Abstract
Osteoclasts are multinucleated giant cells that originate from a monocyte/macrophage lineage, and are involved in the inflammatory bone destruction accompanied by periodontitis. Recent studies have shown that osteoclast precursors reside not only in the bone marrow, but also in the peripheral blood and spleen, though the precise characteristics of each precursor have not been analyzed. We hypothesized that the number of osteoclast precursors in those tissues may increase under pathological conditions and contribute to osteoclast formation in vivo in a mouse model. To test this hypothesis, we attempted to identify cell populations that possess osteoclast differentiation potential in the bone marrow, spleen, and blood by analyzing macrophage/monocyte-related cell surface markers such as CD11b, CD14, and colony-stimulating factor-1 receptor (c-Fms). In the bone marrow, the CD11b− cell population, but not the CD11b+ cell population, differentiated into osteoclasts in the presence of receptor activator of nuclear factor-κB ligand and macrophage colony-stimulating factor. On the other hand, in the spleen and blood, CD11b+ cells differentiated into osteoclasts. Interestingly, lipopolysaccharide (LPS) administration to the mice dramatically increased the proportion of CD11b+ c-Fms+ CD14+ cells, which differentiated into osteoclasts, in the bone marrow and spleen. These results suggest that LPS administration increases the proportion of a distinct cell population expressing CD11b+, c-Fms+, and CD14+ in the bone marrow and spleen. Thus, these cell populations are considered to contribute to the increase in osteoclast number during inflammatory bone destruction such as periodontitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoclasts are multinucleated giant cells that originate from hematopoietic cells of the monocyte/macrophage lineage (Ash et al. 1980; Xing et al. 2005). Stimulation by macrophage colony-stimulating factor (M-CSF, also called CSF-1) and receptor activator of NF-κB ligand (RANKL) produced by osteoblasts induces the differentiation of osteoclast precursors into osteoclasts (Kong et al. 1999; Takahashi et al. 1999; Wong et al. 1997; Yasuda et al. 1998b). M-CSF stimulation, via its receptor M-CSF receptor (c-Fms) and downstream signaling pathway, is required for the formation of osteoclast precursors and subsequent differentiation into mature osteoclasts (Takahashi et al. 1991; Yoshida et al. 1990). RANKL is a membrane-bound cytokine produced by osteoblasts, which plays a role in triggering osteoclast differentiation via its receptor RANK (Takahashi et al. 1999; Yasuda et al. 1998a, b). RANKL- or RANK-deficient mice exhibit osteopetrosis due to impaired differentiation or bone-resorbing function of osteoclasts (Kong et al. 1999). In addition, osteoblasts produce not only RANKL but also osteoprotegerin (OPG), a decoy receptor of RANKL that inhibits osteoclast differentiation by interrupting the RANKL–RANK interaction (Suda et al. 1999; Udagawa et al. 2000; Yasuda et al. 1998a, b).
Osteoclast precursors have been thought to reside in the bone marrow. However, recent studies suggest that osteoclast precursors are also present in the spleen and blood, from which the cells migrate into the bone tissue and differentiate into osteoclasts in response to environmental changes (Fujikawa et al. 1996; Jacome-Galarza et al. 2013; Shi and Pamer 2011). Nakamichi et al. (2012) reported that the spleen preserves osteoclast precursors in M-CSF-mutated op/op mice. Furthermore, Ishii et al. demonstrated the migration of osteoclast precursors from the blood into the bone tissues after sphingosine-1-phosphate stimulation by live imaging (Ishii et al. 2009). Therefore, the existence of various types of osteoclast precursors has been suggested not only in the bone but also in peripheral organs such as the spleen and blood (Walker 1975a, b).
Since osteoclasts are derived from the monocyte/macrophage lineage, several studies have identified cell-surface markers of osteoclast precursors in the bone marrow such as CD11b (MAC1), Ly6c, c-Fms, c-kit, CX3CR1, and RANK (Charles et al. 2012; Jacome-Galarza et al. 2013). Miyamoto et al. (2000) isolated osteoclast precursors bearing the phenotype c-Kit (CD117)+, c-Fms (CD115)+, RANK− from the bone marrow and demonstrated their ability to differentiate into osteoclasts. On the other hand, Takeshita et al. (2000) have reported that the expression of surface markers such as CD14, F4/80, and FcγII/IIIR change during osteoclast differentiation.
In inflammatory conditions such as periodontitis and rheumatoid arthritis, osteoclast precursors are recruited to the site of inflammation and cause pathological bone resorption (Nair et al. 1996; Poltorak et al. 1998). Especially in periodontal disease, bacterial constituents such as lipopolysaccharide (LPS), peptidoglycan, bacterial DNA, and lipoteichoic acid bind to Toll-like receptors (TLRs) and activate immune cells followed by inflammatory factor production (Nair et al. 1996; Poltorak et al. 1998). Activation of the immune system accelerates osteoclastogenesis from the precursors, which results in excess alveolar bone resorption. However, the precise mechanisms by which bacterial constituents increase osteoclast number have not been elucidated.
In the present study, we hypothesized that the number of cells that possess osteoclast differentiation potential may increase and differentiate into mature osteoclasts. In order to examine this hypothesis, we attempted to identify the cells that present osteoclast differentiation potential using cell-surface markers such as CD11b, c-Fms, and CD14. We found that CD11b-negative (CD11b−) cells in the bone marrow and CD11b-positive (CD11b+) cells in the blood differentiated into osteoclasts, while neither CD11b+ nor CD11b− cells did in the spleen. Interestingly, after administration of LPS in the mice, CD11b+ cells in the bone marrow and spleen differentiated into osteoclasts. We further revealed that these cells in the bone marrow and spleen are CD11b+ CD14+ c-Fms+ cells. These results suggest that LPS administration increases the CD11b+ CD14+ c-Fms+ cell population that possesses osteoclast differentiation potential.
Materials and methods
Animals
C57BL/6 male mice (6 weeks old), C3H/Hen male mice (6 weeks old) and C3H/HeJ male mice (6 weeks old) were purchased from Sankyo Laboratories Animal Center (Sankyo Labo Service Corporation; Tokyo, Japan). Mice were housed and acclimated in cages with free access to food and water for 1 week. Mice were subjected to intraperitoneal injection of LPS (500 μg/kg) from Escherichia coli (Sigma-Aldrich; St Louis, MO, USA) or saline. After 24 h, cells from the bone marrow, spleen, and blood were collected and cell-surface marker analysis and cell culture were performed. The Showa University Animal Care and Use Committee and Medical Ethics Committee approved the animal experiments performed in this study.
Cell preparation and separation
Bone marrow cells were collected from the tibiae and femora of the mice. The spleen was removed from the mice and gently smashed in phosphate buffered saline (PBS). Cells were then transferred to a 15-mL tube, spin down, the supernatant was removed, and the pellet was lysed and washed with 10 mL of red blood cell lysing buffer, and then spun down again and resuspended with 1 mL of 2 mM ethylenediaminetetraacetic acid in PBS and counted. Blood cells were prepared from 6-week-old mice and layered onto Lympholyte-mammal® medium (Cedarlane Laboratories; Burlington, ON, Canada). After centrifugation, the monocyte fraction was collected. Subsequently, monocyte/macrophage lineage cells were magnetically separated using a QuadroMACS™ magnet, an MS column (Miltenyi Biotec; Auburn, CA, USA), CD11b microbeads (Miltenyi Biotec), and anti-biotin microbeads (Miltenyi Biotech). The cells that were bound to the microbeads were maintained in the column and constituted the positive cell fraction, whereas those that failed to bind to the microbeads passed through the column and constituted the negative cell fraction. CD11b+ and CD11b− cells were then labeled with an anti-c-Fms antibody (Miltenyi Biotec).
Flowcytometry
For flow cytometric analysis, the cells were labeled with the PE-conjugated c-Fms (CD115) (Miltenyi Biotech), FITC-conjugated CD11b, and biotin-conjugated CD14 antibodies (eBioscience, Poway, CA, USA) followed by APC-conjugated streptavidin secondary antibodies (Southern Biotechnology Associated Inc.; Birmingham, AL, USA). The cells were pre-incubated for 30 min at 4 °C with FcR blocking reagent (Miltenyi Biotec) before specific antibody staining. On every experimental session, isotype-matched antibodies were used as controls (see above). The cells were analyzed or sorted using FACSAriaII or FACSVerse, respectively (Becton-Dickinson & Co; Mountain View, CA, USA). Gating strategy of osteoclast precursors by FACS analysis or sorting are shown in supplemental Fig. 1. In brief, after doublets and dead cells were gated out by 7-AAD, the single living cells were classified into each cell populations using each antibody.
Cell cultures
Cells were cultured in α-modified essential medium (α-MEM; Sigma-Aldrich) containing 10% fetal bovine serum (FBS; Gibco, Life Technologies; Carlsbad, CA, USA) with 50 ng/mL of M-CSF (Leukoprol®, JCR Pharmaceuticals Co.; Hyogo, Japan) and 100 ng/mL of RANKL (R&D Systems; Minneapolis, MN, USA) in 96-well culture plates (1 × 105 cells/0.2 mL/well; Thermo Fisher Scientific Nunc A/S; Roskilde, Denmark) for 5 days. Cells were then fixed and stained for tartrate-resistant acid phosphatase (TRAP), an osteoclast marker enzyme. TRAP-positive multinucleated cells containing 3 or more nuclei were counted as osteoclasts.
Measurement of TRAP activity
Cells in 96-well culture plates were rinsed twice with PBS and dissolved with 150 µL of lysis buffer (50 mM acetic acid buffer (pH 5.0) containing 1% sodium tartrate and 0.1% triton-X 100). The cell lysates were sonicated to completely dissolve the cell constituents, 50 µL of p-nitrophenyl phosphate solution (1 mg/mL in 50 mM acetic acid buffer (pH 5.0) containing 1% sodium tartrate) was added, and the cells were incubated at 37 °C for 30 min. After incubation, 100 µL of 1 M NaOH was added to the lysates and absorbance was measured at 405 nm using an absorption spectrometer (Corona Electric, SH-1000; Ibaraki, Japan).
Statistical analysis
Data were evaluated by the Student t test for statistical analysis (P < 0.05 was considered as statistically significant). All data are presented as the mean ± standard deviation (SD). Results are representative of more than four individual experiments.
Results
The bone marrow CD11b− cells as well as the spleen and blood CD11b+ cells possess osteoclast differentiation potential
CD11b is a typical cell-surface marker of the monocyte/macrophage lineage that is thought to include osteoclast precursors. Thus, we first separated the cells obtained from the bone marrow, spleen, and blood into CD11b+ and CD11b− cell populations and examined their osteoclast differentiation potential. When CD11b+ cells and CD11b− cells from the bone marrow were individually cultured in the presence of M-CSF and RANKL, a significantly larger number of osteoclasts formed in the CD11b− cell culture than that of CD11b+ cell culture (Fig. 1a, b). In contrast, a small number of osteoclasts formed in CD11b+ cell cultures and no osteoclasts formed in CD11b− cell cultures prepared from the spleen (Fig. 1a, b). In blood cell cultures, osteoclasts formed in CD11b+ cell cultures, but not in CD11b− cell cultures (Fig. 1a, b). These results suggest that the bone marrow presents CD11b− cells, while the spleen and blood present CD11b+ cells, which possess osteoclast differentiation potential, respectively.
Osteoclast differentiation potential of CD11b+ and CD11b− cells from the bone marrow, spleen, and blood. Cells from the bone marrow, spleen, and blood were separated into CD11b+ and CD11b− cells using magnetic-labeled anti-CD11b antibodies. The cells (1 × 105) were then cultured in the presence of RANKL (100 ng/mL) and M-CSF (50 ng/mL) in 96-well culture plates. After 5 days of culture, cells were fixed and stained for TRAP, an osteoclast marker enzyme. The cells stained in red are osteoclasts (a). TRAP-positive multinucleated cells were counted (b). Data are presented as the mean values of four independent experiments. The error bars represent the SD. **P < 0.01
LPS increases the CD11b+ cell population possessing osteoclast differentiation potential in the bone marrow and spleen
In order to examine the effects of inflammation on the osteoclast differentiation potential of the cells in the bone marrow, spleen, and blood, we administered LPS to the mice and obtained cells from each tissue after 24 h (Fig. 2a, b). In bone marrow cell cultures, osteoclasts formed not only in CD11b− but also in CD11b+ cell cultures. The number of osteoclasts formed in CD11b+ cell cultures was significantly greater than that of CD11b− cell cultures (Fig. 2a, b). In splenocyte cultures, multinucleated giant osteoclasts formed in CD11b+ cell cultures, but not in CD11b− cell cultures (Fig. 2a, b). In blood cell cultures, osteoclasts formed in CD11b+, but not in CD11b− cell cultures (Fig. 2a, b). These results indicate that LPS administration induces new cell populations expressing CD11b with osteoclast differentiation potential in the bone marrow and spleen.
Effect of LPS administration on the osteoclast differentiation potential of CD11b+ and CD11b− cells in the bone marrow, spleen, and blood. Mice were injected intraperitoneally with LPS (500 μL/kg) or saline. After 24 h, cells from the bone marrow, spleen, and blood were separated into CD11b+ and CD11b− cells using magnetic-labeled anti-CD11b antibodies. The cells (1 × 105) were then cultured in the presence of RANKL (100 ng/mL) and M-CSF (50 ng/mL) in 96-well culture plates. After 5 days of culture, cells were fixed and stained for TRAP, an osteoclast marker enzyme. The cells stained in red are osteoclasts (a). TRAP-positive multinucleated cells were counted (b). Data are presented as the mean values of four independent experiments. The error bars represent the SD. **P < 0.01
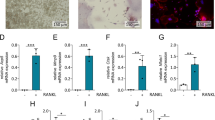
CD11b− c-Fms+ CD14+ cells in the bone marrow and CD11b+ c-Fms+ CD14+ cells in the spleen and blood possess osteoclast differentiation potential
We further attempted to identify the distinct cell populations that possess osteoclast differentiation potential by analyzing additional monocyte/macrophage markers such as c-Fms and CD14 using a cell sorting function of the flow cytometer (Fig. 3). Among the CD11b− cell population from the bone marrow, c-Fms+ CD14+ cells differentiated into osteoclasts. In addition, c-Fms+ CD14− cells also differentiated into osteoclasts though the number of osteoclasts was smaller than that of c-Fms+ CD14+ cells (Fig. 3, upper line panels). Among the CD11b+ cell population from the spleen, c-Fms+ CD14+ cells differentiated into osteoclasts. A smaller number of osteoclasts was also formed in the c-Fms+ CD14− cell population and no osteoclasts formed in the c-Fms− population (Fig. 3, middle line panels). Similar to the spleen, among the blood CD11b+ cell population, c-Fms+ CD14+ cells differentiated into osteoclasts and a smaller number of osteoclasts formed from the c-Fms+ CD14− cell population (Fig. 3, lower line panels). No osteoclasts formed in the c-Fms− cell population from the bone marrow, spleen, and blood.
Analysis of c-Fms and CD14 expression on cells and their osteoclast differentiation potentials. c-Fms and CD14 expression on CD11b− cells from the bone marrow (upper line panels) and on CD11b+ cells from the spleen (middle line panels) or blood (lower line panels) was analyzed (left column panels). Cells were isolated into c-Fms+ CD14− cells, c-Fms+ CD14+ cells, c-Fms− CD14− cells, and c-Fms− CD14+ cells, respectively using a flow cytometer equipped with a cell sorter. The isolated cells (1 × 105) were cultured in the presence of RANKL (100 ng/mL) and M-CSF (50 ng/mL) in 96-well culture plates. After 5 days of culture, cells were fixed and stained for TRAP, a marker enzyme of osteoclasts. TRAP-positive multinucleated cells were counted (middle column panels). The cells stained in red are osteoclasts (right column photos). Data are presented as the mean values of four independent experiments. The error bars represent the SD. **P < 0.01
LPS increases the CD11b+ c-Fms+ CD14+ cell population that possesses osteoclast differentiation potential in the bone marrow and spleen
Since LPS increased alternative populations presenting osteoclast differentiation potential such as CD11b+ cells in the bone marrow and but not in the blood (Figs. 1, 2), we further analyzed the effects of LPS administration on these cell populations of bone marrow and spleen using cell-surface markers, including c-Fms and CD14 (Fig. 4). In cells isolated from the bone marrow of control mice, the proportion of CD11b+ c-Fms+ CD14+ cells was 0.99%, whereas in the bone marrow cells of LPS-administered mice, this proportion increased to 12.34%. This cell population differentiated into osteoclasts (Fig. 4, upper panels). Similarly, the proportion of CD11b+ c-Fms+ CD14+ cells in the spleen of control mice was 0.48%, while that of LPS-administered mice increased to 33.76%. This cell population also differentiated into osteoclasts (Fig. 4, lower panels). These results indicate that LPS increases CD11b+ c-Fms+ CD14+ cells that can differentiate into osteoclasts.
Effects of LPS administration on cell-surface markers and examination of osteoclast differentiation potential of each cell population. c-Fms and CD14 expression levels on CD11b+ cells from the bone marrow (upper line panels) and spleen (lower line panels) were analyzed by FACS (left column panels). CD11b+ c-Fms+ CD14+ cells were isolated using a flow cytometer equipped with a cell sorter. The isolated cells (1 × 105) were cultured in the presence of RANKL (100 ng/mL) and M-CSF (50 ng/mL) in 96-well culture plates. After 5 days of culture, cells were fixed and stained for TRAP, an osteoclast marker enzyme. TRAP-positive multinucleated cells were counted (middle column panels). The cells stained in red are osteoclasts (right column photos). Data are presented as the mean values of four independent experiments. The error bars represent the SD. **P < 0.01
Discussion
During inflammatory bone destruction induced by bacterial infection, an excess amount of bone resorption by osteoclasts is observed (Nair et al. 1996; Taubman et al. 2005). Our results suggest that induction of CD11b+ c-Fms+ CD14+ cells in the bone marrow and spleen by LPS will contribute to increasing the number of osteoclasts. This finding is useful in understanding the mechanisms of bone destruction in periodontitis and for the development of new treatment methods.
Since CD11b is known as a typical cell-surface marker of monocytes and macrophages (Han et al. 2010), we expected that CD11b+ cells would differentiate into osteoclasts. Despite our speculation, CD11b−, but not CD11b+ cells, differentiated into osteoclasts in the bone marrow, while, as speculated, CD11b+ cells differentiated into osteoclasts in the spleen and blood. In addition, we determined that osteoclasts were CD11b+ cells (data not shown). Therefore, CD11b− cells in the bone marrow may be early precursors of osteoclasts that subsequently differentiate into CD11b+ cells, and then ultimately differentiate into osteoclasts. Since CD11b+ cells in the spleen and blood possess osteoclast differentiation potential, these cells may be later precursors derived from the bone marrow.
LPS administration to the mice markedly increased osteoclast numbers in CD11b+ cell cultures prepared from the bone marrow and spleen, suggesting that alternative cells that possess osteoclast differentiation potential increased. On the other hand, the osteoclast number did not increase in CD11b+ cell cultures prepared from the blood. It is possible that the osteoclast precursor number already reached a maximum level before LPS treatment. That may be why no increase in osteoclast number was observed in CD11b+ cell cultures prepared from the blood.
Using additional cell-surface markers such as c-Fms and CD14, we succeeded in identifying cell populations that possess osteoclast differentiation potential in the bone marrow, spleen, and blood. CD11b− c-Fms+ CD14+ cells in the bone marrow and CD11b+ c-Fms+ CD14+ cells in the spleen and blood were suggested as osteoclast precursors residing in those tissues. This also supports the notion that there are different types of cells such as early and later osteoclast precursors.
The most important finding of this study is that we finally identified increased populations that possess osteoclast differentiation potential by LPS administration. In response to LPS administration, the number of CD11b+ c-Fms+ CD14+ cells increased by more than tenfold in the bone marrow and 60-fold in the spleen compared to that of the control mice, respectively. On the other hand, LPS administration to the mice did not affect osteoclast number formed in the cultures of CD11b+ c-Fms+ CD14+ cells isolated from blood (Figs. 1, 2). Thus, regulatory mechanism of CD11b+ c-Fms+ CD14+ population may be somehow different between bone marrow/spleen and blood.
In addition, we preliminary examined the roles of Toll-like receptor 4 (TLR4), an LPS receptor, in bone marrow and spleen cells using C3H/HeJ mice in which TLR4 gene is mutated (supplemental Fig. 2). After LPS administration, osteoclast number increased in the cultures in which CD11b+ cells isolated from bone marrow or spleen of C3H/Hen mice (wild type) were cultured. In contrast, CD11b+ cells isolated from C3H/HeJ mice did not (supplemental Fig. 2). These results suggest that TLR4-mediated signals regulate characteristics of CD11b+ cells in bone marrow and spleen. Further analysis is necessary to reveal the characteristics of CD11b+ cells before and after LPS administration.
A remaining question is how LPS signaling increases CD11b+ c-Fms+ CD14+ cells in the bone marrow and spleen. We do not yet have evidence to explain this mechanism. However, in TLR4-mutated C3H/HeJ mice, LPS failed to increase CD11b+ c-Fms+ CD14+ cells in the bone marrow and spleen (data not shown). This suggests that TLR4 signaling activated by LPS plays a role in increasing this cell population. It is necessary to examine whether inflammatory factors produced by the LPS-TLR4 system such as tumor necrosis factor (TNF) α, interleukin (IL)-1α/β, and prostaglandins increase CD11b+ c-Fms+ CD14+ cells in the bone marrow and spleen. These experiments will offer new insight for the study of inflammatory bone destruction, including periodontitis.
Abbreviations
- IL-1α/β:
-
Interleukin-1α/β
- LPS:
-
Lipopolysaccharide
- M-CSF:
-
Macrophage colony-stimulating factor
- OPG:
-
Osteoprotegerin
- RANK:
-
Receptor activator of nuclear factor-κB
- RANKL:
-
Receptor activator of nuclear factor-κB ligand
- TLR4:
-
Toll-like receptor 4
- TNFα:
-
Tumor necrosis factor α
References
Ash P, Loutit JF, Townsend KM (1980) Osteoclasts derived from haematopoietic stem cells. Nature 283:669–670
Charles JF, Hsu LY, Niemi EC, Weiss A, Aliprantis AO, Nakamura MC (2012) Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J Clin Investig 122:4592–4605
Fujikawa Y, Quinn JM, Sabokbar A, McGee JO, Athanasou NA (1996) The human osteoclast precursor circulates in the monocyte fraction. Endocrinology 137:4058–4060
Han C, Jin J, Xu S, Liu H, Li N, Cao X (2010) Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol 11:734–742
Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN (2009) Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458:524–528
Jacome-Galarza CE, Lee SK, Lorenzo JA, Aguila HL (2013) Identification, characterization, and isolation of a common progenitor for osteoclasts, macrophages, and dendritic cells from murine bone marrow and periphery. J Bone Miner Res 28:1203–1213
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Miyamoto T, Arai F, Ohneda O, Takagi K, Anderson DM, Suda T (2000) An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor κB ligand. Blood 96:4335–4343
Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B (1996) Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun 64:2371–2380
Nakamichi Y, Mizoguchi T, Arai A, Kobayashi Y, Sato M, Penninger JM, Yasuda H, Kato S, DeLuca HF, Suda T, Udagawa N, Takahashi N (2012) Spleen serves as a reservoir of osteoclast precursors through vitamin D-induced IL-34 expression in osteopetrotic op/op mice. Proc Natl Acad Sci USA 109:10006–10011
Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088
Shi C, Pamer EG (2011) Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11:762–774
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357
Takahashi N, Udagawa N, Akatsu T, Tanaka H, Isogai Y, Suda T (1991) Deficiency of osteoclasts in osteopetrotic mice is due to a defect in the local microenvironment provided by osteoblastic cells. Endocrinology 128:1792–1796
Takahashi N, Udagawa N, Suda T (1999) A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun 256:449–455
Takeshita S, Kaji K, Kudo A (2000) Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res 15:1477–1488
Taubman MA, Valverde P, Han X, Kawai T (2005) Immune response: the key to bone resorption in periodontal disease. J Periodontol 76:2033–2041
Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141:3478–3484
Walker DG (1975a) Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science 190:784–785
Walker DG (1975b) Control of bone resorption by hematopoietic tissue. The induction and reversal of congenital osteopetrosis in mice through use of bone marrow and splenic transplants. J Exp Med 142:651–663
Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS 3rd, Frankel WN, Lee SY, Choi Y (1997) TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem 272:25190–25194
Xing L, Schwarz EM, Boyce BF (2005) Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev 208:19–29
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K (1998a) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139:1329–1337
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998b) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442–444
Acknowledgements
We thank Yoichi Miyamoto, Ayako Mochizuki, Akihiro Matsunaga, and Akifumi Matsumoto at Showa University, School of Dentistry for technical support and constructive advice for this study.
Funding
This work was supported by JSPS KAKENHI (Grant Numbers 26293398, 24659830), Industry to Support Private Universities Building up Their Foundations of Strategic Research (S1411009, S1201014, S0801016), and Private University Research Branding Project by MEXT Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Gating strategy for FACS analysis and cell sorting. After doublets and dead cells were gated out using 7-AAD, the single living cells were classified into each cell populations using antibodies. (TIFF 13995 kb)
Supplemental Fig. 2
Effect of LPS administration on the osteoclast differentiation potential of CD11b+ cells in the bone marrow and spleen of C3H/HeN and C3H/HeJ mice. C3H/HeN and C3H/HeJ mice were injected intraperitoneally with LPS (500 μL/kg) or saline. After 24 h, CD11b+cells from the bone marrow and spleen were isolated using magnetic-labeled anti-CD11b antibodies. The CD11b+cells (1 × 105) were then cultured in the presence of RANKL (100 ng/mL) and M-CSF (50 ng/mL) in 96-well culture plates. After 5 days of culture, cells were fixed and stained for TRAP, an osteoclast marker enzyme and TRAP-positive multinucleated cells were counted as osteoclasts. Data are presented as the mean values of four independent experiments. The error bars represent the SD. **P < 0.01 N.S., not significant. (TIFF 13995 kb)
Rights and permissions
About this article
Cite this article
Enomoto, T., Takami, M., Yamamoto, M. et al. LPS administration increases CD11b+ c-Fms+ CD14+ cell population that possesses osteoclast differentiation potential in mice. Cytotechnology 69, 529–537 (2017). https://doi.org/10.1007/s10616-017-0094-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-017-0094-3