Abstract
Purpose
The effects of aerobic exercise on bone metabolism are still unclear. Thus, the main goal of this study was to explore if there was an effect of the short-term aerobic exercise program on the bone remodeling process and if there were sex differences in the effect of the training program on bone metabolism.
Methods
Twenty-one participants (men and women) aged 20–23 performed an 8-week aerobic exercise program three times per week in 1-h sessions with increases in the exercise load every 2 weeks. Bone density, bone mineral content and concentration of markers of bone metabolism: osteocalcin, C-terminal procollagen type I peptide, pyridinoline, parathyroid hormone, osteoprotegerin, and the receptor activator of nuclear kappa B ligand by ELISA were measured at the start and at the end of the study, while changes in body composition were assessed by a bioelectric impedance analysis method 6 times during the study.
Results
The aerobic exercise program increased the concentration of osteocalcin (11.34 vs 14.24 ng/ml), pyridinoline (67.51 vs 73.99 nmol/l), and the receptor activator of nuclear kappa B ligand (95.122 vs 158.15 pg/ml). A statistically significant increase in bone density at neck mean (1.122 vs 1.176 g/cm3) and in bone mineral content at dual femur (33.485 vs 33.700 g) was found in women, while there was no statistically significant change at any site in men.
Conclusion
8 weeks of the aerobic exercise program with increment in intensity increased some of bone remodeling biomarkers and showed different effects for men and women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is strong evidence that regular exercise and physical activity play an important role in preserving bone health, mass, and strength at all stages of life (Santos et al 2017). In childhood and adolescence, regular exercise increases bone accrual and bone mass (Baxter-Jones et al 2008; Hind and Burrows 2007; Meyer et al 2013; Nogueria et al 2014) while for adults and for the elderly exercise can help prevent bone loss (Bolam et al 2013; Howe et al 2011; Kelley et al 2013). This beneficial effect of exercise on bone mass is observed in some athletes who practice certain types of sports, those that consists of high-impact, multi-directional movements, as evidenced by higher bone mass in athletes who compete in these types of sports compared to sedentary controls (Brahm et al 1997; Mantovani et al 2017; Rautainen et al 2014). The mechanical load applied to the bones during exercise evokes the process of bone remodeling to make the bones stronger and more apt to handle the increased stress applied by the exercise. The remodeling process includes bone resorption and formation and leads to an increased resistance to fracture. The best effect on bone mass and strength is achieved by impact-inducing activities (like jumping), while sports like swimming and bicycling could lead to bone loss (Tenforde and Fredericson 2011) if performed exclusively and not combined with impact-inducing exercise. A mechanical load applied to the bone by physical exercise should induce a greater strain to a bone than habitual everyday physical activity, to initiate an osteogenic response. The adaptation of the bone to a mechanical load is very localized, as shown in sports with unilateral loading, e.g. tennis, where differences in bone properties were found between the playing and the non-playing arm (Bass et al 2002). Bone formation depends on strain rates and the applied tension peak, as well as on the frequency of stimulation (Lombardi 2019). However, it remains unclear how much load is necessary and for how long should it be applied to induce bone remodeling (Kelley et al 2013; Santos et al 2017). In addition, there is evidence about different effects of different exercise models on bone metabolism (Guadalupe-Grau et al 2009; Santos et al 2017; Turner and Robling 2003). As Guadalupe-Grau et al (2009) concluded in their review article, the impact and resistance exercise showed best results for the improvement of bone mass, while aerobic training does not have such a potent effect as resistance exercise. A similar conclusion was made by Lombardi (2019) who compared the bone mineral density of athletes performing different sports. He found that adolescents and adults participating in high-volume endurance and non-weight bearing activities have lower bone density than subjects playing ball and power sports.
The measurement of biochemical bone turnover markers is an effective way to study the impact of exercise on bone remodeling, since changes in the concentration of molecules involved in the remodeling process (markers of bone resorption and markers of bone formation) are a very sensitive indicator of remodeling (Ooi and Sahrir 2018; Seibel 2005). They also have their limitations. Many bone metabolism biomarkers are not bone specific. Products of collagen metabolism are found in many connective tissues, not only in bone. Some of them have functions in energy metabolism and muscle activity (Dolan et al 2020). Also, biomarkers are systemic and cannot indicate bone metabolism activity at any particular site, while the effect of exercise on bones is very localized and site specific. There is also a question of the quick changes in the serum concentration of biomarkers of bone remodeling, which makes measurement very difficult regarding sample collection timing. Novel markers appeared in the last decade. MicroRNA are promising markers for bone health, as they were shown to promote osteogenic differentiation of mesenchymal stem cells (MSCs) by suppressing osteogenic inhibitors or by mediating major osteoblastic differentiation and signaling pathways. They are promising markers for the explanation of cellular processes in bone remodeling (Ka-Fai Cheng et al., 2019). Different types of exercise produce different changes in the concentration of bone metabolism markers. For example, an aerobic training program increased bone formation after 8 weeks of practice, while an anaerobic training program resulted in an overall increase in the bone remodeling process, or in the acceleration of bone metabolism (Lester et al 2009; Woitge et al 1998). The metabolic adaptation of bone turnover to exercise depends on age, gender, and the type of exercise performed (Vasikaran et al 2011; Dolan et al 2020). Combined-impact exercises, odd-impact or high-impact combined with resistance training, are recommended for maximizing bone mineral density at a young age (Xu et al 2016; Weaver et al 2016). Intermittent stimulation has better effects compared to continuous stimulation for the increase in bone formation (Santos et al 2017; Martin-St James and Caroll 2010). However, the literature on the influence of interval aerobic training with variations in intensity to the bone remodeling process is scarce (Hind and Burrows 2007; Kelley et al 2013). Aside from improvements in aerobic capacity, aerobic training might also induce changes in body composition, and contribute to health in general. Running is not as potent an osteogenic exercise as jumping, because of its repetitiveness, and it could cause the adaptation of bone cells to routine strain. However, the results on the osteogenic potential of running from literature are somewhat contradictory, as pointed out in the systematic review by Garofolini and Taylor (2019): the outcome suggests that running may increase bone density among other benefits, but it depends on training volume and experience. Some studies included in this review found that long distance runners have lower bone mineral density compared to their non-running peers, but some report opposite results. McCormack et al (2019) found that male cross-country runners have greater bone mineral density at the femoral neck, total hip, and whole body than male non-running controls. Ravnholt et al (2018) found that intense intermittent running with 5 s sprints performed 3 days/week leads to lower body fat mass, as well as higher lean body mass and bone mineral density after only 7 weeks of training. They found an increase in plasma bone remodeling markers. We found no studies exploring increment interval training, which is a good way to start regular exercise for the sedentary population. Since it involves an increment in load, dropout rate is smaller than for high-intensity workouts.
Thus, the main goals of this study were: exploring how a short (8 weeks) interval aerobic exercise training program with increasing intensity affects the bone remodeling process in young untrained men and women and establishing if there was a connection between bone remodeling and body composition. The third goal was to determine if there were any sex differences in the effect of training on bone metabolism.
Based on the results presented in available literature, the main hypothesis of the present study is that there is a stimulating effect of the running program on the bone remodeling process (coupled and synchronized process of bone activation, resorption, reversal and formation), which would be demonstrated by changes in the concentration of markers of bone metabolism.
Materials and methods
General study design
Twenty-one college students voluntarily participated in the study. The inclusion criteria were: age between 20 and 30, non-athlete persons of both sexes. The exclusion criteria were any diagnosed bone disease or chronic illness that could influence bone metabolism, the use of diuretics or antacids, smoking, and any mineral/vitamin supplements taken during the study, which includes multivitamins and vitamin D supplementation in any form. Only women with a regular menstrual cycle were included in the study. The additional exclusion criteria for women was the use of hormone replacement therapy or contraceptives. All participants have completed the exercise protocol and finished the study. Every participant gave its written informed consent for participation in this study. The study protocol and procedures conformed to the standards set by the latest revision of the Declaration of Helsinki and the national legislation. The Ethical Committee of the University of Osijek, Faculty of Medicine approved the study (Approval number 2158–61-07-12-42).
Study protocol
This was a single group intervention. The measurements considering bone metabolism were taken two times: At the beginning of the study—before the start of the exercise program, and at the end of the study—after 8 weeks of exercises. The protocol of exercises, as well as the time points of sampling and measurements, are depicted in Fig. 1.
Measurements of anthropomorphic characteristics
Morphological characteristics were measured 6 times: 3 days before the start of the exercise protocol, after every 6th session, and at the end of the study (7 days after the last session). Weight and height of all participants were measured. The analysis of body composition was done with the use of the bioelectric impedance analysis (BIA) method, using the body composition analyzer GAIA 359 (Jawon Medical, Korea). All participants were informed about the requirements they had to fulfill before the body composition measurement: No food or drinks 4 h prior to the measurements, no exercise 12 h before the measurements, no alcohol consumption within 48 h before the measurements, and emptying the bladder 30 min prior to the measurement. The measurement results included: the body mass index (BMI), lean body mass (LBM), mass of body fat (MBF), percent of body fat (PBF), total body water (TBW), and mineral content (MIN). All measurements were performed in the morning (at 7–9 AM), by the same trained technician. The subjects were positioned to stand barefoot on two electrodes which were built in the pedestal of the instrument and hold another two electrodes in their hands, while electrical current was conducted. Body composition was calculated based on the amount of impedance by the manufacturer’s software. The validity and reproducibility of GAIA 359 Plus has been reported by Jonker (2009), who obtained the correlation for the whole body FFM (fat free mass), measured by BIA and DXA r = 0.96, and that differences for %FFM and PBF (percent body fat) between BIA and DXA are − 0.04% and 0.25%, respectively. The precision and repeatability of the BIA was determined by measuring 10 subjects during 4 consecutive days. %CV and ICC were calculated from these 4 sets of results. We obtained overall results as follows: for MBF: ICC = 0.946, CV = 1.9%, for PBF: CV = 1.93%, ICC = 0.941, for LBM: ICC = 0.742, CV = 1.7%, for MIN: ICC = 0.998, CV = 1.2%, for TBW ICC = 0.743, CV = 1%, for BMI: ICC = 0.895, CV = 1.1%.
Bone density assessment
Bone mass density (BMD) and bone mineral content (BMC) were measured by dual x-ray absorptiometry (DXA) at three sites: at the lumbar spine L1–L4, and at the hips, dual femur (reported as the total mean of both femurs) and femoral neck (reported as the mean of the left and right femoral neck). The measurements were performed in the morning hours (between 7 and 9 am) on two occasions. First (baseline measurement): 3 days before the start of exercise training, and second: On the 7th day after the last training session. A trained technician using Lunar Prodigy 64575 G.E.S. S.A. took the measurements. The technician was blinded to other parameters obtained in the study. It has previously been reported that the precision error of the DXA scanner is between 0.6 and 1.8% (Shepherd et al. 2006).
Bone remodeling markers measurements
Blood samples were taken in the morning hours (between 7 and 9 am), after an overnight fast, on two occasions. At the start of the study: 1 day before the start of the exercise protocol, and at the end of the study: In the morning after the last exercise session (next day). The blood samples were used for the analysis of the bone remodeling biomarkers: osteocalcin (OC), C-terminal procollagen type I peptide (PICP), pyridinoline (PYD), parathyroide hormone (PTH), osteoprotegerin (OPG), and the receptor activator of nuclear kappa B ligand (RANKL). The serum was removed after centrifugation at 1500 g for 10 min and stored at − 80°C before the analysis. The OC concentration was assessed using ELISA (Elabscience Biotechnology Co, Beiging, NR China), CV < 10%. The concentration of PICP was measured by ELISA (USCN Life Science Inc, Hubei, NR China), intra-assay CV < 10%, inter-assay CV < 12%. The PYD concentration was measured by ELISA (Qayee-Bio, Shangai, NR China), CV < 15%. The concentration of RANKL was measured by ELISA (Cusabio Biotech, Hubei, NR China), intra-assay CV < 8%, inter-assay CV < 10%.
The PTH (intact form) concentration was measured by ELISA (Calbiotech, Spring Valley, CA) with intra-assay CV < 10%, inter-assay CV < 12%. The OPG concentration was determined by ELISA (Kamiya Biomedical Company, Seattle, WA, SAD) with intra-assay CV < 12%, inter-assay CV < 14%.
Exercise protocol
The subjects practiced the exercise program for 8 weeks, in 1-h sessions, 3 times per week under supervision at the same time of day. The aerobic exercise consisted of running outside on an asphalt road. The maximum heart rate was calculated according to Tanaka et al (2000) as HRmax = 208–0.7 × years of age. For the first 2 weeks (6 sessions) a low intensity training (60% HRmax) during 45–60 min was conducted. The next 2 weeks (6 sessions) consisted of sessions with changing intensity: A 3 min working interval with 75% HRmax was followed by a 6 min rest. A session started with running at 60% HRmax during 5 min, followed by interchanging intervals (total of 5 intervals per session). The next 6 sessions consisted of 5 min working intervals (75% HRmax), followed by a 5 min low intensity interval (60% HRmax) (the total of 5 interval changes per session). The last 2 weeks (6 sessions) were performed in a way that every first session consisted of working intervals with the intensity of 75% HRmax followed by a rest period; every second session had working intervals with lower intensity of 60% HRmax followed by a rest period, and every third session had working intervals loaded to 80–85% HRmax followed by a rest period. The sessions with maximum load started with 15 min of running at 60% HRmax, followed by 5 intervals of interchanging load, with a 1-min working interval and a 3-min rest. After the completion of the intervals, the running session ended with 15 min of running at 60% HRmax. Every running session ended with a stretching exercise. To evaluate maximum oxygen uptake, the Cooper test (Cooper 1968) was performed before the start and at the end of the exercise program (next day after the last exercise session). The Cooper test was performed by running on a treadmill (Precor 966i, USA) for 12 min, and VO2max was calculated from the distance covered by running in this period of time (Tanaka et al 2000). According to the calculated VO2max expressed as ml/kg/min, the subjects were assigned to one of 5 categories of physical fitness (Strasser 2018): very poor (men < 33, women < 23.6), poor (men 33–36.5; women 23.6–28.9), fair (men 36.5–42.4; women 29.0–32.9), good (men 42.5–46.4; women 33.0–36.9), excellent (men 46.5–52.4; women 37.0–41.0), and superior (men > 52.4; women > 41.0), so that the exercise protocol could be adjusted for every physical fitness category and performed separately for each category. The exercise sessions were practiced for each category separately, with adjusted intensity according to the physical fitness of the group. The exercise intensity was monitored by heart rate monitors Polar RS400. Engstrom et al. (2012) reported the validity and repeatability of the Polar RS400 in comparison with ECG, and they found no differences between the results for the two methods (mean difference of 0.7 bpm). The difference between the two measurements was the mean of 2.6 bpm.
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics 20. The results are presented as the arithmetic mean ± SD. Because the sample size was < 30, non-parametric tests were used. The differences between the initial values and the final values of variables describing bone metabolism (DXA measures and biomarkers of bone metabolism) and VO2max were tested by the Wilcoxon Signed Rank test (for the whole sample and separately for each sex), while the Friedman test with the Bonferroni corrected post hoc tests were used for variables describing body composition (BIA measures) because they were measured on 6 occasions during the study (for the whole sample and separately for each sex). The differences between the sexes at the start of the study and at the end of the study were tested by the Mann Whitney U test. 95% CI for median difference between the sexes or between the initial and final measurements was determined by the appropriate Hodges Lehman estimation (for the difference between sexes, the HL estimation for independent samples, and for the change between pre and post-treatment values, the HL estimation for dependent samples). The effect sizes are calculated based on statistical tests results with the help of an online calculator (Lenhard and Lenhard 2016). The level of statistical significance was set at p = 0.05.
Results
The sample consisted of 11 men and 10 women, with the mean age of 22 (from 20 to 23). The measurement of physical fitness (VO2max) confirmed their improvement in fitness. The VO2max value increased from 35.47 ± 10.8 ml/kg/min at the start of the study to 42.18 ± 9.7 ml/kg/min at the end (p < 0.0001). Men had a better score of VO2max at the start of the study (mean value ± SD = 42.5 ± 8.9 ml/kg/min) compared to women (mean value = 27.7 ± 6.8 ml/kg/min). However, the exercise regime caused a smaller improvement of VO2max in men (mean change = 5.8 ml/kg/min (13.6%), p < 0.0001) than in women (mean change = 7.7 ml/kg/min (28%), p = 0.0002).
The effect of exercise on bone remodeling and body composition
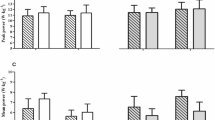
Pre- and post- intervention results and median differences and 95% CI for median difference for the bone remodeling markers and DXA results are presented in Table 1. The results are presented graphically in Figs. 2 and 3. There were no statistically significant changes in bone density and bone mineral content at the measured sites when tested for the whole sample. However, we found a statistically significant increase in the BMD neck mean in women, while there was no statistically significant change in men and in the whole sample. There was also a significant increase in dual femur total BMC in women, while no statistically significant change was observed in men and in the whole sample. Effect sizes (reported in Table 1 as dCohen) indicate large or intermediate effects of the exercise program on the change in bone density and bone mineral content at measured sites. The concentration of some biochemical markers of bone metabolism have also changed significantly. The concentration of pyridinoline (PYD), a marker of bone resorption and the concentration of osteocalcine (OC), a marker of bone remodeling in general, have been significantly increased after 8 weeks of the exercise program compared to their concentrations before the exercises (the baseline value). The concentration of the receptor activator of nuclear kappa B ligand (RANKL) was also significantly increased after the exercise program, compared to the start (baseline) value. All of these changes are observed in the whole sample and separately in both sexes, except for the increase in RANKL, which was not statistically significant in women.
Among the body composition variables (the change of body composition during the study is presented in Table 2, the exercise protocol affected only the mass of body fat (MBF) and the percent of body fat (PBF) significantly. The time course of the changes in the results of the body composition assessment during the study are presented in Fig. 4. Figure 4A represents individual changes in MBF and PBF for each subject, while the boxplots in Fig. 4B represent median and interquartile range overlaid with individual results. MBF was declining as the intensity of exercises increased, but after completing the exercise program, when subjects stopped exercising, it increased. The same was with PBF. Pairwise comparisons showed statistically significant differences between point of measurement 2 and points of measurement 4 and 5 for both variables (Fig. 4A,B). The separate analysis for sexes revealed that the changes in MBF and PBF during the study were statistically significant only in men, and not in women (Table 2).
Results of body composition assessments for MBF (mass of body fat) and PBF (percent of body fat) during the study (6 measurement points labeled with numbers 1 to 6) p < 0.05 # statistically significant change from the point of measurement 2. A Individual changes of each subject during the study. B The boxplots overlaid with individual data points during the study
Sex-related differences in bone metabolism and body composition
Results of bone density, bone mineral content, bone metabolism biomarkers, body composition, and differences between men and women, at the start and at the end of the study, with 95% CI median difference and effect sizes are presented in Table 3. BMD and BMC in most of the measured sites were significantly higher in men compared to women, and these differences were not altered by the exercise regime. The concentration of biochemical markers of bone remodeling were not different between the sexes at the start or at the end of the study. Men had significantly higher values of lean body mass (LBM), total body water (TBW), and body mineral content (MIN), and lower values of the percentage of body fat (PBF) compared to women, while there was no statistically significant difference between sexes for the mass of body fat (MBF) or the body mass index (BMI). The aerobic training program which was performed for 8 weeks did not affect these differences between sexes.
Discussion
The most important finding in the present study is that the applied increment interval aerobic exercise program in the duration of 8 weeks was accompanied by the increase in concentration of biochemical markers of bone metabolism (PYD, OC, and RANKL) at the end of the exercise program, and the increase in BMD neck mean BMD and BMC dual femur total in women, but not in men.
In the present study, the concentration of PYD increased, suggesting increased bone resorption (Banfi et al 2010). PYD is not the preferred biomarker of bone degradation, as it is a very small molecule that is quickly lost in urine, but we believe that the eventual loss of PYD in urine would probably be similar in both occasions of blood sampling (at the start and at the end of the study), since the same persons were tested under the same conditions at both occasions. Nevertheless, PYD was increased after the training period, and the magnitude of the increase of the PYD concentration at the end of the exercise program was more pronounced in women than in men (median increase 8.26 nmol/l vs 3.82 nmol/l). The possible explanation for this observation was that estrogen might influence the mechanism of PYD release from the collagen in the bone matrix during bone resorption. However, this remains to be supported by experimental measurements. Furthermore, the concentration of OC increased, suggesting an increased activity of osteoblasts (Banfi et al 2010), and an increase in the bone remodeling process. Although there is evidence from literature (Weaver et al 1997) that the serum level of OC is highly correlated to bone formation measured by calcium kinetics (Vo +) (r = 0.82, p = 0.001), it is irrefutable that OC can be released during bone resorption, which suggests that this biomarker indicates bone remodeling in general rather than bone forming. The biomarker of bone formation PICP did not change significantly in this study. PICP originates, the same as PINP, mostly from proliferating osteoblasts. PINP is the preferred marker for bone formation, because PICP is cleared by the mannose receptor, which can be regulated by the growth hormone and thyroid hormones, so its interpretation could be complicated in patients with thyroid dysfunction (Shetty et al 2016). Our subjects were healthy young people, and we did not expect this confounding to happen. Although we did not find a statistically significant change in PICP, dCohen (Table 1) indicates an intermediate or large effect size, which means that we cannot disregard the possibility that there could be an effect of the exercise program on the PICP concentration. It seems that this training program, which consisted of interval aerobic exercise with increment intensity, was able to override the effect of everyday physical activity, since we found an increase in markers of bone remodeling, and most of the markers had intermediate or large effect size, which indicates the possibility of change that our study did not observe. These results are different from reported results of Woitge et al (1998), who found no difference in bone degradation markers after 8 weeks of the aerobic exercise program. The present study had a different exercise program, we included increment interval training which could affect bone metabolism through different mechanisms compared to a continuous exercise load and unchanged exercise intensity. It has been shown that intermittent exercise programs are more effective in the acceleration of bone turnover (Robling et al 2001; Ravnholt et al 2018) and the increase of bone formation, since osteocyte desensitization is avoided with the intermittent load. Additionally, the increase in the concentration of RANKL, the biochemical marker of bone remodeling, confirms the enhancement of the bone remodeling process due to the increment intensity of the training protocol. It has been shown that RANKL is involved in the response of the bone to a mechanical load via the increase in differentiation and the activity of osteoclasts, bone degradation cells (Ikeda et al 2004). It is obvious from our results that the range of results for the serum RANKL was much wider at the end than at the start of the study, which could be confounding for result interpretation and reliability. Another stimulus for the increase in RANKL expression is PTH (Bouassida et al 2006). However, PTH concentration was not increased significantly in the present study (Table 1), possibly because the mechanical load of the exercise used in the present study was not strong enough to increase PTH production. This result is in agreement with a similar study by Bouassida et al (2006). Also, the effect size for PTH could indicate that it is possible that PTH changed even though we did not get a statistically significant change, but the direction of this change was towards lower concentration, which is definitely not the result we expected for exercise induced remodeling with PTH involvement. Alternatively, some other mechanism for the increase in RANKL might be involved, or it is possible that the elevated concentration of PTH returned to its baseline value before the collection of blood samples, as it was previously described (Thorsen et al 1997). Interestingly, the literature data on the influence of sex differences on the dynamics of markers of bone metabolism is scarce. For example, some differences in the response to extreme loads could be explained by the role of estrogen in the remodeling process (Joseph et al 2005). In the present study, we found somewhat different results between the sexes when we tested men and women separately for pre-post differences. The increase in RANKL was statistically significant for men, while it was not statistically significant for women. It has been reported that sex hormones influence the mechanisms of bone remodeling and the role of RANKL, by altering its expression (Streicher et al 2017), and our study confirmed different effects in men vs. women regarding exercise induced remodeling. (Fig. 2).
A typical bone remodeling cycle occurs over 120–200 days. It consists of several stages: resorption (2-week duration), reversal (4–5-week duration), formation (up to 4-month duration) and termination at the end of this cycle (Kenkre and Bassett, 2018), with the optimal length of three months for DXA to detect the changes in mineralization. However, the changes in bone density after 9 weeks and even after 7 weeks of exercising were described in literature (McWhannell et al 2008; Ravnholt et al 2018). In the present study, a separate analysis of changes pre-post treatment showed different effects of training for men and women. While there was no change in any of the DXA measurements for men, we found an increase in the femoral neck mean BMD and an increase in BMC dual femur total in women. These changes were small (95%CI median change for femoral neck mean BMD from 0.004 to 0.056 g/cm3, and 95% CI median change for dual femur total BMC from 0.08 to 0.620 g) but statistically significant. These results indicate that the training program had different effect on the sexes. Men had higher bone density and bone mineral content compared to women at the start and at the end of the study. There is some presumption that higher bone density and bone mineralization is connected to higher LBM in men (Vicente-Rodrigez et al 2008), which is evident from the results presented in Table 3, and it is typically considered to be the result of differences in the hormonal status between sexes, but it could also not be disregarded that it could be correlated to physical activity during childhood and adolescence. As Ruiz et al (2011) showed, men are more active than women during adolescence. The higher VO2max in men demonstrated the higher physical fitness level in men than in women at the beginning of the protocol. This could be a reason for the difference in the change of VO2max between men and women after the exercise program in this study. After the study, VO2max improved more for women than for men (delta (value before – value after the protocol) in VO2max for women was on average 7.7 ml/kg/min vs. 5.8 ml/kg/min for men). The performed exercise program brought women from the poor VO2max of 27.68 ml/kg/min to the fair VO2max of 35.37 ml/kg/min, while the improvement for men was from the fair VO2max of 42.55 ml/kg/min to the excellent VO2max of 48.36 ml/kg/min. The difference in physical fitness between the sexes could be the confounding variable for interpreting differences found in other variables, and this can be considered as a limitation of the present study. The effect of the training program on bone metabolism and body composition was not an issue for the main outcome of the present study, since each subject was its own control, and results post-treatment were compared to the same subject pre-treatment.
Aerobic exercise can lead to the reduction of visceral fat which is not correlated to body weight reduction (Johnson et al 2009). In the present study, the only significant effect of the 8 weeks of aerobic exercise was on the mass of body fat (MBF), and consequently on the percent of body fat (PBF). Furthermore, the results demonstrated a decrease in MBF as the intensity of exercises was increasing, until the 4th measurement, while the MBF values were increasing in the last two measurements (Table 2, Fig. 4B). Our results comply with the results of previous studies (Coker et al 2009; Lee et al 2012) and show a negative relationship of aerobic exercise intensity and the mass of body fat. The increase in mass of body fat which was recorded after quitting the exercise protocol was similar to the results of Eastwood et al (2012). However, as King et al (2007) presumed, every individual person responds to the energy deficit produced by aerobic exercises in its own unique way. The difference in MBF between sexes is known and documented in literature (Duncan et al. 2004). Observed changes in body fat mass, although statistically significant, were in a very smallamount, in other words, the changes in MBF and PBF were too small to be clinically important. The median difference between the highest and the lowest value for PBF (2nd and 4th measurement) was 1.1 (IQR 0.45–1.87), which is a decrease of 4.4% from the baseline value for PBF. The least significant change, calculated as 2.77*CV, would be 8.11%. The median decrease in MBF from point of measurement 2 median 17.2 kg to the point of measurement 4 median value 16.5 kg was 1.1 (IQR 0.35–1.77) kg or 6.4% of the baseline value. The least significant change of MBF would be 8%. -Effect sizes (Kendall's W) showed small effect or no effect at all.
There was no statistically significant change in the lean body mass (LBM) after the exercise program. That is in accordance with the results of some studies that demonstrated no effects of aerobic exercise to LBM in overweight persons (Park et al 2003), sedentary women (Antonio et al 2000), and children 11–13 years old (Dashti 2011).
The limitation of this study is that dietary habits of participants were not controlled or recorded. However, the participants were advised not to change dietary or any other lifestyle habits during the study period. We cannot exclude the possibility that the increase in energy expenditure increased their appetite and energy intake in the amount that could influence the results of body composition measurements. Even though the influence of the diet should not be neglected (Bopp et al 2008; Josse et al 2011), some researchers did not notice changes of LBM following aerobic exercise even with a controlled diet (Frykman et al 2003), similar to the results of the present study. Another limitation of the study is a lack of a non-trained control group. All subjects were measured twice: at the start of the study (pre-intervention) and at the end of the study (post-intervention). Pre-intervention measurements served as a control for a comparison with post-intervention measurements, for changes evoked by the study protocol. That way we avoided the biological diversity of the control and the experimental group and avoided the alteration in biomarkers not related to our study protocol. However, the comparison of each individual to their own baseline results does not allow consideration of typical errors or usual variations across the study time period. A control group would be useful for quantifying non-interventional sources of variation and as assistance in isolating results as being due to the exercise program performed in the study, so the lack of such a group is a limitation of this study.
In conclusion, the interval increment aerobic exercise protocol implemented for 8 weeks affected the bone remodeling process, which was evident from the increase in concentration of some bone remodeling biomarkers. The results also revealed somewhat different effects of the exercise program on the bone metabolism in men vs women involved in this study.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
- BIA:
-
Bioelectric impedance analysis
- BMI:
-
Body mass index
- LBM:
-
Lean body mass
- MBF:
-
Mass of body fat
- PBF:
-
Percent of body fat
- TBW:
-
Total body water
- MIN:
-
Mineral content of the body
- BMD:
-
Bone mineral density
- BMC:
-
Bone mineral content
- OC:
-
Osteocalcin
- PICP:
-
C-terminal procollagen type I peptide
- PYD:
-
Pyridinoline
- PTH:
-
Parathyroid hormone
- OPG:
-
Osteoprotegerin
- RANKL:
-
Receptor activator of nuclear kappa B ligand
- ELISA:
-
Enzyme-linked immunosorbent assay
- DF TOTAL:
-
Dual femur total mean
- L1-L4:
-
Lumbar spine L1-L4
- NM:
-
Neck mean (mean from results of left and right femoral neck)
- CV:
-
Coefficient of variability
- DXA:
-
Dual X-ray absorptiometry
References
Antonio J, Sanders MS, Ehler LA, Uelmen J, Raether JB, Stout JR (2000) Effects of exercise training and amino-acid supplementation on body composition and physical performance in untrained women. Nutr 16:1043–1046
Banfi G, Lombardi G, Colombini A, Lippi G (2010) Bone metabolism markers in sports medicine. Sports Med 40(8):697–714
Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S (2002) The effect of mechanical loading on the size and shape of bone in pre-, peri- and postpubertal girls: a study in tennis players. J Bone Miner Res 17:2274–2280
Baxter-Jones ADG, Kontulainen SA, Faulkner RA, Bailey DA (2008) A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone 43(6):1101–1107
Bolam KA, Van Uffelen JGZ, Taaffe DR (2013) The effect of physical exercise on bone density in middle-aged and older men: a systematic review. Osteoporos Int 24:2749–2762
Bopp MJ, Houston DK, Lenchik L, Linda E, Kritchevsky SB, Nicklas BJ (2008) Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J Am Diet Assoc 108:1216–1220
Bouassida A, Latiri I, Bouassida S, Zalleg D, Zaouali M, Feki Y, Gharbi N, Zbidi A, Tabka Z (2006) Parathyroid hormone and physical exercise: a brief review. J Sports Sci Med 5:367–374
Brahm H, Strom H, Piehl-Aulin K, Mallmin H, Ljunghall S (1997) Bone metabolism in endurance trained athletes: a comparison to population-based controls based on DXA, SXA, quantitative ultrasound, and biochemical markers. Calcif Tissue Int 61:448–454
Coker RH, Williams RH, Kortebein PM, Sullivan DH, Evans WJ (2009) Influence of exercise intensity on abdominal fat and adiponectin in elderly adults. Metab Syndr Relat Disord 7:363–368
Cooper KH (1968) A means of assessing maximal oxygen uptake. J Am Med Assoc 203:201–204
Dashti MH (2011) The effect of programmed exercise on body composition and heart rate of 11–13 years old male students. Zahedan J Res Med Sci 13:40–43
Dolan E, Varley I, Ackerman KE, Pereira RMR, Elliot-Sale KJ, Sale C (2020) The bone metabolic response to exercise and nutrition. Exerc Sport Sci Rev 48(2):49–58
Duncan MJ, Woodfieldand L, Al-Nakeeb Y (2004) Differences in body fat of British children from various ethnic groups. Eur Phys Educ Rev 10:41–52
Eastwood A, Bourdon PC, Snowden KR, Gore CJ (2012) Detraining decreases Hb mass of triathletes. Int J Sports Med 33:253–257
Engström E, Ottosson E, Wohlfart B, Grundström N, Wisén A (2012) Comparison of heart rate measured by Polar RS400 and ECG, validity and repeatability. Adv Physiother 14(3):115–122
Frykman PN, Harman EA, Opstad PK, Hoyt RW, DeLany JP, Friedl KE (2003) Effects of a 3-month endurance event on physical performance and body composition: the G2 trans-greenland expedition. Wildern Environ Med 14:240–248
Grofolini A, Taylor S (2019) The effect of running on foot muscles and bones: a systematic review. Hum Mov Sci 64:75–88
Guadalupe-Grau A, Fuentes T, Guerra B, Calbert JAL (2009) Exercise and bone mass in adults. Sports Med 39(6):439–468
Hind K, Burrows M (2007) Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone 40(1):14–27
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev 6(7):CD000333
Ikeda F, Nishimura R, Matsubara T, Tanaka S, Inone J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T, Kukita T, Yoshioka K, Rao A, Yoneda T (2004) Critical roles of c_Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest 114:475–484
Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J (2009) Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 50:1105–1112
Jonker R (2009) Validity and reproducibility of the GAIA 359 PLUS and applicability in chronic wasting. Bachelor thesis Hogelschool van Amsterdam
Joseph C, Kenny AM, Taxel P, Lorenzo JA, Duque G, Kuchel GA (2005) Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Mol Asp Med 26:181–201
Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM (2011) Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J Nutr 141:1626–1634
Ka-Fai Cheng V, Chun-Ming AuP, Tau KCB, Cheung CL (2019) MicroRNA and human bone health. JBMR plus 3(1):2–13
Kelley GA, Kelley KS, Khort NM (2013) Exercise and bone mineral density in men: a meta-analysis of randomized controlled trials. Bone 53:103–111
Kenkre JS, Bassett JHD (2018) The bone remodeling cycle. Ann Clin Biochem 55(3):308–327
King NA, Caudwell P, Hopkins M, Byrne NM, Colley R, Hills AP, Stubbs JR, Blundell JE (2007) Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity 15:1373–1383
Lee MG, Park KS, Kim DU, Choi SM, Kim HJ (2012) Effects of high-intensity exercise training on body composition, abdominal fat loss, and cardiorespiratory fitness in middle-aged Korean females. Appl Physiol Nutr Metab 37:1019–1027
Lenhard W, Lenhard A (2016) Calculation of effect sizes. Retrieved from: https://www.psychometrica.de/effect_size.html. Dettelbach (Germany): Psychometrica. https://doi.org/10.13140/RG.2.2.17823.92329
Lester ME, Urso ML, Evans RK, Pierce JR, Spiering BA, Maresh CM, Hatfield DL, Kraemer WJ (2009) Influence of exercise mode and osteogenic index on bone biomarker responses during short-term physical training. Bone 45(4):768–776
Lombardi G (2019) Exercise-dependent modulation of bone metabolism and endocrine function: new findings and therapeutic perspectives. J Sci Sport Exer 1:20–28
Mantovani AM, de Lima MCS, Gobbo LA, Ronque ERV, Romanzini M, Turi-Lynch BC, Codogno JS, Fernandes RA (2017) Adults engaged in sports in early life have higher bone mass than their inactive peers. J Phys Act Health 15(7):516–522
Martyn-St James M, Carroll S (2010) Effects of different impact exercise modalities on bone mineral density in premenopausal women: a meta-analysis. J Bone Miner Metab 28(3):251–267
McCormack W, Shoepe TC, LaBrie J, Almstedt HC (2019) Bone mineral density, energy availability, and dietary restraint in collegiate cross-country runners and non-running controls. Eur J Appl Physiol 119(8):1747–1756
McWhannell N, Henaghan JL, Foweather L, Doran DA, Batterham AM, Reilly T, Stratton G (2008) The effect of a 9-week physical activity programme on bone and body composition of children aged 10–11 years: an exploratory trial. Int J Sports Med 29:941–947
Meyer U, Ernst D, Zahner L, Schindler C, Puder JJ, Kraenzlin M, Kriemler S (2013) 3-year follow-up results of bone mineral content and density after a school-based physical activity randomized intervention trial. Bone 55(1):16–22
Nogueria RC, Weeks KS, Beck BR (2014) Exercise to improve pediatric bone and fat: a systematic review and meta-analysis. Med Sci Sports Exerc 46:610–621
Ooi FK, Sahrir NA (2018) Physical activity, bone remodelling and bone metabolism markers. J Exerc Sports Orthop 5(2):1–4
Park SK, Park JH, Kwon YC, Kim HS, Yoon MS, Park HT (2003) The effect of combined aerobic and resistance exercise training on abdominal fat in obese middle-aged women. J Physiol Anthropol 22:129–135
Rautainen T, Duckham RL, Suominen H, Heinones A, Alen M, Korhonen MT (2014) Tibial and fibular mid-shaft bone traits in young and older sprinters and non-athletic men. Calcif Tissue Int 95:132–140
Ravnholt T, Tybirk J, Jorgensen NR, Bangsbo J (2018) High-intensity intermittent “5-10-15” running reduces body fat, and increases lean body mass, bone mineral density, and performance in untrained subjects. Eur J Appl Physiol 118:1221–1230
Robling AG, Burr DB, Turner CH (2001) Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol 204(pt19):3389–3399
Ruiz JR, Ortega FB, Martinez-Gomez D, Labayen I, Moreno LA, De Bourdeaudhuij I, Manios Y, Gonzalez-Gross M, Mauro B, Molnar D, WidhalmK MA, Beghin L, Castillo MJ, Sjostrom M (2011) Objectively measured physical activity and sedentary time in european adolescents. The HELENA study. Am J Epidemiol 174:173–184
Santos L, Elliot-Sale KJ, Sale C (2017) Exercise and bone health across the lifespan. Biogerontology 18:931–946
Seibel MJ (2005) Biochemical markers of bone turnover part I: biochemistry and variability. Clin Biochem Rev 26(4):97–122
Shepherd JA, Fan B, Lu Y, Lewiecki EM, Miller P, Genant HK (2006) Comparison of BMD precision for Prodigy and Delphi spine and femur scans. Osteoporos Int 17:1303–1308
Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV (2016) Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocr Metab 20:846–852
Strasser B, Burtscher M (2018) Survival of the fittest: VO2max, a key predictor of longevity? Front Biosci Landm 23:1505–1516
Streicher C, Heyny A, Andrukhova O, Haigl B, Slavic S, Schuler C, Kollman K, Kantner I, Sexl V, Kleiter M, Hofbauer LC, Kostenuik PJ, Erben RG (2017) Estrogen regulates bone turnover by targeting RANKL expression in bone lining cells. Sci Rep 7:6460. https://doi.org/10.1038/s41598-017-06614-0
Tanaka H, Monahan KD, Seals DR (2000) Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37(1):153–156
Tenforde AS, Fredericson M (2011) Influence of sports participation on bone health in young athlete. A review of the literature. PM&R 3(9):861–867
Thorsen K, Kristofferson A, Hultdin J, Lorentzon R (1997) Effects of Moderate Endurance Exercise on calcium, parathyroid hormone, and markers of bone metabolism in young women. Calcif Tissue Int 60:16–20
Turner CH, Robling AG (2003) Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev 31(1):45–50
Vasikaran S, Eastell R, Bruyère O et al (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22(2):391–420
Vicente-Rodrigez G, Urzanqui A, Mesana MI, Ortega FB, Ruiz JR, Ezquerra J, Casajus JA, Blay G, Blay VA, Gonzales-Gros M, Moreno LA (2008) Physical fitness effect on bone mass is mediated by the independent association between lean mass and bone mass through adolescence: a cross-sectional study. J Bone Miner Metab 26:288–294
Weaver CM, Peacock M, Martin BR, McCabe GP, Zhao J, Smith DL, Wastney ME (1997) Quantification of biochemical markers of bone turnover by kinetic measures of bone formation and resorption in young healthy females. JBMR 12(10):1714–1720
Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS (2016) The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27(4):1281–1386
Woitge HN, Friedmann B, Suttner S, Farahmand I, Muller M, Schmidt-Gayk H, Baertsch P, Ziegler R, Seibel MJ (1998) Changes in bone turnover induced by aerobic and anaerobic exercise in young males. J Bone Min Res 13(12):1797–1804
Xu J, Lombardi G, Jiao W et al (2016) Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: an overview of systematic reviews and meta-analyses. Sports Med 46:1165–1182
Funding
No funds, grants or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors were involved in material preparation, data collection and/or analysis. The first draft of the manuscript was written by EDC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Ethical Committee of Faculty of Medicine University of Osijek approved the study (number of approval 2158-61-07-12-42).
Consent to participate
Every participant gave its written informed consent for participation in this study.
Consent for publication
Not applicable.
Additional information
Communicated by Olivier Seynnes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davidović Cvetko, E., Nešić, N., Matić, A. et al. Effects of 8-week increment aerobic exercise program on bone metabolism and body composition in young non-athletes. Eur J Appl Physiol 122, 1019–1034 (2022). https://doi.org/10.1007/s00421-022-04900-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-04900-y