Abstract
There is currently insufficient information on serum 25-hydroxyvitamin D (25OHD) and parathyroid hormone (PTH) concentrations, and bone mineral status in healthy adolescents to allow reference values to be set. This study aimed to provide comparable data on vitamin D status in Japanese adolescents and to assess sex differences in susceptibility to vitamin D insufficiency. Serum 25OHD and PTH concentrations were measured in 1,380 healthy adolescents (aged 12–18 years). Subjects completed a questionnaire on exercise history, diet, and lifestyle factors. Calcaneal stiffness was evaluated by quantitative ultrasound. Serum 25OHD concentrations in boys and girls were 60.8 ± 18.3 and 52.8 ± 17.0 nmol/L, respectively. Approximately 30 % of boys and 47 % of girls had suboptimal 25OHD concentrations (<50 nmol/L). Serum PTH concentration was negatively correlated with serum 25OHD concentration in boys, but negatively correlated with calcium intake rather than serum 25OHD in girls. In contrast, the increment in calcaneal stiffness as a result of elevation of serum 25OHD was higher in girls than in boys. As vitamin D deficiency is common in Japanese adolescents, it was estimated that intakes of ≥12 and ≥14 μg/day vitamin D would be required to reach 25OHD concentrations of 50 nmol/L in boys and girls, respectively. Moreover, the results of the present study indicate that vitamin D deficiency has a greater association with calcaneal stiffness in girls than in boys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D plays an important role in the regulation of calcium homeostasis and bone metabolism. Among the vitamin D metabolites, serum 25-hydroxyvitamin D (25OHD) concentration is the best indicator of vitamin D status. Vitamin D insufficiency is known to cause secondary hyperparathyroidism, which adversely affects bone metabolism in the elderly [1]. Negative correlations between serum 25OHD and parathyroid hormone (PTH) concentrations have been observed in children and adolescents [2–7], while an association between serum 25OHD concentration and bone mineral density (BMD) has also been reported in adolescents [8]. However, there is limited evidence regarding the associations between vitamin D status and serum PTH concentration and BMD in Japanese adolescents. Serum PTH concentration is known to be regulated not only by vitamin D status, but also by dietary calcium intake [9], and dietary calcium intake in Japanese, including adolescents, is lower than in Europeans and Americans [10–12]. These facts suggest that vitamin D status may be more important for regulating serum PTH concentrations and bone metabolism in adolescents with low calcium intake, such as Japanese adolescents.
Differences in the importance of vitamin D for bone growth between boys and girls may be expected to exist during puberty because of differences in patterns of bone growth. However, little is known about sex differences in the associations between vitamin D status and serum PTH concentration and bone metabolism in adolescents.
The aim of this study was to provide comparable data on vitamin D status to allow the establishment of a reference values of serum 25OHD concentration or vitamin D intake in adolescents and to examine sex differences in the association between vitamin D status and bone mineral status.
Materials and methods
Subjects
We recruited adolescents aged 12–18 years (n = 1415) through an annual health examination at a Junior and Senior High School located in urban Tokyo. They were all selected from the same Junior and Senior High School. Subjects who had suffered from acute infection or who had chronic diseases such as diabetes mellitus, kidney disease, bone metabolic disease or inheritable metabolic diseases were excluded. Subjects whose serum parameters could not be measured due to an insufficient blood sample were also excluded. Finally, a total of 1,380 healthy adolescents from first grade of Junior High School (1st JHS) (192 boys and 197 girls, aged 12–13 years), first grade of High School (1st HS) (247 boys and 279 girls, aged 15–16 years), and third grade of High School (3rd HS) (223 boys and 242 girls, aged 17–18 years) were enrolled. The health status of the participants was defined based on health history, questionnaire, and serum biochemical parameters.
Measurements
Blood samples were obtained in May 2003 and 2004. Fasting blood samples were collected by venipuncture at school in the morning, centrifuged at 1,940×g for 15 min at 4 °C, and the supernatant was stored at −35 °C until assayed. Serum 25OHD was determined using the LIAISON 25OH Vitamin D TOTAL assay (DiaSorin Inc, Stillwater, MN, USA), which is an automated competitive chemiluminescence immunoassay (CLIA) [13]. Circulating level of intact PTH was measured by chemiluminescent immunoassay (CLIA) (LIAISON® N-TACT® PTH II Assay). Height, weight, and body mass index were measured for all subjects, and they also completed a questionnaire on exercise history, diet, and lifestyle factors. Vitamin D and calcium intake were assessed using a food-frequency questionnaire (FFQ) [14], based on the semi-quantified FFQ developed by the Drafting Committee of the Ministry of Health and Welfare for Health Index. The FFQ has been shown to be a useful tool for evaluating dietary calcium and vitamin D intake (coefficients of variance of four repeated measurements of intakes throughout 1 year were 14.1 % for calcium and 13.6 % for vitamin D). Calcaneal skeletal status was evaluated by quantitative ultrasound (QUS) measurements at the heel, using the Achilles system A-1000 (GE-Lunar, Madison, WI, USA), which measures speed of sound (SOS) in m/s and broadband ultrasound attenuation (BUA) in dB/MHz. The Achilles software was also used to calculate a stiffness index, which is a combination of both BUA and SOS. Calcaneal stiffness Z score were calculated using Japanese age-matched reference data provided by GE-Lunar (Madison, WI, USA).
Statistical analysis
All statistical analyses were performed using statistical software JMP 7.0J (SAS Institute Inc, Cary, NC, USA). Analysis of variance (ANOVA) was performed to determine the significance of differences in anthropometric parameters, serum 25OHD and intact PTH concentrations, bone mass parameters, and vitamin D and calcium intakes among school grades. Student’s t tests were used to compare parameters between the sexes.
No definite 25OHD threshold for defining vitamin D deficiency/insufficiency has yet been established, and the proposed reference value varies among studies [15–17]. The Institute of Medicine of the National Academies in USA/Canada recently proposed 50 nmol/L 25OHD as a reference value to define vitamin D sufficiency [18]. Based on these reports, we evaluated the frequencies of vitamin D deficiency/insufficiency using the following serum 25OHD concentrations—<12.5 nmol/L, severe vitamin D deficiency; 12.5 to <25 nmol/L, vitamin D deficiency; 25 to <50 nmol/L, mild vitamin D deficiency; 50 to <75 nmol/L, vitamin D insufficiency; ≥75 nmol/L, vitamin D sufficiency.
Serum 25OHD concentrations required to achieve plateau PTH concentrations were calculated using the method reported by Guillemant et al. [3]. The model used was \( {\text{iPTH }}\left( {\text{pg/mL}} \right) = a + b \, \exp \left[ {c \times 2 5\left( {\text{OH}} \right){\text{D}}\left( {\text{nmol/L}} \right)} \right].\) Although this curve is asymptotic, it is usual to estimate that an iPTH ‘plateau’ is reached when \( \left[ { 2 5\left( {\text{OH}} \right){\text{D}}} \right] = 3/c \).
The vitamin D intake required to achieve a serum 25OHD concentration of 50 nmol/L was calculated using a logarithmic regression curve of vitamin D intake and serum 25OHD concentration obtained by Passing and Bablok regression analysis. Generally, an equation of regression is calculated by the least square method. However, in the least square method, errors of variables are considered in the Y axis but are not considered in the X axis. Therefore, variables on the X and Y axes cannot be exchanged because this exchange causes a different relationship between the 2 variables that should be compared. However, error must be considered for each variable of serum 25OHD and PTH concentrations. Thus, we used the Passing and Bablok regression method, which is able to obtain linear regression considering errors in X and Y variables. The merit of this method is that the relationship of 2 variables does not change even if variables in X and Y are exchanged because the calculation of regression is based on the median of the slope between all 2 points that were plotted on a scatter diagram.
Stepwise multiple linear regression analyses were performed to explore the determinants of serum intact PTH concentration or calcaneal stiffness Z score. Significant variables detected in simple linear regression analysis were included in the original model as plausible predictors (for PTH concentration—serum 25OHD concentration, calcium intake, and vitamin D intake; for calcaneal stiffness Z score—body weight, serum 25OHD concentration, calcium intake, vitamin D intake, and exercise). A forward stepwise regression was performed, and a P value >0.10 was used for variable removal.
To assess sex differences in the association between vitamin D status and calcaneal stiffness, subjects were divided into two groups according to serum 25OHD concentration—subjects with serum 25OHD concentrations <50 nmol/L (L-25OHD), and those with serum 25OHD concentrations ≥50 nmol/L (H-25OHD). Differences in calcaneal stiffness Z score between the L-25OHD and H-25OHD groups for each sex were evaluated using Student’s t tests. Subjects were also divided into four groups according to serum 25OHD concentration (same threshold as above) and calcium intake.
Threshold calcium (Ca) intake values were based on the recommended daily allowances (RDAs) according to the Dietary Reference Intakes for Japanese 2010 [19] [boys—1,000 mg/day (12–14 years), 800 mg/day (15–18 years); girls—800 mg/day (12–14 years), 650 mg/day (15–18 years)] (<RDA: L-Ca, ≥RDA: H-Ca). Differences in calcaneal stiffness Z score among the four groups were evaluated by ANOVA and Tukey–Kramer’s honest significant difference test.
Ethical considerations
The comprehensive study protocol, including nutritional evaluation, was reviewed by the ethics committee of Kagawa Nutrition University and comprehensive written informed consent was obtained from all participants.
Results
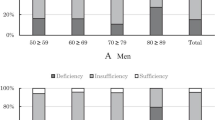
The subject characteristics are summarized in Table 1. Serum 25OHD concentrations in boys and girls were 60.8 ± 18.3 and 52.8 ± 17.0 nmol/L, respectively. Serum 25OHD concentrations of girls were significantly lower than those of boys in all age groups. Approximately 30 % of boys and 47 % of girls had <50 nmol/L 25OHD concentration, and approximately 80 % of boys and 90 % of girls had <75 nmol/L 25OHD concentration. Overall, obvious vitamin D deficiency, defined as serum 25OHD concentration <25 nmol/L, was observed in eight boys and 19 girls (Fig. 1). There was no significant difference in serum 25OHD concentration among school grades in boys, but serum 25OHD levels decreased significantly with age in girls. In 3rd HS girls, serum 25OHD concentration was 50.3 ± 18.5 nmol/L which was approximately 5 nmol/L lower than concentration of 1st JHS girls, and more than half of girls had <50 nmol/L 25OHD concentration. Exercise habit and the ratio of outdoor exercise were much higher in boys than in girls (Table 1). Although vitamin D intake did not differ between boys and girls, serum 25OHD concentrations were lower in girls than in boys.
Distribution of serum 25OHD concentration in adolescent boys and girls. Vitamin D deficiency/insufficiency was defined using serum 25OHD concentration thresholds as follows—<12.5 nmol/L, severe vitamin D deficiency; 12.5 to <25 nmol/L, vitamin D deficiency; 25 to <50 nmol/L, mild vitamin D deficiency; 50 to <75 nmol/L, vitamin D insufficiency; ≥75 nmol/L, vitamin D sufficiency
Intact PTH concentration in boys and girls were 39.0 ± 17.1 and 38.0 ± 15.4 pg/mL, respectively (Table 1). Intact PTH concentration decreased significantly with age in both boys and girls, with no significant difference between the sexes. Decrements of intact PTH concentration from 1st JHS to 3rd HS in both boys and girls were approximately 10 pg/mL. Although calcaneal stiffness was higher in girls than in boys in 1st JHS (12–13 years), the high rate of bone growth in boys led to a reversal of this phenomenon in 3rd HS (17–18 years). Calcium intake among high school students was higher in boys than in girls (Table 1).
In simple regression analysis, a negative correlation between serum 25OHD and PTH concentration was observed in boys. In girls, however, a significant correlation was observed only in 3rd HS (Fig. 2). Therefore, we evaluated the serum 25OHD concentrations required to achieve plateau PTH concentrations in boys (12–18 years) and 3rd HS girls (17–18 years) using the method reported by Guillemant et al. (Fig. 3). The equations of the regression curves of boys (12–18 years) was \( {\text{intactPTH }}\left( {{\text{pg}}/{\text{mL}}} \right) = 1 5. 2 1 + 3 8. 2 5\,\,{\text{exp}}\left[ { - 0.00 7 9 2 \times 2 5 {\text{OHD }}\left( {{\text{nmol}}/{\text{L}}} \right)} \right], \) and that of girls (17–18 years) was \( {\text{intactPTH }}\left( {{\text{pg}}/{\text{mL}}} \right) = 2. 8 9 + 3 4. 7 6\,\,{\text{exp}}\left[ { - 0.00 3 4 4 \times 2 5 {\text{OHD }}\left( {{\text{nmol}}/{\text{L}}} \right)} \right] \). Plateau was considered to be reached for a concentration of 25OHD equal to 3 divided by 0.00792 or 0.00344. Those values were 379 nmol/L for boys and 872 nmol/L for girls, which were unrealistic concentrations. To be compared with other studies, the regression curves of French boys [3] and Finnish girls [4] are shown together in this figure. The serum 25OHD concentrations required to achieve plateau PTH concentrations are 83 nmol/L for French boys [3] and 273 nmol/L for Finnish girls [4].
Comparison of the relationship between serum 25-hydroxyvitamin D (25(OHD) and intact PTH concentrations in boys and girls. The equations of the regression curves are—boys (12–18 years): \( {\text{intactPTH }}\left( {{\text{pg}}/{\text{mL}}} \right) = 1 5. 2 1 + 3 8. 2 5\,\,{\text{exp}}\left[ { - 0.00 7 9 2 \times 2 5 {\text{OHD }}\left( {{\text{nmol}}/{\text{L}}} \right)} \right] \); girls (17–18 years): \( {\text{intactPTH }}\left( {{\text{pg}}/{\text{mL}}} \right) = 2. 8 9 + 3 4. 7 6\,\,{\text{exp}}\left[ { - 0.00 3 4 4 \times 2 5 {\text{OHD }}\left( {{\text{nmol}}/{\text{L}}} \right)} \right] \). Plateau was considered to be reached for a concentration of 25OHD equal to 3 divided by 0.0198 or 0.0083. Those values were 379 nmol/L for boys and 872 nmol/L for girls. To be compared with other studies, the regression curves of French boys and Finnish girls are shown in this figure. These equations of the regression curves are—French boys (14–16 years): \( {\text{intactPTH }}\left( {{\text{pg}}/{\text{mL}}} \right) = 20. 3 7 + 3 4. 7 8\,\,{\text{exp}}\left[ { - 0.0 3 6 2 \,\times\, 2 5 {\text{OHD }}\left( {{\text{nmol}}/{\text{L}}} \right)} \right] \); Finnish girls (13–16 year): \( {\text{intactPTH }}\left( {{\text{pg}}/{\text{mL}}} \right) = 1 2.1 + 27.0\,\,{ \exp }\left[ { - 0.0 1 1\,\times\,2 5 {\text{OHD }}\,\,\left( {{\text{nmol}}/{\text{L}}} \right)} \right] \). Plateau was considered to be reached for a concentration of 25OHD equal to 3 divided by 0.0362 or 0.011. Those values were 83 nmol/L for French boys and 273 nmol/L for Finnish girls. * Guillemant et al. [3], ** Outila et al. [4]
Negative correlations between serum 25OHD and calcaneal stiffness in simple regression analysis were observed in both boys (P = 0.029, r 2 = 0.007) and girls (P < 0.001, r 2 = 0.049) (Fig. 4).
Figure 5 shows the association between serum 25OHD concentration and vitamin D intake. Significant positive associations were observed in all subjects (all boys and all girls). Based on these associations, the vitamin D intake required to reach a serum 25OHD concentration of 50 nmol/L was estimated by the logarithmic equation \( { \log }\left( { 2 5 {\text{OHD}}} \right) = a + {\text{b}}\,\,{ \log }\left( {\text{vitamin D intake}} \right) \) or \( { \log }\left( { 2 5 {\text{OHD}}} \right) = {\text{a}} + {\text{b}}^{\prime }\,\, { \log }\left( {\text{vitamin D intake}} \right) \). Slope b is the ordinary slope (solid line) and slope b′ is the lower slope limit of the 95 % confidence interval (dashed line). The results indicated that an intake of approximately 10 μg/day of vitamin D was required on average, and that approximately >12, >12 and >14 μg/day vitamin D would be required to reach 50 nmol/L serum 25OHD concentrations for all subjects, boys and girls, respectively.
Correlations between vitamin D intake and serum 25OHD concentrations in boys and girls. Solid lines show the logarithmic equation \( { \log }\left( { 2 5 {\text{OHD}}} \right) = a + b\,\,{ \log }\left( {\text{vitamin D intake}} \right) \). Dashed lines show the logarithmic equation \( { \log }\left( { 2 5 {\text{OHD}}} \right) = a + b^{\prime }\,\, { \log }\left( {\text{vitamin D intake}} \right) \) using the lower slope limit (b′) of the 95 % confidence interval. a All subjects: a = 0.409, b = 1.568, b′ = 1.406, b boys: a = 1.190, b = 1.254, b′ = 1.080, c girls: a = −0.101, b = 1.765, b′ = 1.509. Vitamin D intakes required to achieve a serum 25OHD concentration of 50 nmol/L were estimated using these equations. 25OHD 25-hydroxyvitamin D
Table 2 shows the independent parameters associated with serum intact PTH concentration and calcaneal stiffness Z score evaluated by stepwise multiple regression analysis. First, stepwise multiple linear regression analyses were performed to explore determinants of serum intact PTH concentration. Plausible predictors (plasma 25OHD concentration, calcium intake, and vitamin D intake) were included in the original model. Forward stepwise regression was performed, and a P value of >0.1 was used for adjustment/removal of the variable. Only significant independent variables are shown in Table 2. In boys, serum 25OHD concentration (P < 0.001) and daily calcium intake (P = 0.011) were independently and negatively associated with serum intact PTH concentration. Additionally, serum 25OHD concentration was negatively associated with serum intact PTH concentration in boys in all age groups. In contrast, only calcium intake was independently and negatively associated with serum intact PTH concentration in girls. Calcium intake was a significant variable in the 1st JHS and 1st HS groups in girls in all age groups, whereas 25OHD concentration was a significant variable only in 3rd HS girls.
Next, stepwise multiple linear regression analyses were performed to explore determinants of calcaneal stiffness Z score. Plausible predictors (body weight, serum 25OHD concentration, calcium intake, vitamin D intake, and exercise) were included in the original model. Forward stepwise regression was performed as described above. Body weight, exercise, and serum 25OHD concentration were independently associated with calcaneal stiffness Z score in all boys and girls (Table 2). 25OHD concentration was a significant variable affecting calcaneal stiffness in girls in all age groups, while calcium intake was also identified as a significant variable except in the 3rd HS group. These results suggest that serum 25OHD concentration has a greater association with calcaneal stiffness in girls than in boys.
Figure 6 shows the association between vitamin D status and calcium intake and calcaneal stiffness Z score. The Z scores in the H-25OHD groups were significantly higher than in the L-25OHD groups for both sexes (Fig. 6a). The difference between L-25OHD and H-25OHD groups was more significant in girls than in boys. Moreover, subgroup analysis identified significant and stronger associations between both vitamin D status and calcium intake in girls compared with boys (Fig. 6b, c). These results suggest that calcaneal stiffness might be more susceptible to 25OHD concentration and calcium intake in girls than in boys. Furthermore, Fig. 6 indiates that vitamin D status has more impact on bone than calcium intake in both sexes.
Associations between vitamin D status and calcium intake and calcaneal stiffness in boys and girls. Subjects were divided according to serum 25OHD concentration and calcium intake. L-25OHD and H-25OHD—<50 nmol/L and ≥50 nmol/L serum 25OHD concentration, respectively. L-Ca and H-Ca—<RDA and ≥RDA calcium intake, respectively. a L-25OHD and H-25OHD groups were compared using Student’s t tests. *P < 0.05, ***P < 0.001. b, c Differences in calcaneal stiffness Z score among the four groups were evaluated by ANOVA and Tukey–Kramer’s honest significant difference test. Significant differences are between the groups which are not connected by the same letter (a, b or c). Values given are means and standard errors (SE)
Discussion
Associations between serum 25OHD concentration and serum PTH concentration and BMD in adolescents have mainly been reported in the USA and Europe [3–5, 20–30]. However, scientific evidence regarding vitamin D status during adolescence remains scarce, not only in the USA and Europe, but also in Asian countries, including Japan. We therefore assessed vitamin D status in Japanese adolescents. Serum 25OHD concentrations in Japanese adolescents were similar to those reported in USA and European adolescents [3, 4, 9, 22, 28–31], and higher than those in Indian and Chinese adolescents [31–33]. The serum 25OHD concentration in girls was significantly lower than in boys, and decreased significantly with age. However, Gonzalez-Gross et al. reported that the 25OHD concentration was higher in girls than in boys and increased with age [27], while other studies showed a significant reduction in serum 25OHD concentration according to increasing age in adolescents [17], or lack of an association between 25OHD concentration and age [29]. These inconsistent results in adolescents suggest that region-specific lifestyles may be an important factor influencing 25OHD concentration during adolescence. The present study found that, although vitamin D intake did not differ between boys and girls, serum 25OHD concentration was lower in girls than in boys. One possible explanation for this may be the higher percentage of boys taking exercise, and the higher ratio of outdoor exercise compared with girls.
The average vitamin D intake in Japanese adolescents was approximately 10 μg/day. This is two to three times higher than the adequate intake (AI) according to the Dietary Reference Intakes for the Japanese population (AI—3.5 μg/day for 12–14-year-olds, 4.5 μg/day for 15–17-year-olds, 5.5 μg/day for 18–29-year-olds) [34]. However, approximately 30 % of boys and 47 % of girls had a blood concentration <50 nmol/L 25OHD. We tried to estimate the vitamin D intake required to achieve a serum 25OHD concentration of 50 nmol/L using the regression curve of vitamin D intake and serum 25OHD concentration (Fig. 5). Based on the lower limit of the slope, it was estimated that approximately >12, >12 and >14 μg/day vitamin D would be required to reach 50 nmol/L serum 25OHD concentrations for all subjects, boys and girls, respectively. However, it was difficult to know the exact vitamin D intake required to reach a concentration of 50 nmol/L serum 25OHD, because the correlation coefficients between vitamin D intake and serum 25OHD concentration were extremely low. Exposure to sunlight is the most important factor affecting serum 25OHD concentration. Serum samples in our study were collected in early May. Therefore, serum 25OHD concentrations would be affected by sunlight exposure experienced in April. The level of UVB radiation in April is lower than that in summer, but the strength of UVB is approximately 60 % compared with that in mid-summer in Japan (latitude N36°). Therefore, the difference in sunlight exposure among subjects would result in a low correlation coefficient between vitamin D intake and serum 25OHD concentration. The Institute of Medicine of the National Academies (IOM) has reported that vitamin D intake correlates well with serum 25OHD concentration in people with limited sunlight exposure living at northern latitudes in Europe and Antarctica during their winter season [35]. When the slope of lower confidence interval (CI) of regression equation was used, they reported that an intake of 400 or 600 IU (10 or 15 μg) in children and adolescents was associated with a predicted serum 25OHD concentration of 52 and 56 nmol/L, respectively [35]. Interestingly, although correlation coefficients between vitamin D intake and serum 25OHD concentration were low in our study, the vitamin D intake estimated to reach a concentration of 50 nmol/L serum 25OHD was the same as that reported by the IOM. These results suggest that higher vitamin D intake or much more sun exposure is needed to improve the status of vitamin D deficiency in Japanese adolescents.
A negative correlation between serum 25OHD and PTH concentration was observed in Japanese adolescents, in accordance with other studies [3–7]. Serum 25OHD concentrations required to achieve plateau PTH concentrations have been reported in adults [7, 36, 37] and adolescents [3, 7]. Guillemant et al. [3] reported that a 25OHD concentration >82.5 nmol/L was needed to reach the plateau PTH concentration in adolescent boys, while Hills et al. reported that a 25OHD concentration of approximately 60 nmol/L could reach the plateau PTH concentration in girls, but not in boys [7]. Using a similar method, we were unable to obtain realistic information on the serum 25OHD concentration required to reach the plateau PTH concentration, as reported in a study in Finnish girls [4]. However, we did identify a sex difference in the relationship between serum PTH and 25OHD concentrations. PTH concentration was more susceptible to serum 25OHD concentration in boys than in girls. Serum PTH concentration is thought to be a useful marker of vitamin D insufficiency in adolescent Japanese boys aged 12–18 years. However, calcium intake had a greater association than serum 25OHD concentration on serum PTH in girls aged 12–16 years, although the reasons for the lack of an apparent relationship between PTH and 25OHD concentration in 12–16-year-old girls is not clear. One possibility is that an extremely low calcium intake (471 ± 199 mg/day) in Japanese adolescents may affect the relationship between PTH and 25OHD concentrations. The calcium intake of Japanese girls was approximately one-third lower than that of Finnish girls [8]. These results suggest that serum PTH concentration may not be a useful marker of vitamin D insufficiency in girls aged 12–16 years who have a low calcium intake, such as Japanese adolescents.
Serum 25OHD concentration was significantly positively associated with calcaneal stiffness in adolescents, based on multiple regression analysis. In contrast to the relationship between serum 25OHD and PTH concentration, serum 25OHD concentration was significantly associated with calcaneal stiffness in girls of all age groups, while a significant association was only observed in 3rd HS boys. The present study also suggested that both vitamin D status and calcium intake would have a greater association with calcaneal stiffness in adolescent girls. The reason why the association between 25OHD concentration and calcaneal stiffness was weaker in boys is unclear. After the growth spurt, bone mineral content increments were much higher in boys than in girls [38]. We also observed a much higher increment in calcaneal stiffness in boys. From body height, it could be assumed that boys aged 12–13 years are still in the early stages of puberty, while most of the girls at this age are near menarche. Furthermore, it could be assumed that girls reach final height by age 15, but boys do not reach final height until age 17. Sex differences in bone growth may therefore be one reason why calcaneal stiffness was hardly affected by 25OHD concentration in adolescent boys.
The RDA of calcium according to the Dietary Reference Intakes for Japanese 2015 [19] is 1,000 mg/day for 12–14-year-olds and 800 mg/day for 15–18-year-olds in boys, and 800 mg/day for 12–14-year-olds and 650 mg/day for 15–18-year-olds in girls. The calcium intake of 450–550 mg/day in Japanese adolescents of both sexes was thus regarded as very low. Improvement of the low calcium status, especially in girls, should be emphasized for bone health in Japan.
Secondary hyperparathyroidism induced by vitamin D deficiency is known to be associated with bone resorption and bone loss. However, previous reports have indicated that PTH has anabolic effects on bone formation [39] and that serum PTH concentrations are usually raised during adolescence, when bone development is promoted actively [40, 41]. We speculate that a mild elevation in PTH concentration could compensate for vitamin D deficiency in boys, and that this response may be another reason why calcaneal stiffness was largely unaffected by 25OHD concentration in boys.
To the best of our knowledge, the present study represents the first evaluation of vitamin D status in Japanese adolescents and could thus provide comparable data for establishing reference values for serum 25OHD concentration and vitamin D intake. This study is also the first to report a sex difference in the relationship between vitamin D status and PTH concentration and calcaneal stiffness in adolescents. However, there were methodological limitations. The study was a cross-sectional study and subjects were recruited from an urban area in the eastern part of Japan. Further studies involving more subjects of all age groups, from rural as well as urban areas in different parts of the country, are needed to verify the results. Additionally, although the secretion of sex hormones and growth hormone would be expected to be associated with the sex difference, we were unable to measure these. Furthermore, although pubertal status is associated with bone growth, we could not obtain information about pubertal status (such as menstrual status or Tanner stage). There is another limitation in the estimation of the vitamin D intake required to achieve a serum 25OHD concentration of 50 nmol/L. We used the regression curve of vitamin D intake and serum 25OHD concentration to obtain the vitamin D intake required. Although the association between 25OHD and vitamin D intake was significant, the correlation coefficients were too small. To confirm the required vitamin D intake for the prevention of vitamin D deficiency, intervention trials in Japanese adolescents with the same sunlight exposure conditions will be needed in the future.
Another limitation of our study was the fact that bone measurements were carried out using QUS, which is less accurate than dual energy X-ray absorptiometry (DXA); however, it is difficult to perform DXA in the annual health examinations carried out in schools. However, we found significant associations between bone status measured by QUS, anthropometry, and serum 25OHD concentrations. Furthermore, recent data demonstrated that QUS is an inexpensive and excellent method for screening for future risk of hip fracture [42, 43]. QUS data not only provide information associated with BMD but may also incorporate information associated with other bone properties, such as bone structure and fragility [44–46].
Despite its limitations, the present study was able to conclude that vitamin D deficiency is common in Japanese adolescents, and that an intake of at least 12 and 14 μg/day vitamin D would be required to improve vitamin D deficiency in Japanese adolescent boys and girls, respectively. We also confirmed that serum PTH concentration is a useful biomarker of vitamin D deficiency in Japanese adolescents, except in girls aged 12–16 years with low calcium intake. Moreover, the results of present study suggest that vitamin D status has a greater association with calcaneal stiffness in girls than in boys.
References
Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G (1991) Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med 115:505–512
Oliveri MB, Ladizesky M, Mautalen CA, Alonso A, Martinez L (1993) Seasonal variations of 25 hydroxyvitamin D and parathyroid hormone in Ushuaia (Argentina), the southernmost city of the world. Bone Miner 20:99–108
Guillemant J, Taupin P, Le HT, Taright N, Allemandou A, Pérès G, Guillemant S (1999) Vitamin D status during puberty in French healthy male adolescents. Osteoporos Int 10:222–225
Outila TA, Kärkkäinen MU, Lamberg-Allardt CJ (2001) Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr 74:206–210
Harkness L, Cromer B (2005) Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int 16:109–113
Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D, Srinivasarao PV, Sarma KV, Kumar EG (2007) High prevalence of low dietary calcium, high phytate consumption, and vitamin D deficiency in healthy south Indians. Am J Clin Nutr 85:1062–1067
Hill TR, Cotter AA, Mitchell S, Boreham CA, Dubitzky W, Murray L, Strain JJ, Flynn A, Robson PJ, Wallace JM, Kiely M, Cashman KD (2009) Vitamin D status and parathyroid hormone relationship in adolescents and its association with bone health parameters: analysis of the Northern Ireland Young Heart’s Project. Osteoporos Int 21:695–700
Lehtonen-Veromaa MK, Möttönen TT, Nuotio IO, Irjala KM, Leino AE, Viikari JS (2002) Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study. Am J Clin Nutr 76:1446–1453
Kärkkäinen MU, Wiersma JW, Lamberg-Allardt CJ (1997) Postprandial parathyroid hormone response to four calcium-rich foodstuffs. Am J Clin Nutr 65:1726–1730
Hirota T, Kusu T, Hirota K (2005) Improvement of nutrition stimulates bone mineral gain in Japanese school children and adolescents. Osteoporos Int 16:1057–1064
The National Health and Nutrition Survey Japan (2007) http://www.mhlw.go.jp/bunya/kenkou/eiyou09/01.html. Accessed 15 Apr 2015
The National Health and Nutrition Survey in Japan (2009) Ministry of Health, Labour and Welfare, Japan. Daiichishuppan, Japan
Ersfeld DL, Rao DS, Body JJ, Sackrison JL Jr, Miller AB, Parikh N, Eskridge TL, Polinske A, Olson GT, MacFarlane GD (2004) Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem 37:867–874
Uenishi K, Ishida H, Nakamura K (2008) Development of a simple food frequency questionnaire to estimate intakes of calcium and other nutrients for the prevention and management of osteoporosis. J Nutr Sci Vitaminol 54:25–29
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930
Rosen CJ (2011) Vitamin D insufficiency. N Engl J Med 364:248–254
Absoud M, Cummins C, Lim MJ, Wassmer E, Shaw N (2011) Prevalence and predictors of vitamin D insufficiency in children: a Great Britain population based study. PLoS One 6:e22179
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58
Uenishi K, Ishimi Y, Nakamura K, Kodama H, Esashi T (2013) Dietary reference intakes for Japanese 2010: macrominerals. J Nutr Sci Vitaminol 59:S83–S90
Harkness LS, Cromer BA (2005) Vitamin D deficiency in adolescent females. J Adolesc Health 37:75
Lehtonen-Veromaa M, Möttönen T, Nuotio I, Irjala K, Viikari J (2002) The effect of conventional vitamin D2 supplementation on serum 25(OH)D concentration is weak among peripubertal Finnish girls: a 3-y prospective study. Eur J Clin Nutr 56:431–437
Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS (2007) Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr 86:150–158
Ginty F, Cavadini C, Michaud PA, Burckhardt P, Baumgartner M, Mishra GD, Barclay DV (2004) Effects of usual nutrient intake and vitamin D status on markers of bone turnover in Swiss adolescents. Eur J Clin Nutr 58:1257–1265
Das G, Crocombe S, McGrath M, Berry JL, Mughal MZ (2006) Hypovitaminosis D among healthy adolescent girls attending an inner city school. Arch Dis Child 91:569–572
Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ (2004) Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 158:531–537
Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR (2002) Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777
González-Gross M, Valtueña J, Breidenassel C, Moreno LA, Ferrari M, Kersting M, De Henauw S, Gottrand F, Azzini E, Widhalm K, Kafatos A, Manios Y, Stehle P (2012) Vitamin D status among adolescents in Europe: the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr 107:755–764
Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA (2008) Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 88:1519–1527
Dong Y, Pollock N, Stallmann-Jorgensen IS, Gutin B, Lan L, Chen TC, Keeton D, Petty K, Holick MF, Zhu H (2010) Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics 125:1104–1111
Rovner AJ, O’Brien KO (2008) Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med 162:513–519
Zhu K, Zhang Q, Foo LH, Trube A, Ma G, Hu X, Du X, Cowell CT, Fraser DR, Greenfield H (2006) Growth, bone mass, and vitamin D status of Chinese adolescent girls 3 y after withdrawal of milk supplementation. Am J Clin Nutr 83:714–721
Du X, Greenfield H, Fraser DR, Ge K, Trube A, Wang Y (2001) Vitamin D deficiency and associated factors in adolescent girls in Beijing. Am J Clin Nutr 74:494–500
Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, Saluja B, Ganie MA, Singh S (2005) Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr 82:477–482
Tanaka K, Terao J, Shidoji Y, Tamai H, Imai E, Okano T (2013) Dietary reference intakes for Japanese 2010: fat-soluble vitamins. J Nutr Sci Vitaminol 59:S57–S66
Institue of medicin of the National Academies (2011) Dietary reference intakes: calcium, vitamin D. The National Academies Press, pp 370–384. http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ (1997) Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443
Okazaki R, Sugimoto T, Kaji H, Fujii Y, Shiraki M, Inoue D, Endo I, Okano T, Hirota T, Kurahashi I, Matsumoto T (2011) Vitamin D insufficiency defined by serum 25-hydroxyvitamin D and parathyroid hormone before and after oral vitamin D load in Japanese subjects. J Bone Miner Metab 29:103–110
Krabbe S, Christiansen C, Rødbro P, Transbøl I (1979) Effect of puberty on rates of bone growth and mineralization: with observations in male delayed puberty. Arch Dis Child 54:950–953
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Krabbe S, Transbøl I, Christiansen C (1982) Bone mineral homeostasis, bone growth, and mineralisation during years of pubertal growth: a unifying concept. Arch Dis Child 57:359–363
Cadogan J, Blumsohn A, Barker ME, Eastell R (1998) A longitudinal study of bone gain in pubertal girls: anthropometric and biochemical correlates. J Bone Miner Res 13:1602–1612
Bauer DC, Glüer CC, Cauley JA, Vogt TM, Ensrud KE, Genant HK, Black DM (1997) Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. A prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 157:629–634
Schott AM, Hans D, Duboeuf F, Dargent-Molina P, Hajri T, Bréart G, Meunier PJ (2005) Quantitative ultrasound parameters as well as bone mineral density are better predictors of trochanteric than cervical hip fractures in elderly women. Results from the EPIDOS study. Bone 37:858–863
Yamamoto K, Nakatsuji T, Yaoi Y, Yamato Y, Yanagitani T, Matsukawa M, Yamazaki K, Matsuyama Y (2012) Relationships between the anisotropy of longitudinal wave velocity and hydroxyapatite crystallite orientation in bovine cortical bone. Ultrasonics 52:377–386
Frost ML, Blake GM, Fogelman I (2001) Quantitative ultrasound and bone mineral density are equally strongly associated with risk factors for osteoporosis. J Bone Miner Res 16:406–416
Nicholson PH, Müller R, Cheng XG, Rüegsegger P, Van Der Perre G, Dequeker J, Boonen S (2001) Quantitative ultrasound and trabecular architecture in the human calcaneus. J Bone Miner Res 16:1886–1892
Acknowledgments
This work was partly supported by a Grant-in-Aid from the Japan Osteoporosis Foundation, a Grant-in-Aid (#20590078) from the Ministry of Education, Culture, Science, Sports and Technology of Japan, a Grant-in-Aid for Comprehensive Research on Cardiovascular Diseases and Research on Dietary Reference Intakes in the Japanese from the Ministry of Health Labor, and Welfare of Japan. We would like to thank Kyowa Medex Co., Ltd for the measurement of serum 25OHD concentrations.
Conflict of interest
Although Naoko Tsugawa and Toshio Okano were funded by Kyowa Medex Co., Ltd, it played no part in the design of the study, collection and analysis of data and decision to publish. The other authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tsugawa, N., Uenishi, K., Ishida, H. et al. Association between vitamin D status and serum parathyroid hormone concentration and calcaneal stiffness in Japanese adolescents: sex differences in susceptibility to vitamin D deficiency . J Bone Miner Metab 34, 464–474 (2016). https://doi.org/10.1007/s00774-015-0694-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0694-y