biomarkers have been classically used, but their clinical utility has limitations in specificity and sensitivity. Recently, various biomarkers based on pathophysiological studies of bone metastasis have been proposed, but extensive further research is still required before clinical application. Meanwhile, liquid biopsy-based diagnostic techniques recently are spotlighted and currently tried as specific diagnostic methods for bone metastatic cancer. In particular, cOB may be a strong candidate marker specifically for bone metastasis compared with CTCs and other cell-based markers. Providing rationale for further research and development.
References
Hofbauer LC, Rachner TD, Coleman RE, Jakob F (2014) Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol 2:500–512. https://doi.org/10.1016/S2213-8587(13)70203-1
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593. https://doi.org/10.1038/nrc867
Eriksen EF, Axelrod DW, Melsen F (1994) Bone histomorphometry. Raven Press
Shiozawa Y, Pedersen EA, Havens AM et al (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121:1298–1312. https://doi.org/10.1172/JCI43414
Shiozawa Y, Pienta KJ, Taichman RS (2011) Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res 17:5553–5558. https://doi.org/10.1158/1078-0432.CCR-10-2505
Jeong HM, Cho SW, Park SI (2016) Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment. Endocrinol Metab 31:485–492. https://doi.org/10.3803/EnM.2016.31.4.485
Cook GJR, Azad GK, Goh V (2016) Imaging bone metastases in breast cancer: staging and response assessment. J Nucl Med 57:27S-33S. https://doi.org/10.2967/jnumed.115.157867
Goyal N, Kalra M, Soni A et al (2019) Multi-modality imaging approach to bone tumors - State-of-the art. J Clin Orthop Trauma 10:687–701. https://doi.org/10.1016/j.jcot.2019.05.022
Koizumi M, Matsumoto S, Takahashi S et al (1999) Bone metabolic markers in the evaluation of bone scan flare phenomenon in bone metastases of breast cancer. Clin Nucl Med 24:15–20. https://doi.org/10.1097/00003072-199901000-00004
Hirahata T, Ul Quraish R, Quraish AU et al (2022) Liquid biopsy: a distinctive approach to the diagnosis and prognosis of cancer. Cancer Inform 21:11769351221076062. https://doi.org/10.1177/11769351221076062
Coleman R, Costa L, Saad F et al (2011) Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol 80:411–432. https://doi.org/10.1016/j.critrevonc.2011.02.005
Coleman RE, Lipton A, Costa L et al (2013) Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumours and poor prognostic features-An exploratory analysis of placebo-controlled trials. J bone Oncol 2:70–76. https://doi.org/10.1016/j.jbo.2013.01.002
Tamiya M, Tokunaga S, Okada H et al (2013) Prospective study of urinary and serum cross-linked N-telopeptide of type I collagen (NTx) for diagnosis of bone metastasis in patients with lung cancer. Clin Lung Cancer 14:364–369. https://doi.org/10.1016/j.cllc.2012.11.006
Coleman RE, Major P, Lipton A et al (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23:4925–4935. https://doi.org/10.1200/JCO.2005.06.091
Brown JE, Cook RJ, Major P et al (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 97:59–69. https://doi.org/10.1093/jnci/dji002
Lara PNJ, Ely B, Quinn DI et al (2014) Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J Natl Cancer Inst 106:dju013. https://doi.org/10.1093/jnci/dju013
Jung K, Lein M, Stephan C et al (2004) Comparison of 10 serum bone turnover markers in prostate carcinoma patients with bone metastatic spread: diagnostic and prognostic implications. Int J cancer 111:783–791. https://doi.org/10.1002/ijc.20314
Brown J, Rathbone E, Hinsley S et al (2018) Associations between serum bone biomarkers in early breast cancer and development of bone metastasis: results from the AZURE (BIG01/04) trial. J Natl Cancer Inst 110:871–879. https://doi.org/10.1093/jnci/djx280
Lipton A, Chapman J-AW, Demers L et al (2011) Elevated bone turnover predicts for bone metastasis in postmenopausal breast cancer: results of NCIC CTG MA.14. J Clin Oncol 29:3605–3610. https://doi.org/10.1200/JCO.2010.31.5069
Kong QQ, Sun TW, Dou QY et al (2007) Beta-CTX and ICTP act as indicators of skeletal metastasis status in male patients with non-small cell lung cancer. Int J Biol Markers 22:214–220. https://doi.org/10.5301/jbm.2008.3777
Koopmans N, de Jong IJ, Breeuwsma AJ, van der Veer E (2007) Serum bone turnover markers (PINP and ICTP) for the early detection of bone metastases in patients with prostate cancer: a longitudinal approach. J Urol 178:849–853. https://doi.org/10.1016/j.juro.2007.05.029
Wada N, Fujisaki M, Ishii S et al (2001) Evaluation of bone metabolic markers in breast cancer with bone metastasis. Breast Cancer 8:131–137. https://doi.org/10.1007/BF02967492
Lumachi F, Basso SMM, Camozzi V et al (2016) Bone turnover markers in women with early stage breast cancer who developed bone metastases. A prospective study with multivariate logistic regression analysis of accuracy. Clin Chim acta 460:227–230. https://doi.org/10.1016/j.cca.2016.07.005
Lyubimova NV, Pashkov MV, Tyulyandin SA et al (2004) Tartrate-resistant acid phosphatase as a marker of bone metastases in patients with breast cancer and prostate cancer. Bull Exp Biol Med 138:77–79. https://doi.org/10.1023/b:bebm.0000046945.95479.d6
Salminen E, Ala-Houhala M, Korpela J et al (2005) Serum tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of skeletal changes in prostate cancer. Acta Oncol (Madr) 44:742–747. https://doi.org/10.1080/02841860500327586
Elfar GA, Ebrahim MA, Elsherbiny NM, Eissa LA (2017) Validity of Osteoprotegerin and Receptor Activator of NF-κB Ligand for the Detection of Bone Metastasis in Breast Cancer. Oncol Res 25:641–650. https://doi.org/10.3727/096504016X14768398678750
Oremek G, Sauer-Eppel H, Klepzig M (2007) Total procollagen type 1 amino-terminal propeptide (total P1NP) as a bone metastasis marker in gynecological carcinomas. Anticancer Res 27:1961–1962
Westerhuis LW, Delaere KP (1997) Diagnostic value of some biochemical bone markers for the detection of bone metastases in prostate cancer. Eur J Clin Chem Clin Biochem 35:89–94. https://doi.org/10.1515/cclm.1997.35.2.89
Brown JE, Cook RJ, Lipton A et al (2010) Prognostic factors for skeletal complications from metastatic bone disease in breast cancer. Breast Cancer Res Treat 123:767–779. https://doi.org/10.1007/s10549-010-0981-1
Du W-X, Duan S-F, Chen J-J et al (2014) Serum bone-specific alkaline phosphatase as a biomarker for osseous metastases in patients with malignant carcinomas: a systematic review and meta-analysis. J Cancer Res Ther 10:C140–C143. https://doi.org/10.4103/0973-1482.145842
Lipton A, Smith MR, Fizazi K et al (2016) Changes in bone turnover marker levels and clinical outcomes in patients with advanced cancer and bone metastases treated with bone antiresorptive agents. Clin cancer Res 22:5713–5721. https://doi.org/10.1158/1078-0432.CCR-15-3086
Zhao H, Han K-L, Wang Z-Y et al (2011) Value of C-telopeptide-cross-linked Type I collagen, osteocalcin, bone-specific alkaline phosphatase and procollagen Type I N-terminal propeptide in the diagnosis and prognosis of bone metastasis in patients with malignant tumors. Med Sci Monit 17:CR626-633. https://doi.org/10.12659/msm.882047
Arai Y, Takeuchi H, Oishi K, Yoshida O (1992) Osteocalcin: is it a useful marker of bone metastasis and response to treatment in advanced prostate cancer? Prostate 20:169–177. https://doi.org/10.1002/pros.2990200302
Terpos E, Kiagia M, Karapanagiotou EM et al (2009) The clinical significance of serum markers of bone turnover in NSCLC patients: surveillance, management and prognostic implications. Anticancer Res 29:1651–1657
Bayrak SB, Ceylan E, Serter M et al (2012) The clinical importance of bone metabolic markers in detecting bone metastasis of lung cancer. Int J Clin Oncol 17:112–118. https://doi.org/10.1007/s10147-011-0266-7
Lee K-H, Lee KJ, Kim T-Y et al (2020) Circulating Osteocalcin-positive cells as a novel diagnostic biomarker for bone metastasis in breast cancer patients. J Bone Miner Res 35:1838–1849. https://doi.org/10.1002/jbmr.4041
Larson SR, Zhang X, Dumpit R et al (2013) Characterization of osteoblastic and osteolytic proteins in prostate cancer bone metastases. Prostate 73:932–940. https://doi.org/10.1002/pros.22639
Hesse E, Schröder S, Brandt D et al (2019) Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. https://doi.org/10.1172/jci.insight.125543
Rachner TD, Göbel A, Thiele S et al (2014) Dickkopf-1 is regulated by the mevalonate pathway in breast cancer. Breast cancer Res 16:R20. https://doi.org/10.1186/bcr3616
Kasoha M, Bohle RM, Seibold A et al (2018) Dickkopf-1 (Dkk1) protein expression in breast cancer with special reference to bone metastases. Clin Exp Metastasis 35:763–775. https://doi.org/10.1007/s10585-018-9937-3
Qiao R, Zhong R, Chang Q et al (2017) Serum dickkopf-1 as a clinical and prognostic factor in non-small cell lung cancer patients with bone metastases. Oncotarget 8:79469–79479. https://doi.org/10.18632/oncotarget.18446
Ramankulov A, Lein M, Kristiansen G et al (2007) Elevated plasma osteopontin as marker for distant metastases and poor survival in patients with renal cell carcinoma. J Cancer Res Clin Oncol 133:643–652. https://doi.org/10.1007/s00432-007-0215-z
Ramankulov A, Lein M, Kristiansen G et al (2007) Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate 67:330–340. https://doi.org/10.1002/pros.20540
Hiraki A, Ueoka H, Bessho A et al (2002) Parathyroid hormone-related protein measured at the time of first visit is an indicator of bone metastases and survival in lung carcinoma patients with hypercalcemia. Cancer 95:1706–1713. https://doi.org/10.1002/cncr.10828
Washam CL, Byrum SD, Leitzel K et al (2013) Identification of PTHrP(12–48) as a plasma biomarker associated with breast cancer bone metastasis. Cancer Epidemiol Biomark Prev 22:972–983. https://doi.org/10.1158/1055-9965.EPI-12-1318-T
Abuzallouf S, Dayes I, Lukka H (2004) Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol 171:2122–2127. https://doi.org/10.1097/01.ju.0000123981.03084.06
Briganti A, Suardi N, Gallina A et al (2014) Predicting the risk of bone metastasis in prostate cancer. Cancer Treat Rev 40:3–11. https://doi.org/10.1016/j.ctrv.2013.07.001
Sutherland A, Forsyth A, Cong Y et al (2016) The role of prolactin in bone metastasis and breast cancer cell-mediated osteoclast differentiation. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv338
D’Amico L, Belisario D, Migliardi G et al (2016) C-met inhibition blocks bone metastasis development induced by renal cancer stem cells. Oncotarget 7:45525–45537. https://doi.org/10.18632/oncotarget.9997
Lee SK, Park K-K, Kim H-J et al (2017) Human antigen R-regulated CCL20 contributes to osteolytic breast cancer bone metastasis. Sci Rep 7:9610. https://doi.org/10.1038/s41598-017-09040-4
Wang J, You H, Qi J et al (2017) Autocrine and paracrine STIP1 signaling promote osteolytic bone metastasis in renal cell carcinoma. Oncotarget 8:17012–17026. https://doi.org/10.18632/oncotarget.15222
Tiedemann K, Sadvakassova G, Mikolajewicz N et al (2019) Exosomal release of L-plastin by breast cancer cells facilitates metastatic bone osteolysis. Transl Oncol 12:462–474. https://doi.org/10.1016/j.tranon.2018.11.014
Helo P, Cronin AM, Danila DC et al (2009) Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with Cell Search assay and association with bone metastases and with survival. Clin Chem 55:765–773. https://doi.org/10.1373/clinchem.2008.117952
De Giorgi U, Valero V, Rohren E et al (2010) Circulating tumor cells and bone metastases as detected by FDG-PET/CT in patients with metastatic breast cancer. Ann Oncol 21:33–39. https://doi.org/10.1093/annonc/mdp262
Cheng M, Liu L, Yang H-S, Liu G-F (2014) Circulating tumor cells are associated with bone metastasis of lung cancer. Asian Pacif J cancer Prev 15:6369–6374. https://doi.org/10.7314/apjcp.2014.15.15.6369
Pantano F, Rossi E, Iuliani M et al (2020) Dynamic changes of receptor activator of nuclear factor-κB expression in circulating tumor cells during Denosumab predict treatment effectiveness in Metastatic Breast Cancer. Sci Rep 10:1288. https://doi.org/10.1038/s41598-020-58339-2
Rizzo FM, Vesely C, Childs A et al (2019) Circulating tumour cells and their association with bone metastases in patients with neuroendocrine tumours. Br J Cancer 120:294–300. https://doi.org/10.1038/s41416-018-0367-4
Zhu L, Loo WTY, Chow LWC (2005) Circulating tumor cells in patients with breast cancer: possible predictor of micro-metastasis in bone marrow but not in sentinel lymph nodes. Biomed Pharmacother 59:S355–S358. https://doi.org/10.1016/s0753-3322(05)80077-0
Zhu L, Loo WTY, Cheng CWN, Chow LWC (2006) Possible predictive markers related to micro-metastasis in breast cancer patients. Oncol Rep 15:1217–1223
Trapp EK, Fasching PA, Fehm T et al (2022) Does the presence of circulating tumor cells in high-risk early breast cancer patients predict the site of first metastasis-results from the adjuvant SUCCESS a trial. Cancers (Basel). https://doi.org/10.3390/cancers14163949
Iuliani M, Simonetti S, Ribelli G et al (2020) Current and emerging biomarkers predicting bone metastasis development. Front Oncol 10:789. https://doi.org/10.3389/fonc.2020.00789
Plaks V, Koopman CD, Werb Z (2013) Cancer circulating tumor cells. Science 341:1186–1188. https://doi.org/10.1126/science.1235226
Tian R, Li X, Zhang H et al (2022) Ulex Europaeus agglutinin-I-based magnetic isolation for the efficient and specific capture of SW480 circulating colorectal tumor cells. ACS Omega 7:30405–30411. https://doi.org/10.1021/acsomega.2c03702
Kolostova K, Spicka J, Matkowski R, Bobek V (2015) Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am J Transl Res 7:1203–1213
Yoon J, Yoon H-S, Shin Y et al (2017) Ethanol-dispersed and antibody-conjugated polymer nanofibers for the selective capture and 3-dimensional culture of EpCAM-positive cells. Nanomedicine 13:1617–1625. https://doi.org/10.1016/j.nano.2017.02.015
Eghbali-Fatourechi GZ, Lamsam J, Fraser D et al (2005) Circulating osteoblast-lineage cells in humans. N Engl J Med 352:1959–1966. https://doi.org/10.1056/NEJMoa044264
Dumez B, Van Damme K, Casteleyn L (2007) Research on the socio-ethical impact of biomarker use and the communication processes in ECNIS NoE and NewGeneris IP. Int J Hyg Environ Health 210:263–265. https://doi.org/10.1016/j.ijheh.2007.01.018
Suda RK, Billings PC, Egan KP et al (2009) Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells 27:2209–2219. https://doi.org/10.1002/stem.150
De Potter P, von Weymarn C, Zografos L (1991) In vivo phosphorus 31 magnetic resonance spectroscopy of human uveal melanomas and other intraocular tumors. Am J Ophthalmol 111:276–288. https://doi.org/10.1016/s0002-9394(14)72310-4
Undale A, Srinivasan B, Drake M et al (2010) Circulating osteogenic cells: characterization and relationship to rates of bone loss in postmenopausal women. Bone 47:83–92. https://doi.org/10.1016/j.bone.2010.03.018
Rubin MR, Manavalan JS, Dempster DW et al (2011) Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism. J Clin Endocrinol Metab 96:176–186. https://doi.org/10.1210/jc.2009-2682
Egan KP, Duque G, Keenan MA, Pignolo RJ (2018) Circulating osteogentic precursor cells in non-hereditary heterotopic ossification. Bone 109:61–64. https://doi.org/10.1016/j.bone.2017.12.028
Johnson RW (2020) The search for a bone metastasis biomarker may have a new find: circulating osteocalcin-positive cells. J Bone Miner Res 35:1836–1837
Lee C, Whang YM, Campbell P et al (2018) Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis. Cancer Lett 414:205–213. https://doi.org/10.1016/j.canlet.2017.11.016
Park SI, Lee C, Sadler WD et al (2013) Parathyroid hormone-related protein drives a CD11b+Gr1+ cell-mediated positive feedback loop to support prostate cancer growth. Cancer Res 73:6574–6583. https://doi.org/10.1158/0008-5472.CAN-12-4692
Zheng Y, Chow S-O, Boernert K et al (2014) Direct crosstalk between cancer and osteoblast lineage cells fuels metastatic growth in bone via auto-amplification of IL-6 and RANKL signaling pathways. J Bone Miner Res 29:1938–1949. https://doi.org/10.1002/jbmr.2231
Swami S, Johnson J, Bettinson LA et al (2017) Prevention of breast cancer skeletal metastases with parathyroid hormone. JCI Insight. https://doi.org/10.1172/jci.insight.90874
Devignes C-S, Aslan Y, Brenot A et al (2018) HIF signaling in osteoblast-lineage cells promotes systemic breast cancer growth and metastasis in mice. Proc Natl Acad Sci USA 115:E992–E1001. https://doi.org/10.1073/pnas.1718009115
Masucci MT, Minopoli M, Carriero MV (2019) Tumor associated neutrophils their role in tumorigenesis, metastasis Prognosis and Therapy. Front Oncol 9:1146. https://doi.org/10.3389/fonc.2019.01146
Thio QCBS, Goudriaan WA, Janssen SJ et al (2018) Prognostic role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with bone metastases. Br J Cancer 119:737–743. https://doi.org/10.1038/s41416-018-0231-6
Wang S, Zhang Z, Fang F et al (2011) The neutrophil/lymphocyte ratio is an independent prognostic indicator in patients with bone metastasis. Oncol Lett 2:735–740. https://doi.org/10.3892/ol.2011.304
Caliskan B, Korkmaz AN (2016) Can Neutrophil/Lymphocyte Ratio be a Predictor for Bone Metastases of Solid Tumors? World J Nucl Med 15:196–199. https://doi.org/10.4103/1450-1147.174711
Camerer E, Qazi AA, Duong DN et al (2004) Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 104:397–401. https://doi.org/10.1182/blood-2004-02-0434
Felding-Habermann B, Fransvea E, O’Toole TE et al (2002) Involvement of tumor cell integrin alpha v beta 3 in hematogenous metastasis of human melanoma cells. Clin Exp Metastasis 19:427–436. https://doi.org/10.1023/a:1016377114119
Bakewell SJ, Nestor P, Prasad S et al (2003) Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci USA 100:14205–14210. https://doi.org/10.1073/pnas.2234372100
Boucharaba A, Serre C-M, Grès S et al (2004) Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest 114:1714–1725. https://doi.org/10.1172/JCI22123
Park SI, Liao J, Berry JE et al (2012) Cyclophosphamide creates a receptive microenvironment for prostate cancer skeletal metastasis. Cancer Res 72:2522–2532. https://doi.org/10.1158/0008-5472.CAN-11-2928
Lee EJ, Jung S, Park KH, Park SI (2022) Flow cytometry-based immunophenotyping of myeloid-derived suppressor cells in human breast cancer patient blood samples. J Immunol Methods 510:113348. https://doi.org/10.1016/jj.im.2022.113348
Funding
This study was supported by the National R&D Program for Cancer Control, the Ministry of Health and Welfare, the Republic of Korea (HA17C0040) and the National Research Foundation of Korea (2019R1A2C1085672, 2020R1A2C1012966 and 2022R1A6A3A01087551).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Sun Wook Cho is a co-founder and CEO of Cellus, Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Song, MK., Park, S.I. & Cho, S.W. Circulating biomarkers for diagnosis and therapeutic monitoring in bone metastasis. J Bone Miner Metab 41, 337–344 (2023). https://doi.org/10.1007/s00774-022-01396-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01396-6