Abstract
Osteolytic disorders cause serious problems for quality of life with aging. Osteolysis is performed by osteoclasts of the hematopoietic lineage that share some characteristics with monocytes and macrophages. As osteoclast precursors (pOCs) are present in peripheral blood, their characterization in osteolytic diseases may help us to understand risk factors. Although essential factors for osteoclastogenesis have been reported, the effective induction from pOCs in human peripheral blood mononuclear cells (PBMCs) to mature osteoclasts in culture requires further improvement. The aim of this study was development of an efficient culture system for human osteoclastogenesis and providing a simple system for the enrichment of pOCs from PBMCs. We employed coculturing of human PBMCs with a mouse stromal cell line. Significant numbers of tartrate-resistant acid phosphatase-positive (TRAP+) multinucleated osteoclasts (MNCs), which could resorb dentine slices, were efficiently induced in this culture condition. pOCs were enriched in an anti-CD16 antibody column-passed anti-CD14 antibody-bound cell population isolated by magnetic cell sorting. We compared the percentage of the CD14high CD16dull cell population, which mainly contained pOCs in PBMCs, from age-matched patients with rheumatoid arthritis (RA) and osteoporosis (OP), but it was comparable. However, the mean number of TRAP+ MNCs generated in cultures from PBMCs of RA was higher. In contrast, the frequency of pOCs in PBMCs from OP was relatively higher. These results suggest the characteristics of pOCs from RA and OP may be different, because single pOCs from OP gave rise to lower numbers of osteoclasts than those from RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) and osteoporosis (OP) cause bone loss, resulting in serious problems for the quality of life with aging [1–4]. RA is a chronic systemic inflammatory disorder with an unknown etiology characterized by invasive synovial hyperplasia leading to progressive joint destruction. Radiographic studies have shown that bone erosion in RA begins at an early stage of the disease and gradually or rapidly exacerbates. Bone erosion results in severe deformities of the affected joints and impairs normal activity and quality of life [5, 6].
Osteoporosis is a skeletal disorder characterized by low bone mass and loss of bone tissue integrity that causes weak and fragile bones. Although the exact etiology of osteoporosis remains unknown, it is now widely accepted that the normal balance between bone formation and bone resorption is impaired in osteoporotic patients. The primary cause of postmenopausal osteoporosis is estrogen deficiency, and bone destruction takes place at a faster rate after menopause [4]. Recent evidence has shown that bone remodeling rates were increased in postmenopausal women and osteoporotic patients [7]. The regulation leans toward bone resorption compared to osteogenesis, although these osteolytic disorders might be induced by different mechanisms [1, 4].
Osteoclasts are in the hematopoietic lineage, sharing some characteristics with monocytes and macrophages, and perform bone resorption and remodeling [8, 9]. Osteoclasts function only in bone tissues; however, osteoclast precursors (pOCs) are also present in peripheral blood [10]. Recently, it has been reported that macrophage-colony stimulating factor (M-CSF) [11] and receptor activator of nuclear factor (NF)-kappa B (RANK) ligand (RANKL) [12, 13] are essential factors for osteoclastogenesis. RANKL is expressed mainly as a membrane-bound form on osteoblasts and induces the signaling essential for precursor cells to differentiate into osteoclasts, whereas M-CSF, secreted by osteoblasts, provides the survival signal to these cells [14, 15]. Therefore, the upregulation of their production and the reduction of a decoy receptor of RANKL, named osteoprotegerin (OPG), are critical for bone disorders [16, 17].
In RA patients, inflammation-induced bone loss is thought to result from increased activity of bone-resorbing osteoclasts [3, 18]. Synovial fibroblasts, activated T lymphocytes, and/or dendritic cells aberrantly produce RANKL, which triggers bone destruction in RA [3, 19]. Increased bone resorption is observed after estrogen withdrawal. It is well documented in menopausal women with OP that estrogen is involved in RANKL production and that reduction of estrogen causes OP [4, 20]. Therefore, studies of these diseases have focused on this factor [14, 21].
If the characteristics of pOCs in peripheral blood influence these diseases and indicate the pathological hallmarks of each disease, investigation of these factors may help us to understand risk factors of osteolytic disorders. In a mouse culture system, sufficient osteoclast development is induced by only M-CSF and RANKL [11–13, 22]; however, the efficient induction of pOCs in human peripheral blood mononuclear cells (PBMCs) to form mature osteoclasts in culture needs to be improved because many references showed that 10–20 times as many PBMCs as in this study were needed for the induction of mature osteoclasts from PBMCs [23, 24].
In this study, to develop an efficient culture system for human osteoclastogenesis, we employed a coculture system of human PBMCs with the mouse bone marrow (BM)-derived ST2 stromal cell line, which is available worldwide and can also be purchased [25]. These stromal cells are known to produce M-CSF continuously, and dexamethasone and 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] regulate the production of RANKL and the reduction of OPG [13, 26]. Osteoclast development from human PBMCs required not only coculturing with ST2 stromal cells but also the addition of human RANKL and M-CSF. Therefore, the efficient induction of osteoclasts from pOCs in human PBMCs by using this culture system may be accounted for by their presence as well as by molecule(s) other than M-CSF, RANKL, or OPG, expressed in ST2 stromal cells [27, 28].
To enrich pOCs from human PBMCs, we selected two cell-surface molecules: CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS-binding protein [29, 30], and CD16, a granulocyte marker, which is the low-affinity receptor for the Fc region of IgG [31]. pOCs were enriched in the anti-CD16 antibody column-passed (CD16-passed) anti-CD14 antibody-bound (CD14-bound) cell population by magnetic cell sorting [32, 33]. Using this culture system, the osteoclastogenesis of PBMCs from age-matched patients carrying rheumatoid arthritis (RA) and osteoporosis (OP) was compared.
Materials and methods
Reagents
1α,25(OH)2D3 (BIOMOL Research Laboratories, Plymouth Meeting, PA, USA), Dexamethasone (Sigma Chemical, St Louis, MO, USA), and recombinant human soluble RANKL (sRANKL; Pepro-Tech EC, London, UK) were purchased. Recombinant human M-CSF was a kind gift from Dr. M. Takahashi (Otsuka Pharmaceutical, Tokushima, Japan). Microbead-conjugated monoclonal mouse antihuman CD14, and rat antimouse IgG1 antibodies, were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Monoclonal mouse fluorescein isothiocyanate (FITC)-conjugated antihuman CD14 (CLB-Mon/1) and phycoerythrin (PE)-conjugated antihuman CD16 (3G8) antibodies were purchased from Nichirei (Tokyo, Japan).
Cells were cultured in alpha-minimal essential medium (α-MEM; Gibco-BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Tace Scientific, Melbourne, Australia), 50 U/ml streptomycin, and 50 μg/ml penicillin (Meiji, Tokyo, Japan) (10% FBS/α-MEM) with or without 10−8 M 1α,25(OH)2D3 and 10−7 M dexamethasone. The numbers of TRAP+ MNCs were expressed as the mean ± SD of triplicate cultures. A mouse BM-derived stromal cell line ST2 was maintained in RPMI-1640 (Gibco-BRL) supplemented with 5% FBS, 50 μM 2-mercaptoethanol (Wako Pure Chemical Industries, Osaka, Japan), streptomycin, and penicillin [25]. One day before responder cells were added, ST2 cells were harvested by treatment with 0.05% trypsin/0.54 mM ethylenediaminetetraacetic acid (EDTA) (Gibco-BRL) for 5 min and plated into a 24-well plate (Corning Costar, Corning, NY, USA) at 37°C with 5% CO2 in a humidified incubator.
Patients and healthy controls
Human peripheral blood was collected from RA patients (n = 9, 55–78 years of age; mean age 63.4 ± 8.8 years) and OP patients (n = 14, 57–77 years of age; mean age 66.4 ± 6.3 years) diagnosed according to the criteria of the American College of Rheumatology (ACR) and the Japanese Society for Bone and Mineral Research (JSBMR), respectively. As controls, peripheral blood was collected from healthy controls (HC) (n = 10, 60–79 years of age; mean age 67.6 ± 5.6 years). All patients and healthy controls were women. All participants gave written informed consent to participate in this study, and the Tottori University Faculty of Medicine Review Board for Research Involving Human Subjects at each study site approved the protocol.
Most of the RA patients were receiving nonsteroidal antiinflammatory drugs (NSAIDs) and disease-modifying antirheumatic drugs (DMARDs). None of the RA patients had been receiving steroids or methotrexates for a half year. Most of the OP patients were receiving calcium and/or vitamin D supplements, but none of them had been receiving bisphosphonates for the previous half year. All clinical and biochemical data measured by routine techniques were obtained from hospital clinical laboratories.
Cell preparation
To assess the culture condition for the osteoclastogenesis from PBMCs, blood samples were prepared from five healthy male volunteers (21–35 years old) in our laboratory. Heparinized human peripheral blood (10 ml) was diluted 1:1 in phosphate-buffered saline (PBS), layered on Ficoll–paque Plus (Amersham Biosciences, Tokyo, Japan), and centrifuged at 176g for 20 min at 4°C. The PBMC layer was collected and washed twice in 10 vol PBS. In preparation of PBMCs, when red blood cells (RBCs) still remained in fractionated cells, we treated them with Tris–buffered NH4Cl solution for lysis of RBCs. Cells were resuspended in 10% FBS/α-MEM.
To enrich CD14+ cells from PBMCs, antihuman CD14 antibody-conjugated MACS microbeads (20 μl) were added to 107 PBMCs in 80 μl PBS containing 0.5% bovine serum albumin and 2 mM EDTA (pH 7.2, washing buffer) and incubated for 15 min at 6°C. PBMCs were then washed with 10 vol washing buffer and resuspended in 500 μl washing buffer. The cell suspension was applied to a mass spectroscopy (MS) positive-selection column on a magnetic separator (Miltenyi Biotec). CD14+ cell-enriched (CD14-bound) and CD14– cell-enriched (CD14-passed) PBMCs were collected according to the manufacturer’s instructions. CD14-bound cells were used in experiments for improvement of the culture system for human osteoclastogenesis and osteoclast development from PBMCs from the three groups. For further investigation of pOCs, in the experiment for enrichment and characterization of circulating human pOCs from the three groups, magnetic cell sorting by anti-CD16 and bead-conjugated antimouse IgG1 antibodies was performed before that by the anti-CD14 antibodies. Cells were stained with FITC-conjugated anti-CD14 and PE-conjugated anti-CD16, and were analyzed using an EPICS XL flow cytometer (Coulter Electronics, Hialeah, FL, USA).
Induction of osteoclasts and pit formation assay
PBMCs or CD14+ cell-enriched PBMCs (5 × 104/well) from three groups (RA, OP, and HC) were cultured on an ST2 cell layer in 24-well tissue culture plates containing 1 ml 10% FBS/α-MEM with or without 10−8 M 1α,25(OH)2D3, 10−7 M dexamethasone, 50 ng/ml human M-CSF, and 25 ng/ml human sRANKL for 14 days. Cultures were fed every 3–4 days by replacing spent medium with fresh medium. After cultivation for 14 days, histochemical staining for tartrate-resistant acid phosphatase (TRAP) was carried out and TRAP+ multinucleated cells (MNCs; more than three nuclei) were counted by light microscopy as osteoclasts [34].
For the analysis of pit formation, cells were cultured on 6-mm-diameter dentine slices (a gift from Dr. N. Udagawa, Matsumoto Dental University) in 96-well tissue culture plates (Corning Costar) for 21 days. The cells were removed from the dentine slices with 2 N NaOH, and then the pits formed on the dentine slices were visualized by staining with Coomassie brilliant blue R250 (Wako) [35].
Frequency analysis of pOCs in PBMCs
The frequency of pOCs in human PBMCs was determined by a limiting dilution assay based on the conditions described previously [36]. Various numbers of PBMCs were inoculated into wells of 96-well plates pre-seeded with ST2 cells and were cultured for 21 days in the presence of human M-CSF, sRANKL, 1α,25(OH)2D3, and dexamethasone. Culture wells with pOCs present were determined by TRAP staining. Regardless of whether cells were mononuclear or multinuclear, we decided that the wells where TRAP+ cells were detected were pOC positive.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
To determine the expression of human TRAP (encoded by ACP6), human CD51/αv integrin (ITGAV), and human β-actin (ACTB) genes, RT-PCR was performed [37–39]. Total RNA was isolated using Isogen (Nippon Gene, Toyama, Japan) and reverse transcribed using Reverse TraAce (Toyobo, Osaka, Japan). The DNA fragments were amplified from the mouse cDNAs by PCR. Hot-lid PCR amplification of cDNA equivalent to 50 ng (ACP6 and ITGAV) and 25 ng (ACTB) of total RNA was carried out in 1× PCR buffer (1.5 mM MgCl2) containing 0.2 mM dNTPs (Takara, Shiga, Japan), 0.75 U rTaq DNA polymerase (Toyobo), and primers were used at 1.2 μM. Amplifications were carried out on DNA thermal cyclers (MJ Research, Watertown, MA, USA). Following an initial 3 min denaturing step (94°C), each PCR cycle consisted of 1 min denaturing (94°C), 1 min annealing (55°C for ITGAV, 58°C for ACTB, or 60°C for ACP6), and 1 min elongation (72°C). After the final cycle, the reaction was held for 3 min at 72°C. The PCR products were then separated on a 2% agarose gel, stained with ethidium bromide, and photographed. The primers used here were as follows: ACP6, 5′-CTG GCT GAT GGT GCC ACC CCT G-3′ and 5′-CTC TCA GGC TGC AGG CTG AGG-3′ (470 bp); ITGAV, 5′-GTT GGG AGA TTA GAC AGA GGA-3′, and 5′-CAA AAC AGC CAG TAG CAA CAA-3′ (288 bp); ACTB, 5′-GAC TAC CTC ATG AAG ATC CT-3′ and 5′-CCA CAT CTG CTG GAA GGT GG-3′ (510 bp: the primers also recognized mouse Actb).
Statistical analysis
All experimental results were expressed as means ± SD of triplicate cultures. Fisher’s protected least significant difference procedure was performed after a repeated measures analysis of variance (ANOVA) for the comparison between groups. Statistical analysis was performed using Stat View software (Version 5.0; SAS Institute, Cary, NC, USA). A P value less than 0.05 was considered significant.
Results
Improvement of the culture system for human osteoclastogenesis
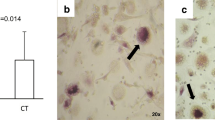
Osteoclastogenesis from healthy human PBMCs was induced by human M-CSF (50 ng/ml) and human sRANKL (25 ng/ml) for 14 days; however, efficient induction was not observed (Fig. 1a, left). To improve the culture system, a mouse stromal cell line, ST2, was used as a feeder cell layer. ST2 stromal cells are known to produce M-CSF continuously, and to induce RANKL production and reduce OPG production by addition of dexamethasone and 1α,25(OH)2D3 [13]. Human PBMCs cocultured with ST2 cells did not efficiently develop osteoclasts with or without human sRANKL (Fig. 1a, left). Although we added 10−7 M dexamethasone and 10−8 M 1α,25(OH)2D3 in the presence of human M-CSF, osteoclastogenesis was not accelerated. When PBMCs were cultured on ST2 cell layers with both human M-CSF and sRANKL, significant numbers of TRAP+ MNCs were generated with or without dexamethasone and 1α,25(OH)2D3 (Fig. 1a, left). The developed TRAP+ MNCs resorbed dentine slices and formed pits (Fig. 1b, c). When mouse BM cells were used as pOCs, significant numbers of TRAP+ MNCs developed in the presence of human M-CSF and human sRANKL, or of cocultures with ST2 cells supplemented with dexamethasone and 1α,25(OH)2D3 (data not shown).
Comparison of culture systems for human osteoclastogenesis from peripheral blood mononuclear cells (PBMCs) and CD14 + cell-enriched population. a Whole (left) or CD14-magnetic bead-bound (right) PBMCs from healthy donors were cultured for 14 days with or without human macrophage-colony stimulating factor (M-CSF) (50 ng/ml), human receptor activator of nuclear factor (NF)-kappa B ligand (sRANKL) (25 ng/ml), 10−7 M dexamethasone (Dex), and/or 10−8 M 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) [26]. Columns 3–7 were cocultured with ST2 cells. Data from one of maximal responses shown represents the mean ± SD of triplicate cultures. Typical tartrate-resistant acid phosphatase-positive multinucleated osteoclasts (MNCs) (b) and pit formation (c) were observed in the culture on ST2 cells with M-CSF, RANKL, dexamethasone, and 1α,25(OH)2D3. d The gene expression of human TRAP (ACP6), CD51/αv integrin (ITGAV), and β-actin (ACTB) was detected by reverse transcription-polymerase chain reaction (RT-PCR). RNA samples were prepared from four culture wells in ST2 without PBMCs or in the indicated culture conditions as in a
To assess the expression of other markers for the osteoclast lineage, RT-PCR was performed, and detected the gene expression of human TRAP (ACP6), and CD51/αv integrin (ITGAV) (Fig. 1d).
Enrichment of circulating human pOCs
Since Komano et al. reported that pOCs in PBMCs were enriched in CD14+ cell fraction [33], we prepared CD14+ cells by magnetic cell sorting using microbeads conjugated with an antihuman CD14 antibody. The sorted (column-bound) population contained 94.7 ± 7.3% CD14+ cells in representative experiments. The CD14+ cell-enriched populations were cocultured with ST2 cells in the presence of human M-CSF and sRANKL for 14 days. Compared with the osteoclastogenesis from unfractionated PBMCs, up to 30-fold-higher numbers of TRAP+ MNCs were induced from the CD14+ cell-enriched fraction (Fig. 1a, right). Interestingly, in cultures without dexamethasone and 1α,25(OH)2D3, more TRAP+ MNCs were observed than in those with these reagents. A small number of TRAP+ MNCs were observed when human PBMCs were cocultured with ST2 cells in the presence of dexamethasone and 1α,25(OH)2D3 for 14 days (data not shown).
In this experiment, because human PBMCs were cocultured with mouse stromal cells for 2 weeks, responses directed to xenogeneic antigens by immunocompetent cells in the human PBMCs might be considered. Compared with whole PBMCs, a CD14+ cell-enriched population, which contained less than 2.4% of CD4+ or CD8+ cells, efficiently differentiated into osteoclasts, meaning that immune responses to mouse antigens probably did not play a major role in this osteoclastogenesis. Whole PBMCs contained more T and B cells than the CD14+ cell-enriched population. However, the following experiments mainly used this coculture system with dexamethasone and 1α,25(OH)2D3 unless otherwise indicated, because these reagents are also known to be immunosuppressants [40].
As the aim of this study was to produce a simple system for the enrichment of pOCs, we prepared cell populations only by using the MACS system. To characterize CD14+ populations in PBMCs, we performed magnetic bead-cell sorting by using anti-CD14 antibody. Anti-CD14 antibody-column bound (CD14-bound) and -passed (CD14-passed) populations were further stained with FITC-anti-CD14 and PE-anti-CD16 antibodies and analyzed by a flow cytometer. CD14-bound cells mainly consisted of two populations, CD14high CD16dull and CD14low CD16high (Fig. 2a). Therefore, PBMCs from young volunteers were purified with the depletion of CD16+ cells before selection of CD14+ cells by MACS (Fig. 2b).
Osteoclast precursor (pOC) enrichment from PBMCs by magnetic cell sorting. a CD14-bound cells consisted of CD14high CD16dull and CD14low CD16high populations. b Fractionated cell populations using anti-CD16 and anti-CD14 antibody beads were analyzed; forward (FS)/side (SS) scatter profile (upper), CD14 and CD16 profile (middle), and cytospinning samples (bottom). c Fractionated cell populations were cultured on ST2 cells with M-CSF, RANKL, dexamethasone, and 1α,25(OH)2D3 for 14 days, and the numbers of TRAP+ MNCs were counted
To assess the purity, flow cytometry analysis was carried out, and each fractionated cell population was cultured on ST2 stromal cells with human M-CSF, human sRANKL, dexamethasone, and 1α,25(OH)2D3 for 14 days. The majority of osteoclasts were generated from the CD16-passed CD14-bound fraction (Fig. 2c). This fraction consisted mainly of CD14high CD16dull monocyte-like cells (Fig. 2b, bottom). The CD16-passed CD14-passed PBMC fraction consisted of lymphocytes, and the CD16-bound PBMC fraction consisted of granulocytes (Fig. 2b, bottom). The developed TRAP+ MNCs from the CD16– CD14+ cells only resorbed dentine slices and formed pits (data not shown).
Presence of comparable cell populations in RA and OP PBMCs
As shown in Fig. 2, pOCs were enriched in the CD16-passed CD14-bound fraction. To characterize PBMCs from age matched RA (n = 9), OP (n = 14), and HC (n = 10) individuals, flow cytometry analysis was performed by indicating the expression of CD14 and CD16. The ratio of the CD14+ (Table 1, 5th line) and CD16+ (Table 1, 7th line) populations was comparable among the three groups. The numbers of PBMCs were comparable (Table 1, 4th line), and the cell numbers of each population in PBMCs were also within the range of HCs. We compared the percentage of CD14high and CD16high cell populations among the three groups, but no difference was detected (Table 1, 6th and bottom lines).
Osteoclast development from PBMCs in OP, RA, and HC individuals
To assess the relationship of two bone diseases, RA (n = 9) and OP (n = 14) with pOCs in PBMCs, we cultured PBMCs (5 × 104/well) from OP and RA patients on ST2 stromal cells with human M-CSF, human sRANKL, dexamethasone, and 1α,25(OH)2D3 for 14 days. PBMCs from HC (n = 10) were used as a control. The mean number of TRAP+ MNCs from RA patient cultures seemed higher than those from OP or HC cultures (Table 2, 1st line). OP patient cultures generated lower numbers of TRAP+ MNCs than those from HC cultures (Table 2, 1st line). However, a variety of results from patient to patient were obtained, and therefore statistically significant differences between RA and HC (P = 0.070) and between OP and HC (P = 0.679) groups were not present. Only the difference between the RA and OP groups was significant (P = 0.019).
Using a limiting dilution assay [36], we assessed the frequency of pOCs in PBMCs. The presence of pOCs in the well was indicated by the generation of TRAP+ cells regardless of mononuclear or multinuclear cells in the 14-day cultures. The means of the frequencies were higher in OP than in RA or HC patients; however, there were no significant differences among the three groups (see Table 2). To assess the relationship of osteoclast generation and frequency of pOCs, the numbers of TRAP+ MNCs generated in the cultures were divided by the cultured cell number (5 × 104/well) and the frequency. These results suggested that OP patients increased the number of pOCs in PBMCs (Table 2, third line), but their single pOCs might give rise to lower numbers of osteoclasts than pOCs in PBMCs of RA and HC patients, although a significant difference was not present with the RA group (Table 2, bottom line). Furthermore, we observed that PBMCs from RA patients gave rise to more TRAP+ MNCs than those from OP patients, although a significant difference was only observed between RA and OP patients (Table 2, first line). We also found the frequency of pOCs in PBMCs and calculated the number of osteoclasts from single pOCs. The frequency of PBMCs from RA and OP patients appeared higher than that from HC patients; however, significant differences were not observed (see Table 2, second line).
Discussion
In this study, we developed a culture system for osteoclastogenesis from human PBMCs. Employing coculture with a mouse stromal cell line ST2, osteoclast development could be induced efficiently. CD14+ cell enrichment from PBMCs by using magnetic cell sorting further accelerated osteoclastogenesis. In this culture system, we compared the characteristics of RA and OP PBMCs, and observed that pOCs in PBMCs might be not identical in each disease.
There have been many reports of induction of TRAP+ MNCs in cultures from PBMCs [41, 42]. However, the induction from human PBMCs was not effective compared with that from mouse samples. Here, we showed the coculture system of human PBMCs with mouse ST2 cells supplemented with human M-CSF and sRANKL, dexamethasone, and 1α,25(OH)2D3 effectively induced osteoclastogenesis. Our culture system employed lower numbers of cells in cultures compared with previous reports. In this condition, only M-CSF and RANKL might not be sufficient for inducing osteoclastogenesis from PBMCs. Because our focus is also counting pOCs, including for frequency analysis, our system was adjusted for low numbers (5 × 104 cells/well in our cultures; 50–100 × 104 cells/well in standard cultures). Concentration of M-CSF and RANKL in our cultures is lower than in standard cultures (e.g., M-CSF 100 ng/ml and RANKL 100 ng/ml); however, low numbers of cells in a higher volume of medium and larger-sized wells (24 well plates) seem to induce more precise numbers of osteoclastogenesis from pOCs than those in standard cultures. We have experienced that numbers of TRAP+ MNCs generated in cultures were not linearly increased over numbers of cells added in cultures, especially when large numbers of cells were cultured.
Interestingly, the effect of ST2 cells on human osteoclast development might not result from the production of mouse M-CSF or RANKL, but the expression of other molecule(s) on the stromal cells. In fact, the large numbers (>500) of TRAP+ MNCs, including more than 60 cells with more than ten nuclei, were induced from mouse BM cells (1 × 104/well) by 50 ng/ml human M-CSF and 25 ng/ml human sRANKL for 7 days culture. The osteoclastogenesis from mouse BM cells induced by 50 ng/ml human M-CSF and 25 ng/ml human sRANKL was completely inhibited by the addition of 100 ng/ml human OPG, but was not inhibited by the same dose of mouse OPG (data not shown), suggesting that other molecule(s) on ST2 cells might play an important role in this augmentation. As the results were very interesting but the reason remained unsolved, we will continue to assess the problem.
The candidates supplied from ST2 cells may contain adhesion molecules directed to integrin αvβ3 on pOCs and/or signals to FcRγ or DAP12 [43–45]. Enrichment of CD14+ cells increased osteoclastogenesis, indicating that immune reactions might not be involved in this response, although human responder cells were cocultured with xenogeneic stromal cells in vitro. ST2 cells are well known BM-derived pre-adipocytic cells, which are distributed worldwide and can be purchased from RIKEN Cell Bank (Tsukuba, Japan) [25]. Experiments using individual human osteoblast-like cells have to consider the different influences from each stromal cell. Therefore, ST2 cells enable us to supply relatively constant conditions and assess the differences in pOC characteristics in each osteolytic disorder.
The aim of this study was development of an efficient culture system for human osteoclastogenesis and providing a simple system for the enrichment of pOCs. Therefore, we employed the MACS system for cell preparations as previously reported by Komano et al. [33]. PBMCs contained two CD14+ cell populations, CD14high CD16dull cells and CD14low CD16high cells, and pOCs were enriched in the CD14high CD16dull cell population, which mainly consisted of monocytes (see Fig. 2). Although in a recent study it appeared that the number of CD14+ CD16+ monocytes increased in a host of inflammatory and infectious diseases in humans, our study showed that RA and OP patients did not increase the number or the ratio of this CD14high CD16dull cell fraction compared with healthy donors (see Table 1) [46].
We induced the TRAP+ MNCs from PBMCs under these culture conditions, and observed that PBMCs from RA patients gave rise to more TRAP+ MNCs than those from OP patients, although a significant difference was only observed between RA and OP patients (see Table 2). We also assessed the frequency of pOCs in PBMCs and calculated the number of osteoclasts from single pOCs. The frequency of PBMCs from RA and OP patients seemed higher than that from HC patients; however, significant differences were not observed. Compared with RA and HC groups, single pOCs in the OP group might give rise to lower numbers of TRAP+ MNCs (see Table 2), resulting in lower numbers of TRAP+ MNCs in the culture. Both RA and postmenopausal OP cause bone loss. However, each state may have a different route to develop into osteoporosis, which may mean that the capacity of differentiation and proliferation of pOCs in OP patients is not identical to that in HC or RA patients. Although recent studies showed that osteoclast differentiation, function, and survival were strongly influenced by local inflammatory cytokines and growth factors produced at sites of joint inflammation, in this study we propose that the osteoclast precursors themselves in RA were different from those in OP and HC. These different processes resulting in activated osteoclasts may make it difficult to investigate osteoporosis. It is important to determine the activities of mature osteoclasts generated from peripheral blood mononuclear cells by a pit assay. However, the aim of this study was to examine the capacity of differentiation and proliferation in osteoclast precursors.
It is still not clear why numbers of TRAP+ MNCs made an enormous difference between PBMCs and the CD14+-enriched fraction (see Fig. 1), although one-fourth to one-third of PBMCs were CD14+ cells, and we showed data of maximal responses examined in this study. This finding may mean that fractionation by using magnetic beads not only enriched CD14+ cells, but also removed CD14– cell fractions that inhibit osteoclastogenesis.
In conclusion, we developed a novel culture system for osteoclastogenesis from human PBMCs and observed that the numbers of mature OCs from single pOCs in the OP group were lower than in the RA group. The characteristics of pOCs may differ between RA and OP. Further investigation of this process will accelerate the analysis of the pathology of osteoporosis and the development of preventative therapies and treatments.
References
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Mundy GR (1999) Bone remodeling and its disorders, 2nd edn. Martin Dunitz, London
Walsh NC, Crotti TN, Goldring SR, Gravallese EM (2005) Rheumatic diseases: the effects of inflammation on bone. Immunol Rev 208:228–251
Clowes JA, Riggs BL, Khosla S (2005) The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 208:207–227
Goldring SR, Granallese EM (2000) Pathogenesis of bone erosions in rheumatoid arthritis. Curr Opin Rheumatol 12:195–199
American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines (2002) Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum 46:328–346
De Martinis M, Di Benedetto MC, Mengoli LP, Ginaldi L (2006) Senile osteoporosis: is it an immune-mediated disease? Inflamm Res 55:399–404
Suda T, Udagawa N, Takahashi N (1996) Cells of bone: osteoclast generation. In: Bilezikian JP, Raisz LG, Roden GA (eds) Principles of bone biology. Academic Press, New York, pp 87–102
Hayashi SI, Yamane T, Miyamoto A, Hemmi H, Tagaya H, Tanio Y, Kanda H, Yamazaki H, Kunisada T (1998) Commitment and differentiation of stem cells to the osteoclast lineage. Biochem Cell Biol 76:911–922
Fujikawa Y, Quinn JM, Sabokbar A, McGee JO, Athanasou NA (1996) The human osteoclast precursor circulates in the monocyte fraction. Endocrinology 137:4058–4060
Yoshida H, Hayashi SI, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature (Lond) 345:442–444
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Theill LE, Boyle WJ, Penninger JM (2002) RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 20:795–823
Lagasse E, Weissman IL (1997) Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell 89:1021–1031
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K (1998) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139:1329–1337
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Hirayama T, Danks L, Sabokbar A, Athanasou NA (2002) Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology 41:1232–1239
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature (Lond) 402:304–309
Carlsten H (2005) Immune responses and bone loss: the estrogen connection. Immunol Rev 208:194–206
Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y (2006) Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 24:33–63
Tanaka S, Nakamura K, Takahasi N, Suda T (2005) Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev 208:30–49
Tsurukai T, Udagawa N, Matsuzaki K, Takahashi N, Suda T (2000) Roles of macrophage-colony stimulating factor and osteoclast differentiation factor in osteoclastogenesis. J Bone Miner Metab 18:177–184
Holmes SG, Still K, Buttle DJ, Bishop NJ, Grabowski PS (2004) Chemically modified tetracyclines act through multiple mechanisms directly on osteoclast precursors. Bone 35:471–478
Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T (1989) The bone marrow-derived stromal cell line MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology 125:1805–1813
Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M, Kurino T, Hayashi SI, Sakano S (2003) Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood 101:2227–2234
Kollet O, Dar A, Lapidot T (2007) The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol 25:51–69
Takayanagi H (2005) Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med 83:170–179
Massey HM, Flanagan AM (1999) Human osteoclasts derived from CD14-positive monocytes. Br J Haematol 106:167–170
Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC (1990) CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431–1433
Unkeless J (1989) Function and heterogeneity of human Fc receptors for immunoglobulin G. J Clin Invest 83:355–361
Nicholson GC, Malakellis M, Collier FM, Cameron PU, Holloway WR, Gough TJ, Gregorio-king C, Kirkland MA, Myers DE (2000) Induction of osteoclasts from CD14-positive human peripheral blood mononuclear cells by receptor activator of nuclear factor κB ligand (RANKL). Clin Sci 99:133–140
Komano Y, Nanki T, Hayashida K, Taniguchi K, Miyasaka N (2006) Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res Ther 8:R152
Shevde N, Anklesaria P, Greenberger JS, Bleiberg I, Glowacki J (1994) Stromal cell-mediated stimulation of osteoclastogenesis. Proc Soc Exp Biol Med 205:306–315
Tamura T, Takahashi N, Akatsu T, Sasaki T, Udagawa N, Tanaka S, Suda T (1993) New resorption assay with mouse osteoclast-like multinucleated cells formed in vitro. J Bone Miner Res 8:953–960
Hayashi SI, Miyamoto A, Yamane T, Kataoka H, Ogawa M, Sugawara S, Nishikawa S, Nishikawa S, Sudo T, Yamazaki H, Kunisada T (1997) Osteoclast precursors in bone marrow and peritoneal cavity. J Cell Physiol 170:241–247
Yang CR, Wang JH, Hsieh SL, Wang SM, Hsu TL, Lin WW (2004) Decoy receptor 3 (DcR3) induces osteoclast formation from monocyte/macrophage lineage precursor cells. Cell Death Differ 11:S97–S107
Hofmann G, Bernabei PA, Crociani O, Cherubini A, Guasti L, Pillozzi S, Lastraioli E, Polvani S, Bartolozzi B, Solazzo V, Gragnani L, Defilippi P, Rosati B, Wanke E, Olivotto M, Arcangeli A (2001) HERG K+ channels activation during β1 integrin-mediated adhesion to fibronectin induces an up-regulation of αvβ3 integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem 276:4923–4931
Schoeler D, Grützkau A, Henz BM, Küchler J, Krüger-Krasagakis S (2003) Interleukin-6 enhances whereas tumor necrosis factor α and interferons inhibit integrin expression and adhesion of human mast cells to extracellular matrix proteins. J Invest Dermatol 120:795–801
Swanson C, Lorentzon M, Conaway HH, Lemer UH (2006) Glucocorticoid regulation of osteoclast differentiation and expression of receptor activator of nuclear factor-kappaB (NF-κB) ligand, osteoprotegerin, and receptor activator of NF-κB in mouse calvarial bones. Endocrinology 147:3613–3622
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature (Lond) 423:337–342
Quinn JM, Neale S, Fujikawa Y, McGee JO, Athanasou NA (1998) Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int 62:527–531
Ross FP, Teitelbaum SL (2005) αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev 208:88–105
Maekawa TL, Takahashi TA, Fujihara M, Urushibara N, Kadowaki-Kikuchi E, Nishikawa M, Ikebuchi K, Asano S, Ozawa K, Sekiguchi S (1997) A novel gene (drad-1) expressed in hematopoiesis-supporting stromal cell lines, ST2, PA6 and A54 preadipocytes: use of mRNA differential display. Stem Cells 15:334–339
Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature (Lond) 428:758–763
Ziegler-Heitbrock L (2007) The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81:584–592
Acknowledgments
We thank Drs. N. Takakura (Osaka University) and M. Yoshino (Tottori University) for helpful suggestions, M. Takahashi (Otsuka Pharmaceutical Co. Ltd) for M-CSF, and N. Udagawa (Matsumoto Dental University) for dentine slices. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and from the Molecular Medical Science Institute, Otsuka Pharmaceutical Co., Ltd, Tokushima, Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nose, M., Yamazaki, H., Hagino, H. et al. Comparison of osteoclast precursors in peripheral blood mononuclear cells from rheumatoid arthritis and osteoporosis patients. J Bone Miner Metab 27, 57–65 (2009). https://doi.org/10.1007/s00774-008-0011-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-008-0011-0