Abstract
This study aimed to evaluate the 24-week effects of a high-intensity aquatic exercise program on bone remodeling markers and bone mass of postmenopausal women. In this randomized, controlled trial we studied 108 women (58.8 ± 6.4 years), randomized into Aquatic Exercise Group (AEG), n = 64, performing 24 weeks of aquatic exercises, and Control Group (CG), n = 44, sedentary. They had their fasting morning blood sample collected for the measures of intact parathyroid hormone (iPTH), procollagen type 1 amino-terminal propeptide (P1NP) and carboxy-terminal cross-linking telopeptide of type I collagen (CTx). Bone mass was measured by dual-energy X-ray absorptiometry before and after the intervention. Participants of both groups received a daily supplementation of 500 mg of elementary calcium and 1,000 IU of vitamin D (cholecalciferol). Results showed an augment in bone formation marker (P1NP) only in the AEG (15.8 %; p = 0.001), and although both groups experienced significant enhancements in bone resorption marker (CTx), this increase was less considerable in the AEG (15 % in the AEG and 29 % in the CG). IPTH was increased by 19 % in the CG (p = 0.003) at the end. The femoral trochanter BMD presented a 1.2 % reduction in the CG (p = 0.009), whereas in the AEG no change was observed (p = 0.069). The proposed aquatic exercise program was efficient in attenuating bone resorption raise and enhancing bone formation, which prevented the participants in the AEG from reducing the femoral trochanter BMD, as happened in the CG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis caused by estrogen deficiency in postmenopausal women is a worldwide public health problem [1]. Osteoporotic fractures represent 80 % of all fractures in women after menopause over the age of 50 years [2] and are the cause of mortality and morbidity, as well as chronic pain and substantial economic costs. Preventing and treating osteoporosis is of major importance to women’s health in an aging society like ours. Many strategies have been researched and are already recognized as effective against osteoporosis, such as the maintenance of optimum levels of serum calcium and vitamin D, in addition to the avoidance of tobacco and alcohol, appropriate drug therapy and engagement in a regular physical exercise program [3].
Studies analyzing the effects of physical activity on bone mass showed that dynamic resistance exercises, likewise, impact exercises, can stimulate bone metabolism in postmenopausal women [4–7]. These studies demonstrated a reduction in bone loss or even a small gain in bone mass in older women documented by a bone densitometry exam. Although bone mineral density (BMD) is one of the best parameters for the assessment of the risk of fractures, it reflects a static condition of bone mass and, therefore, does not reproduce the bone dynamic changes around the time of the examination. To overcome this limitation and to understand the dynamic responses of bone remodeling due to exercise, biochemical markers of bone remodeling are being used.
A few studies have analyzed the variation of bone remodeling markers after a period of regular physical exercise; nevertheless, the mechanism by which these markers are stimulated is not clear yet. Some scientific findings show that chronic exercise can improve bone formation with no change in bone resorption [8]. Contrarily, other results showed that exercise can reduce bone resorption rate with no change in bone formation markers [9]. The different types of bone markers as well as exercise protocols, together with the variety of exercise intensities and diverse studied population make it difficult to compare the results and comprehend the physiology of bone metabolism after chronic physical training.
To our knowledge, no study has focused on the effects of aquatic exercises on bone mass (DXA) and bone remodeling markers of postmenopausal women. In Brazil, a tropical country, aquatic exercises are most appreciated by older people [10]. In water the mechanical stress on joints is greatly reduced, blood circulation is facilitated by fluctuation and hydrostatic pressure, thermoregulation is improved, and the risk of injuries and falls is dramatically decreased [11]. Considering these characteristics, aquatic exercises could be regarded as the ideal modality of exercise for older people. However, there are doubts whether the reduction of the mechanical load on the immersed body would diminish its effect on bone metabolism. We hypothesize that even with the reduction of impact forces in water, other factors such as the muscle tension generated by water resistance, as well as the multidirectional and high-speed movements performed by the participants, are capable of stimulating bone metabolism in postmenopausal women. Therefore, this study aimed to evaluate the 24-week effects of a high-intensity aquatic exercise program on bone remodeling markers and bone mass of postmenopausal women.
Materials and methods
The present study was approved by the ethics committee of the Federal University of São Paulo (n° 1771/2008) and all subjects signed a written informed consent before entry.
Subjects
This research took place in the city of Barueri, in the state of Sao Paulo, Brazil. Women were invited to participate through advertisements in public places of the city. The volunteers were included when their physical activity status was classified as sedentary by the IPAQ short version questionnaire [12], if they were post-menopausal for at least 5 years and only when their cognition function allowed them to understand and respond to the authors’ questions and commands during the questionnaires. Participants were excluded if they presented: any physical conditions that might affect performance during aquatic exercises (hypothyroidism; primary hyperparathyroidism; osteoarthritis and/or rheumatic arthritis, only in the severe phase, with inflamed synovium and restrictive pain; edema or ulcer in the lower limbs); chronic kidney disease (serum creatinine >1.4 mg/dl); history of recent hip fracture (in the last 2 years); dependency on alcohol or illicit drugs; chronic therapy with corticosteroids, bisphosphonates, calcitonin, calcium, vitamin D and its metabolites; estrogen, selective estrogen receptor modulators and strontium in the earlier 6 months; use of any medications that might interfere with vitamin D metabolism; systolic blood pressure >200 mmHg and/or diastolic blood pressure >100 mmHg.
Design
Subjects were randomized using a restricted randomization procedure (urn design). Sample size was calculated with reference to total femur BMD (primary outcome) and according to the Graphpad State 2 Software, a sample size of 33 in each group would have a 99 % power to detect a 1.2 % difference between means with a significance level (alpha) of 0.05 (two-tailed).
In this randomized, prospective, controlled trial, we studied 108 postmenopausal women (58.8 ± 6.4 years), that went through the following study phases: (a) questionnaire about clinical history, family history, ethnicity, dietary habits, consumption of alcohol and cigarettes; (b) measures of blood pressure (mmHg), height (cm) and body mass (kg); (c) blood collection of 8-h fasting morning blood samples (between 7 and 8 a.m.) for the biochemical analyses; and (d) electrocardiographic exercise stress testing for cardiac risk assessment.
Fasting morning blood samples were used to measure all the biochemical parameters. Creatinine was measured by alkaline picrate assay (ADVIA 1650; Bayer, Tokyo, Japan). Intact parathyroid hormone (iPTH) was measured by a chemiluminescence commercial assays (Elecsys 1010, Roche Diagnostics, Indianapolis, IN, USA), presenting intra- and inter-assay coefficients of variability (CV) of 3.0 and 3.5 %, respectively. Total calcium (Ca) was measured by the enzymatic colorimetric assay (ADVIA 1650; Bayer), with intra- and inter-assay CV of 1.30 and 1.95 %, respectively. 25-hydroxyvitamin D (25OHD) was determined by an immunoradiometric assay (Diasorin, Stillwater, MN, USA), presenting intra- and inter-assay CV of 5.6 and 10 %, respectively. Procollagen type 1 amino-terminal propeptide (P1NP) (intra and inter coefficients of variation: 1.8 and 2.7 %, respectively) and carboxy-terminal cross-linking telopeptide of type I collagen (CTx) (intra and inter coefficients of variation: 4.6 and 4.7 %, respectively), were both determined by electrochemiluminescence assay (Roche Diagnostics, Indianapolis, IN, USA).
The participants were instructed to keep their dietary routine unchanged throughout the study. Daily calcium intake was estimated by a nutritionist using the 24-h food intake recall [13] that confirmed a similar calcium intake among the volunteers (both groups were receiving 600 mg of calcium/day) and irrelevant vitamin D ingestion from diet.
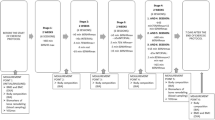
The 108 participants (58.8 ± 6.4 years) were randomized into two groups: Aquatic Exercise Group (AEG), n = 64, performing 24 weeks of aquatic exercises, and Control Group (CG), n = 44, with no regular physical exercises. The study design and the flow of subjects can be seen in Fig. 1.
Participants of both groups received a daily supplementation of 500 mg of elementary calcium and 1,000 IU of vitamin D (cholecalciferol), combined in the same pill (Silícea Handling Pharmacy, São Paulo, Brazil). For ethical reasons, we could not have a control group without any supplementation; as there were women with osteoporosis in the groups, we had to treat them with at least calcium and vitamin D. By the end of the 24-week intervention, from the first 108 participants, only 100 remained in the groups (Fig. 1), resulting in an adherence rate of 92.6 % (95 % confidence interval, 85–98 %).
Exercise intervention
The control group remained sedentary for 24 weeks and at the end of the study the IPAQ questionnaire’s result showed that the participants of the CG had the same sedentary lifestyle from the beginning.
Volunteers of the AEG attended the aquatic exercise sessions 3 times a week, for 24 weeks, in a covered swimming pool, with depth varying between 1.10 and 1.30 m and water temperature between 30 and 31 °C. For the aquatic exercise sessions no equipment was used and the sessions lasted from 50 to 60 min, starting with 10 min of warm-up exercises, then strength/power training, followed by cardiorespiratory training, and finishing with 10 min of stretching and balance exercises. As many of the subjects were not familiar with the aquatic exercises, the first 4 weeks were designed for their adaptation to the water characteristics and to the movements’ execution, breathing and posture techniques. In this phase they exercised at 55 % of their maximum heart rate (MHR). After the adaptation period we started the protocol itself that consisted of four mesocycles of 5 weeks each. The complete aquatic exercise protocol is shown in Table 1.
During the exercise sessions participants of the AEG were instructed to do each repetition with maximal effort (without shortening the range of motion) to achieve the highest possible movement speed and consequent resistance that they could maintain during the entire execution time. Verbal encouragement was constantly provided by the instructor during the sessions. Exercise intensity was controlled by the use of the modified Borg Scale of Perceived Exertion [14]. In addition, heart rate of participants was monitored by a polar heart rate monitor (FT1 model) during the aquatic exercise sessions.
During all 24 weeks the neuromuscular exercises were performed with subjects standing in the pool (feet on the floor), with water at the chest line, in the following order: elbow extension/flexion with shoulder abduction, hip abduction/adduction, shoulder horizontal flexion/extension with elbow extended, knee extension (high kick)/hip extension with knee extended. To control the intensity of the cardiorespiratory exercises the Borg CR10 category scale of perceived exertion (ranging from 0—nothing at all, to 10—very, very hard) was used (a big panel with the Borg Scale was disclosed on the wall in front of the pool) along with the measurement of heart rate of the participants during the sessions, as the following: level 6 in Borg Scale (≈60 % of MHR) during 16 min of the session in weeks 5–9, level 7 in Borg Scale (≈70 % of MHR) during 13 min of the session in weeks 10–14, level 8 in Borg Scale (≈80 % of MHR) during 9 min of the session in weeks 15–19, and level 9 in Borg Scale (≈90 % of MHR), during 7 min of the session in weeks 20–24.
The presence of each volunteer in exercise sessions was controlled and the least acceptable adherence rate was 85 %.
Markers of bone turnover
For the analysis of the 24-week effects of the aquatic exercise protocol on bone metabolism, before and after the study all the participants had their 8-h fasting morning blood sample collected for the measures of the iPTH, together with the P1NP (bone formation marker) and the CTx (bone resorption marker).
Bone mass
Bone mass of lumbar spine (L1–L4), proximal femur and total body was measured before and after the study by dual-energy X-ray absorptiometry (DXA), in a Hologic QDR 4500A densitometer (Waltham, MA, USA). This assessment was performed at the Bone Evaluation Laboratory of the Endocrinology Division at Universidade Federal de Sao Paulo. In our study the CV % for lumbar spine and total femur is 1 %, for trochanter is 1.1 % and for femoral neck is 1.5 % (data not shown). Bone status of participants was classified according to the World Health Organization [15] criteria for classification of patients with BMD measured by DXA: normal (T score −1.0 or greater), osteopenia (T score between 1–0.0 and 0.2.5) and osteoporosis (T score 2.5 and below).
Statistical analysis
For all statistical tests, a per-protocol analysis was used and results were considered significant when p ≤ 0.05. The Kolmogorov–Smirnov test was used to test the hypothesis of normality and showed that all analyzed parameters were normally distributed, so the baseline comparison between the groups was done using the Student’s t test for independent samples. The Chi-square test for independence was used to verify the distribution of bone mass status (normal, osteopenia and osteoporosis) in the spine and the femur in both groups, before the intervention. For the evaluation of bone mass parameters and bone remodeling markers we used repeated measures analysis of variance (ANOVA) to determine differences within and between groups over time. Once we found a significant (group × time) interaction, then multiple comparisons were used to establish if and where there was a difference between the group means.
Results
The percentage of participants with osteoporosis, osteopenia and normal bone mass in spine and femur was equivalent in the CG and the AEG at baseline (Table 2).
Except for the creatinine, there were no significant difference in the studied variables between the groups at the beginning and all subjects presented measures within the normal range (Table 3).
There was a significant time effect for both groups after the 24-week-supplementation with cholecalciferol (p = 0.001), showing a 25(OH)D raise by 21 % in the CG and 23 % in the AEG (58.2 ± 28.5 and 63.7 ± 31.3 nmol/L, respectively). As in the beginning, the groups did not differ at the end of the study (p = 0.265).
A significant time x group interaction was verified in the P1NP analysis (p = 0.006) and after multiple comparisons we could observe that only the AEG showed an improvement in this variable (Fig. 2).
A time effect was found (p < 0.001) in the evaluation of the CTx, revealing that both groups experienced significant enhancements in this bone resorption marker (15 % in the AEG, p = 0.019 and 29 % in the CG, p = 0.004). At the end of the study CTx serum values were slightly lower in AEG compared to CG, but with only a borderline significance (0.379 ± 0.220 and 0.454 ± 0.222 ng/mL, respectively, p = 0.064). A significant interaction effect was observed for the iPTH (p = 0.003) and further multiple comparisons demonstrated that after intervention only the CG increased the iPTH levels (19 %, p = 0.003), although this increase was not enough to make the groups differ at the end of the study (AEG: 43.77 ± 17.71 × CG: 51.91 ± 25.95 pg/mL; p = 0.069). Nevertheless, total calcium levels at the end of the study were 9.40 ± 0.39 for the AEG and 9.20 ± 0.31 for the CG, with no interaction effects (p = 0.548), as well as no difference between the two moments (p = 0.078) or the two groups (p = 0.911).
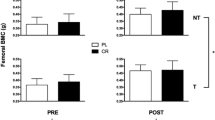
After the 24 weeks of protocol, there were no BMD differences within or between the groups in most skeletal sites, excepted for the trochanter, which showed a significant decrease only in the CG. In the evaluation of this variable the ANOVA showed a significant time × group interaction effect (p = 0.001) and the multiple comparisons demonstrated that while the values were stable in the AEG, there was a significant reduction in the femoral trochanter BMD in the CG (Fig. 3).
At the end of study, the L1L4 BMD was 0.912 ± 0.150 in the AEG and 0.90 ± 0.150 in the CG (p = 0.803), femoral neck BMD was 0.830 ± 0.134 in the AEG and 0.799 ± 0.126 in the CG (p = 0.069), total femur BMD was 0.911 ± 0.140 in the AEG and 0.890 ± 0.128 in the CG (p = 0.589) and total body BMD was 1.036 ± 0.115 in the AEG and 1.030 ± 0.106 in the CG (p = 0.861).
There were no injuries in the participants that could be related to the exercise practice.
Discussion
This study presents an aquatic exercise program called HydrOS, with reference to specific high-intensity aquatic exercises for the prevention and treatment of osteoporosis. Analyzing the entire group profile at baseline we can observe that the prevalence of vertebral osteopenia and osteoporosis in our group (54.6 and 12.3 %, respectively) was very similar to the one reported for Latin American women over 50 years (45.5–49.7 % with osteopenia and 12.1–17.6 % with osteoporosis) [16]. The same comparison considering the femur also revealed an analogous occurrence of osteoporosis (5 % in this study and 7.9–22 % in Latin America), whereas the prevalence of osteopenia in the femur in our group was lower than the one previously found in Latin America (11.1 % × 46–57.2 %, respectively). Showing that the bone status of the femur in our volunteers was better than the average reported for Latin American older women.
The HydrOS program significantly changed bone remodeling markers in the participants who exercised gradually changing the intensity from moderate (levels 6–7 at Borg CR10 Scale or 60–70 % of MHR) to high (levels 8–9 at Borg CR10 Scale or 80–90 % of MHR). A significant increase in bone resorption marker (CTx) was observed in both groups after 24 weeks. As CTx is one of the bone markers that most increases during the first years after menopause [17], the fact that the studied group was not so old (58.8 ± 6.4 years) might have contributed to this high bone turnover. Also, the participants were not engaged in any treatment that could benefit bone metabolism, apart from calcium and vitamin D supplementation. The CTx raise in the AEG (15 %) was smaller than the one in the CG (29 %), almost reaching statistical significance. The increase in CTx can be in part due to seasonal variance described by others, once the protocol began in the summer and finished in the winter, and the CTx increase could be following some PTH oscillation [18], despite the vitamin D supplementation. Moreover, some authors found that, regarding bone metabolism response to a power exercise training, it is assumed that bone resorption does not play a major role; on the contrary, the benefits for BMD occur by promotion of bone formation [19]. After the aquatic exercise intervention the treated group showed a 15.8 % increase of the bone formation marker (P1NP), with no changes observed in the CG. Contrarily, other authors showed that in situations in which bone formation markers decreased or did not change, bone resorption probably makes a difference in bone health. Klentrou et al. [20] analyzed the effects of 12 weeks of moderate multimodal exercise training using weighted vests on bone turnover of postmenopausal women and found that the bone resorption marker (NTx) significantly decreased by 14.5 % in the exercise group, with no significant changes in bone formation marker osteocalcin (OC). In another study [4], moderate walking exercise in postmenopausal women with osteopenia/osteoporosis also led to a decrease in the NTx followed by a decrease in the serum bone-specific alkaline phosphatase (BAP). Although bone formation marker was reduced, the decrease in bone resorption marker was reflected in an improvement in lumbar BMD of the exercise participants. This study supports the idea that a reduction in the bone resorption markers, even without an increase in the bone formation ones, can improve BMD status in postmenopausal women. In our study, although the intervention did not decrease bone resorption markers, we could observe a less expressive increase in this parameter when compared to the CG.
Vincent and Braith [21] examined the effect of 6 months of high- or low-intensity resistance exercise (50 and 80 % of 1 maximum repetition, respectively) on BMD and biochemical markers of bone turnover in adults aged 60–83 years. BMD of the femoral neck significantly increased by 1.96 % for the high intensity exercise group. No other significant changes in BMD were found. Also in this study, OC increased by 25.1 and 39.0 % for low- and high-intensity exercise, respectively. BAP significantly increased by 7.1 % only for the high-intensity exercise group and no changes were seen in the marker of bone resorption (serum pyridinoline cross-links) in any of the groups. As in our study, the kind of physical activity that seems to most stimulate bone formation markers is high-intensity exercise.
At the end of our study, we could observe a 1.2 % decrease in the femoral trochanter BMD in the CG, so susceptible to fractures, while the AEG preserved BMD at this bone site. The increase in bone formation marker, as well as a less expressive bone resorption rate compared to the CG, contributed to the maintenance of the femoral trochanter BMD in the AEG, in just 24 weeks. For such a short period of observation, it was unexpected to detect any significant variation on BMD. In fact, our results showed a decrease in trochanter BMD only for the control group, which was reinforced by an almost significant decrease in femoral neck BMD in the same group. According to some studies, it appears that the femoral trochanter might be more sensitive to mechanical load than other proximal femur regions. Marques et al. [22] observed that older women who participated in an 8-month resistance exercise intervention, 3 times a week, showed a better improvement in the trochanter BMD (2.9 %) than the one observed in the total hip BMD (1.5 %). Recently, the same group examined the effects of 32 weeks of exercise training on the BMD and other parameters of forty-seven healthy older adults (women = 24, men = 23; mean age 68.2 years) who participated in an exercise intervention (60 min/session) that included resistance exercise training (2 days/week) at 75–80 % of maximum plus a multicomponent weight-bearing impact exercise training (1 day/week). Again, after 32 weeks, results showed a significant improvement in trochanter BMD (0.7 %), with no change in femoral neck BMD. In our study, if the intervention duration were longer maybe we could see some changes in femoral neck or total hip BMD.
Our results showed no gain in lumbar spine BMD after the exercise protocol. Corroborating our findings, Martyn-St and Carroll [23] published a meta-analysis including only randomized controlled trials and a random-effects model and found out a little but significant exercise effect on femoral neck BMD (0.004 g/cm2), whereas no statistically significant benefit was observed at the lumbar spine in postmenopausal women. In another study [24], although a significant exercise effect was observed both at femoral neck (g = 0.29) and lumbar spine BMD, (g = 0.18), results were more convincing for femoral neck BMD.
Contradicting our results, in 1994, Goldstein et al. [25] conducted one of the first studies aiming to use water resistance as a bone load. Sixty-four postmenopausal women were divided into water and land exercise groups. The protocol consisted of 5 months of aquatic exercises, 3 times a week, with sessions of 45 min (10 min of warm-up, 25 min of weight training and 10 min of cool-down and stretches). Bone density was measured at the distal end of the radius and in the wrist, using Compton Scattering Technique. At the end of the study, results showed no difference in bone density in the land exercise group and a 1 % significant increase in the water exercise group. Nevertheless, a major limitation of this study was that there was no control of the physical activities performed by the participants outside the study; consequently the results might not have been due to the intervention. Also in 1994, Tsukahara et al. [26] found that the BMD of the lumbar spine in women trained with water exercises (35.2 months of practice) was significantly higher than that in women initiating in this activity (3 or 4 weeks of practice) and the control group (sedentary). The participants trained in water showed a 1.55 % enhance in spinal BMD whereas the control group presented a 0.92 % decline. Again, the limitation of this study was the lack of control of the further physical activity in which the participants might have participated, a bias that prevents us from relying on the results.
It is important to point out that the improvement in bone metabolism and bone mass observed in our study was not a result of impact exercises in the pool, but a consequence of resistive muscle training against water resistance. Bravo et al. [27], in 1997, studied the effects of successive jumps and endurance exercises (low to moderate intensity) on seventy-seven postmenopausal women (50–70 years) during 12 months of waist-high water exercise sessions, 3 days a week, and found a significant decrease in spinal BMD and no significant change in femoral neck BMD. The level of the water (waist line) made the exercises much easier in terms of resistance than they could be if it were on chest line, due to the lack of water mass resisting against the participants’ movements around the upper body. In addition, the exercises of the aquatic protocol were based on land exercises, not adapted to the water’s characteristics.
As in our study, two earlier researches evidenced the positive effects of aquatic exercises on femoral bone mass. Littrell et al. [28] compared the BMD (spine, hip and femoral neck) of 59 postmenopausal women, before and after a 12-month aquatic exercise program, consisting water exercise classes, 3 times a week, at moderate to high intensity. Results revealed that over the 12 months, femoral neck BMD decreased 1.7 % in controls (p < 0.0l) and did not change in exercisers (p = 0.98). The same way, Harush et al. [29] studied 35 postmenopausal women (55.45 ± 3.97 years) over a 7-month period of a water training program that included three 1-h sessions per week. Although no significant differences in bone density were found in the vertebrae for the experimental and the control groups, a significant interaction was found between the variables Time and Group for all four measures of BMD, BMC, T Score and Z Score, indicating that water exercise had a positive effect on bone density and allowed women in the experimental group to preserve bone density in comparison to the control group. On the other hand, for hip bone density a significant interaction was found between the variable Time and the variable Group only for BMC in the right leg (p < 0.01).
Recently, Ay and Yurtkuran [30] studied hormonal markers (insulin-like growth factor-1, growth hormone and calcitonin) together with calcaneous ultrasound in postmenopausal women and observed a positive effect of an aquatic exercise program on these variables. These results made the authors conclude that aquatic resistance exercise can maintain BMD in postmenopausal women.
Our findings suggest that improvement in the bone formation marker found in the AEG, along with the attenuated bone resorption raise, were reflected into the preservation of the femoral trochanter BMD at the end of the study in this group, while a significant bone loss was detected in the CG in this bone site. The mechanism by which exercise stimulates bone formation is not completely identified yet, although it is already known that impact as well as resistance exercises may have a positive effect on bone mass. In a meta-analysis on the effects of impact exercises on bone mass of postmenopausal women, the authors observed that exercise programs that combine impact activities such as jumping, jogging and climbing stairs are effective in preserving bone mass in hip and spine, especially if combined with other less demanding multi-directional impact exercises, like squash and soccer [23]. Along with impact loads, the resistance exercises are considered as another way of mechanically stimulate bone mass, once BMD can be increased by an improved muscle contraction, via the stimulation of bone formation and a possible inhibition of bone resorption [31, 32]. Many studies have reported that non-impact resistance exercises (weight-lifting), that require a great amount of muscle tension, can maintain and even increase the BMD of postmenopausal women [33, 34]. In our study, as the participants exercised in chest-high water the impact on bones was greatly minimized. However, the resistance imposed by water during the execution of the high-speed movements might have been enough stimuli for the bone metabolism of the studied women.
It has already been reported that exercise performed at high speed is more effective in terms of bone stimulation than the same exercise executed in slow motion [35]. Bone osteogenic response is incremented by just a few loading cycles if loading forces are high, applied at a fast rate and through a multidirectional odd strain distribution [36, 37]. Robling et al. [38] explained that exercises which involve forceful muscle contractions, but with few repetitions (as in the HydrOS program), target type II or fast twitch muscle fibers and have the potential to promote osteogenesis.
There are three noteworthy limitations of this study. The first limitation concerns the comparison of the bone markers’ results in our study to results of other studies on land exercises, as we found no other research on aquatic exercises and bone remodeling; this might have harmed the correct understanding of bone metabolism response after the proposed aquatic exercise program. A second limitation would be the short duration of the exercise program (24 weeks). Regarding the femoral trochanter BMD, if we had had a 1-year or longer duration exercise program, maybe we would have seen significant changes in the AEG. And finally, the third limitation of this trial would be the fact that, for ethical reasons, we could not have a control group without any calcium + vitamin D supplementation. If this could be possible we would certainly have a better understanding of the isolate effects of the supplementation on the measured variables.
We conclude that the HydrOS program was safe and efficient in attenuating the raise in bone resorption marker and increasing bone formation marker, which prevented the participants of the AEG from reducing the femoral trochanter BMD, as happened in the CG.
References
Clarke BL, Khosla S (2010) Female reproductive system and bone. Arch Biochem Biophys 503:118–128
Bessette L, Ste-Marie LG, Jean S, Davison KS, Beaulieu M, Baranci M, Bessant J, Brown JP (2008) The care gap in diagnosis and treatment of women with a fragility fracture. Osteoporos Int 19:79–86
Melendez-Ortega A (2007) Osteoporosis, falls and exercise. Eur Rev Aging Phys Activity 4:61–70
Yamazaki S, Ichimura S, Iwamoto J, Takeda T, Toyama Y (2004) Effect of walking exercise on bone metabolism in postmenopausal women with osteopenia/osteoporosis. J Bone Miner Metab 22:500–508
Chien MY, Wu YT, Hsu AT, Yang RS, Lai JS (2000) Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women. Calcif Tissue Int 67:443–448
Bemben DA, Bemben MG (2011) Dose-response effect of 40 weeks of resistance training on bone mineral density in older adults. Osteoporos Int 22:179–186
Vainionpää A, Korpelainen R, Leppäluoto J, Jämsä T (2005) Effects of high-impact exercise on bone mineral density: a randomized controlled trial in premenopausal women. Osteoporos Int 16:191–198
Hinton PS, Rector RS, Thomas RT (2006) Weight-bearing, aerobic exercise increases markers of bone formation during short-term weight loss in overweight and obese men and women. Metabolism 55:1616–1618
Evans EM, Racette SB, Van Pelt RE, Peterson LR, Villareal DT (2007) Effects of soy protein isolate and moderate exercise on bone turnover and bone mineral density in postmenopausal women. Menopause 14:481–488
Moreira LDF, Teixeira RN, Teixeira LR (2005) O que motiva idosos de classe média à prática de uma atividade física? Coleção Pesquisa em Educação Física 4:199–202
Becker BE (2000) Princípios Físicos da Água. In: Ruoti RG, Morris DM, Cole AJ (eds) Reabilitação Aquática. Manole, São Paulo, pp 17–27
Pardini R, Matsudo SMM, Matsudo VKR, Araujo T, Andrade E, Braggion G (1997) Validation of the international physical actyvity questionnaire (IPAQ): Pilot study in Brazilian young adults. Med Sci Sports Exerc 29:s5–s9
Buzzard M (1998) 24-hours dietary recall and food record methods. In: Willett WC (ed) Nutritional epidemiology. Oxford University Press, Oxford, pp 50–73
Borg GAV (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
World Health Organization (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. In Technical report series 843.WHO, Geneva
Morales-Torres J, Gutierrez-Urena S, Osteoporosis Committee of Pan-American League of Associations of Rheumatology (PANLAR) (2004) The burden of osteoporosis in Latin America. Osteoporos Int 15:625–632
Fink E, Cormier C, Steinmetz P, Kindermans C, Le Bouc Y, Souberbielle JC (2000) Differences in the capacity of several biochemical bone markers to assess high boneturnover. Osteoporos Int 11:295–303
Pasco JA, Henry MJ, Kotowicz MA, Sanders KM, Seeman E, Pasco JR, Schneider HG, Nicholson GC (2004) Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: the Geelong Osteoporosis Study. J Bone Miner Res 19:752–758
Ishikawa T, Sakuraba K (2009) Biochemical markers of bone turnover. New aspect. Bone metabolism movement in various sports and physical activities. Clin Calcium 19:1125–1131
Klentrou P, Slack J, Roy B, Ladouceur M (2007) Effects of exercise training with weighted vests on bone turnover and isokinetic strength in postmenopausal women. Aging Phys Act 15:287–299
Vincent KR, Braith RW (2002) Resistance exercise and bone turnover in elderly men and women. Med Sci Sports Exerc 34:17–23
Marques EA, Wanderley F, Machado L, Sousa F, Viana JL, Moreira-Gonçalves D, Moreira P, Mota J, Carvalho J (2011) Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp Gerontol 46:524–532
Martyn-St JM, Carroll S (2009) A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programs. Br J Sports Med 43:898–908
George AK, Kristi SK, Wendy MK (2012) Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 13:177
Goldstein J, Simkin E (1994) The influence of weight-bearing water exercise on bone density of postmenopausal women. Mov J Phys Educ Sport Sci 2:4–7
Tsukahara I (1994) The influence of water exercise on bone density in postmenopausal women. J Nutr Sci Vitaminol 40:37–47
Bravo G, Gauthier P, Roy PM, Payette H, Gaulin P (1997) A weight-bearing, water- based exercise program for osteopenic women: its impact on bone, functional fitness, and well-being. Arch Phys Med Rehab 78:1375–1380
Littrell TR, Snow CM (2004) Bone density and physical function. In Postmenopausal women after a 12-month water exercise intervention. Med Sci Sports Exerc 36:S289–S290
Harush M, Rotstein A (2005) The effect of a water exercise program on bone density among postmenopausal women. Israel, University of Haifa, School Education, Department of Education, thesis
Ay A, Yurtkuran M (2003) Evaluation of hormonal response and ultrasonic changes in the heel bone by aquatic exercise in sedentary postmenopausal women. Am J Phys Med Rehabil 82:942–949
Menkes A, Mazel S, Redmond RA, Koffler K, Libanati CR, Gundberg CM, Zizic TM, Hagberg JM, Pratley RE, Hurley BF (1993) Strength training increases regional bone mineral density and bone remodeling in middle-aged and older men. J Appl Physiol 74:2478–2484
Andreoli A, Monteleone M, Van Loan M, Promenzio L, Tarantino U, De Lorenzo A (2001) Effects of different sports on bone density and muscle mass in highly trained athletes. Med Sci Sports Exerc 33:507–511
Bocalini DS, Serra AJ, dos Santos L, Murad N, Levy RF (2009) Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J Aging Health 21:519–527
Engelke K, Kemmler W, Lauber D, Beeskow C, Pintag R, Kalender WA (2006) Exercise maintains bone density at spine and hip EFOPS: a 3-year longitudinal study in early postmenopausal women. Osteoporos Int 17:133–142
von Stengel S, Kemmler W, Kalender WA, Engelke K, Lauber D (2007) Differential effects of strength versus power training on bone mineral density in postmenopausal women: a 2-year longitudinal study. Br J Sports Med 41:649–655
Guadalupe-Grau A, Fuentes T, Guerra B, Calbet JA (2009) Exercise and bone mass in adults. Sports Med 39:439–468
Khan K, McKay H, Kannus P, Bailey D, Wark J, Bennell K (2001) Physical activity and bone health. Human Kinetics, Champaign
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455–498
Acknowledgments
This study was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), grant No. 08/50179-9.
Conflict of interest
All authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Moreira, L.D.F., Fronza, F.C.A.O., dos Santos, R.N. et al. The benefits of a high-intensity aquatic exercise program (HydrOS) for bone metabolism and bone mass of postmenopausal women. J Bone Miner Metab 32, 411–419 (2014). https://doi.org/10.1007/s00774-013-0509-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0509-y