Abstract
A certified reference material (CRM) specifically designed for C-reactive protein (CRP) measurement in clinical laboratories was produced in this study. CRP, a protein synthesized by the liver, serves as an indicator of inflammation when present in elevated concentrations in the bloodstream. The primary goal is to ensure accurate measurement and quality control in accredited clinical laboratories. To achieve this, a recombinant CRP solution was selected as the most suitable candidate material for the CRM due to its exceptional purity. The homogeneity and stability of the developed CRM were thoroughly examined using size exclusion chromatography coupled with ultraviolet detection and high-performance liquid chromatography, (SEC-UV-HPLC). Additionally, traceable amino acid analysis coupled with isotope dilution mass spectrometry (ID-LC/MS-AAA) was employed to determine the protein concentration of CRP in the CRM solution. The evaluation of uncertainties originating from factors such as characterization, homogeneity, long-term stability, and short-term stability data was incorporated to assess the uncertainty associated with the certified CRP value. Consequently, the certified value of the CRM, denoted as UME CRM 1008, was determined to be 43.2 ± 2.2 μmol kg−1, with an expanded uncertainty at a coverage factor of k = 2. The CRP-certified reference material resulting from this study is intended to be used as a primary reference material to enable SI traceable measurement of CRP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
C-reactive protein (CRP) is a human serum protein widely used for diagnostic purposes as an acute-phase reactant [1]. It serves as a biomarker, exhibiting a significant increase, up to 100-fold, in response to inflammation, injury, or infection [2]. Additionally, CRP is present in normal human serum at very low concentrations, typically below 1 mg/L, as an acute-phase protein [2]. Abnormal levels of CRP have been associated with cardiovascular and cerebrovascular diseases [3, 4]. The measurement of CRP presents challenges due to the lack of standardization in measurement methods and the diversity of reagents used, leading to significant deviations in measurement results [5]. The Joint Committee for Traceability in Laboratory Medicine (JCTLM) presently lists the secondary certified reference material (CRM) ERM DA-474/IFCC. This CRM, along with two general immunoassay-based method principles, serves as the foundation for establishing metrological traceability in the measurement of CRP using end-user measurement procedures commonly employed in medical laboratories. The assigned value of ERM-DA-470 is traceable to World Health Organization (WHO) 85/506, international standard for CRP, as outlined in the certification report. However, it remains uncertain whether primary calibrators prepared as solutions based on WHO 85/506 exhibit commutability with clinical samples [6]. Even the most commonly accepted CRP measurement assays show considerable discrepancies when using the IFCC CRP secondary reference material ERM-DA474/IFCC [7]. Furthermore, the clinical testing procedures for CRP measurements are highly dependent on the instrumentation and procedure used, further contributing to the lack of standardization in metrological procedures [8]. To address these issues and enhance diagnostic accuracy, the establishment of a reference measurement system for CRP is crucial. In the effort to establish precise metrological traceability for C-reactive protein (CRP) measurements, a consensus summary from the “Joint Committee for Traceability in Laboratory Medicine Workshop” has recently been documented [6]. The discussion highlighted that the current metrological traceability framework for CRP immunoassays has resulted in a satisfactory alignment between clinical sample results, thereby fulfilling clinical requirements. This alignment has been demonstrated across a representative selection of end-user measurement techniques [6, 9]. From a metrology perspective, a desirable aim is to create a hierarchy of calibrations that includes a primary pure substance reference material and a reference measurement procedure (RMP). Such a system would enable the standardization of measurement results and provide traceability for in vitro diagnostics. Primary reference materials can be single compounds or mixtures used to verify and calibrate the primary reference method, with the measurement procedure defined by the Consultative Committee for the Amount of Substance (CCQM). On the other hand, secondary reference materials are employed to validate the calibration of serum-based protein standards and provide control materials for national metrology institutes or clinical diagnostic laboratories during the development of immunoassay measurement methods for CRP. Currently, there are candidate CRP primary reference materials, such as CRM 6201-b and SRM 2924, produced by the National Metrology Institute of Japan and the National Institute of Standards and Technology, respectively [10, 11]. When proposing a new candidate CRM or RMP for inclusion in its database, the JCTLM mandates a validation of extent-of-equivalence against an already existing CRM or RMP [12, 13]. However, for CRP, there is currently an absence of any pure primary CRM or RMP documented for the targeted measurement.
In this study, a certified primary reference material of CRP was developed by TUBITAK National Metrology Institute of Türkiye (TUBITAK UME). The establishment of traceability in measurement methods played a crucial role in the development of the reference material, ensuring consistent and reliable results. Two measurement methods were employed: amino acid analysis coupled with isotope dilution mass spectrometry (ID-LC/MS-AAA) and size exclusion chromatography coupled with ultraviolet detection and high-performance liquid chromatography, (SEC-UV-HPLC). ID-LC/MS-AAA is a highly preferred technique for protein quantification, providing traceable results aligned with the International System of Units (SI) [14, 15]. The hydrolysis process, a critical step in amino acid analysis [9, 12], was optimized, and vapor-phase hydrolysis was chosen for its efficiency and the purity of the obtained samples. ID-LC/MS-AAA was used for characterization study and value assignment, while SEC-HPLC was performed for stability assessment and homogeneity analysis. Statistical analysis, including ANOVA, was performed, and uncertainty contributions were determined to establish an uncertainty budget for the experimental process [14]. The CRP-certified reference material developed through this research aims to function as a primary reference material for ensuring CRP measurements are traceable to the International System of Units (SI). The value of the CRM was established through traceable amino acid analyses that adhere to the SI traceable standards.
Materials and methods
Chemicals and materials
The reagents used in the study included NMIJ CRMs for the natural amino acid standards or calibration standards: L-alanine (Ala, CRM 6011-a, 99.9 ± 0.2 %), L-leucine (Leu, CRM 6012-a, 99.9 ± 0.2 %), L-phenylalanine (Phe, CRM 6014-a, 99.9 ± 0.2 %), L-proline (Pro, CRM 6016-a, 99.9 ± 0.2 %), and L-valine (Val, CRM 6015-a, 99.8 ± 0.2 %). For the ID-LC/MS method, internal standards of isotopically labeled amino acids were obtained from Cambridge Isotope Laboratories. The internal standards used were as follows: Ala (13C3, 99 %; 15N, 99 %), Leu (13C6, 99 %; 15N, 99 %), Phe (13C9, 99 %; 15N, 99 %), Pro (13C5, 99 %; 15N, 99 %), and Val (13C5, 99 %; 15N, 99 %). Furthermore, as quality control in the study, standard reference materials from the National Metrology Institute of Japan (NMIJ CRM 6201-c) and the National Institute of Standards and Technology in Gaithersburg, MD (NIST SRM 2924) were employed.
Preparation of CRP
Recombinant human CRP was purchased from OYC Americas (Andover, MA) in a 1.25-L bottle containing a buffer solution of 20 mM Tris (pH 7.5), 140 mM sodium chloride, 2 mM calcium chloride, and 0.05 % (w/v) sodium azide. The recombinant CRP was produced in Escherichia coli as described by Tanaka et al. [16] and purified using affinity binding to 6-aminohexanoic acid. The 1 250 ml of recombinant CRP was divided into 1 ml aliquots. These aliquots were placed into 1250 polypropylene SRM vials and labeled accordingly, following the order of the vials. The material, which was shipped at 4 °C, was subsequently stored at − 80 °C after being bottled.
SEC-UV-HPLC parameters
The Thermo ScientificTM UHPLC system with a Dionex Ultimate 3000 RS HPLC detector and RS diode array, Dionex Ultimate 3000 RS autosampler, Dionex Ultimate 3000 RS pump, column compartment, and column heater, along with an Agilent Bio-SEC33µm, 150 Å 4.6 × 300 mm HPLC column was used for size exclusion chromatography analysis of CRP in an aqueous solution. The Thermo Scientific™ Chromeleon 7 software was employed for data analysis, and the peak areas of the analyte were determined. Prior to the analysis, the autosampler was set up with specific parameters, including a column temperature of 30 °C, an injection volume of 10 µl, a loop volume of 100 µl, and the partial injection mode. The autosampler washing solution consisted of a 50:50 % mixture of isopropanol and water (v/v). For the analysis, the reference material bottles were thawed at room temperature (20–25 °C) for one hour. A 10 µl injection of the CRP sample, derived from a 40 µM CRP concentrated stock solution, was introduced onto the system column. The elution mobile phase contained 0.1 M Tris HCl (pH 7.5) with 0.15 M NaCl and 1 mM EDTA. A gradient elution method was employed over 20 min with a flow rate of 0.3 ml.min−1 at a temperature of 30 °C. The absorption peaks of the proteins were monitored at 225 nm and 280 nm by adjusting the parameters of detector A and detector B, respectively.
Homogeneity analysis
Homogeneity analysis of the candidate reference material was conducted using the size exclusion chromatography (SEC) method. The analysis was performed in triplicate using 12 randomly selected bottles from the pre-prepared 1250 vials, each containing 1 ml of the CRP solution. The concentration values of the 12 samples were determined based on their UV absorption at 225 nm and by analyzing the peak areas of their main peaks. The resulting data were then subjected to further statistical analysis.
Stability testing
In the long-term stability studies for UME CRM 1008, two temperature conditions, − 20 °C and +4 °C, were selected, and the time points for testing were set at the 1st, 3rd, 6th, 9th, and 12th months. For each time interval at the designated temperatures, two units were placed in the respective temperature chambers, resulting in a total of 10 units for each temperature condition. As for the reference point in the stability test, two units were allocated and stored directly at the reference temperature of −80 °C. At the end of each test period, two units were transferred from each test temperature environment to the reference temperature. After completing the 12-month test period, all units transferred to the reference temperature were analyzed together with the units designated as reference.
Similarly, in the short-term stability studies for UME CRM 1008, two temperature conditions, +4 °C and −20 °C, were selected, and the time points for testing were set at the 0th, 1st, 2nd, 3rd, and 4th weeks. For each time interval at the designated temperatures, two units were placed in the respective temperature chambers, resulting in a total of eight units for each temperature condition. As for the reference point in the stability test, two units were allocated and stored directly at the reference temperature of −80 °C. At the end of each test period, two units were transferred from each test temperature environment to the reference temperature. After completing the 4-week test period, all units transferred to the reference temperature were analyzed together with the units designated as reference. For testing, the UV absorption values or peak areas at 225 nm in HPLC–UV were measured for each sample using size exclusion chromatography (SEC). Also, as part of the stability testing, the freeze and thaw cycle was repeated 5 times in duplicate to be able to observe the degradation level.
Molecular weight determination
The intact molecular weight analysis of the CRP monomer was conducted through high-resolution HR-LC/MS. Reverse phase (RP) chromatography was carried out using a Dionex Ultimate 3000 HPLC system. The mobile phase consisted of 0.01 % trifluoroacetic acid (TFA) in Milli-Q water (solvent A) and 0.01 % TFA in acetonitrile (solvent B). A protein sample of five microliters was introduced into a PLRP-S column with dimensions of 2.1 mm × 150 mm and a particle size of 8 μm (Polymer Laboratories, Varian, Shropshire, UK). Elution took place at a flow rate of 0.2 mL min−1 while maintaining a temperature of 40 °C.
For mass spectrometry analysis, an Orbitrap Q-Exactive HF-X instrument (Thermo Scientific, USA) was utilized, operating in the High Mass Range Mode. This enabled the determination of the molecular weight of the CRP monomer. The acquired data underwent processing using BioPharma Finder software (Thermo Scientific, USA) for the purposes of deconvolution and resolving the molecular masses.
To determine the approximate molecular weight of the pentameric candidate reference material, a calibration curve was constructed using the isocratic SEC-UV-HPLC method and the Sigma Molecular Weight Kit (Sigma MWGF1000). The analysis involved injecting 3 µl of the candidate reference material solution into an Agilent BioSec-3 column with dimensions of 4.6 × 300 mm and a particle size of 3 µm (PN: 5190–2513 3 µM). The mobile phase used was phosphate buffer with a pH of 7.6. Elution was performed isocratically at a flow rate of 0.25 ml min−1, and the column temperature was maintained at room temperature.
Amino acid analysis
The natural amino acid mixture was prepared gravimetrically in a ratio that closely matched the concentration of each amino acid in CRP. All these preparations were made fresh and independently for each hydrolysis reaction. Amino acid concentrations corresponding to 1.25 µM, 2.5 µM, 5.0 µM, 10 µM, 20 µM, and 40 µM CRP were used for each amino acid calibration standard, and each solution was prepared accordingly. The same procedure was applied for the preparation of isotopically labeled amino acid solutions. A six-point calibration curve was prepared gravimetrically for each hydrolysis method by mixing the natural and isotopically labeled amino acid mixtures. The final concentration of each isotopically labeled amino acid in the calibration mixtures was adjusted to be nearly the same as in the sample blends. Additionally, the concentration of the natural amino acids in the calibration sample, which coincided with the midpoint of the calibration curve, was carefully aligned with the anticipated amino acid concentrations in the sample blends.
Vapor-phase acid hydrolysis
Six calibration standards were prepared, consisting of a mixture of natural and isotopically labeled amino acid solutions, and five candidate reference material solutions were prepared, containing isotopically labeled amino acids. After weighing the solutions in the glass tubes, they were placed into separate Pico-Tag vials along with the calibration mixtures. The calibration and sample mixtures were completely dried under vacuum using a Pico-Tag Workstation (Waters, Milford, MA, USA). Then, they were hydrolyzed under inert atmosphere conditions at 130 °C for 24 h in hydrolysis tubes containing 200 µl of 6 M constant boiling HCl and 1 % phenol. After the completion of hydrolysis, the samples were evaporated under vacuum until completely dry, and then reconstituted with 200 µl of 20 mM HCl for the derivatization process of amino acids.
Derivatization for LC/MS
The dissolved samples (200 µl each) were mixed with 200 µl of Propanol:Pyridine (7:1) reagent and vortexed. Then, they were mixed with 200 µl of Isooctane:PCF (5:1) and incubated for 1 min. Subsequently, 100 µl of CHCl3:Isooctane:PCF (24:16:1) reagent was added, vortexed, and incubated for 1 min. Finally, 200 µl of 5 % HCl was added, vortexed, and centrifuged. The upper phase of each sample was separated and transferred to clean tubes. The samples were then evaporated using the Caliper TurboVap LV evaporator under nitrogen. Lastly, each sample and calibrants in the tubes were dissolved by adding 50 µl of H2O:acetonitrile (95:5)–0.1 % FA sample solution and transferred to suitable vials for LC/MS analysis.
LC/MS analysis
LC analysis was carried out using a Dionex UPLC™ system (Thermo Scientific, Bremen, Germany) coupled with a Q-Exactive HF-X Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Scientific, Bremen, Germany) equipped with an electrospray ion source. Amino acids were injected and separated on Phenomenex EZ:faast 4 µ AAA column. The mobile phase was composed of, A: 10 mM ammonium formate, 50:50 methanol:water in water, B: 10 mM ammonium formate, and methanol. The autosampler wash solvent included a mixture of water, acetonitrile, and NH4OH in a ratio of 40:50:10 (v:v:v) for both weak and strong washes. The injection volume was 10 µl. The gradient program used involved an increase in the B phase from 35 to 55 % over 12 min, followed by an increase to 100 % B. The flow rate was maintained at 0.250 µl.min.−1 during a 5-min wash with 100 % B. Finally, equilibration was achieved by washing with a 35 % B solution for 5-min. The mass spectrometry analysis was performed in Full MS mode, and the MS parameters for amino acids following PCF derivatization are presented in Table 1
All data were acquired in positive ion mode. The following acquisition parameters were set for the instrument: The spray voltage was set to 3.5 kV, sheath gas flow rate to 45, sweep gas flow rate to 2 and capillary temperature to 250 °C. Full MS resolution was set to 120000 for m/z 200 with maximum injection time of 200 ms. The Full MS AGC target was set to 3E6, and the mass range was defined as 100 − 500. Additionally, mass data extraction and analysis were performed using QuanBrowser™ Software. Following the MS measurements, the mass fractions of peptide samples were calculated for Leu, Pro, Val, Gly, and Ala amino acids based on the integral values of signal peak areas using calibration curves. The assignment process was completed by considering the gravimetric dilution factors.
Results and discussion
The structural analysis
The study utilized recombinant human C-reactive protein (rhCRP) obtained from Oriental Yeast Co., Ltd. as the reference material due to its attributes of high purity, and low heterogeneity [16]. The rhCRP monomer is composed of 206 amino acids and shares the same amino acid sequence as its natural counterpart CRP [16,17,18]. This confirmation was based on factors such as molecular weight determination by mass spectrometry, ability to bind to phosphorylcholine in a calcium-dependent manner, N-terminal amino acid analysis, electrophoretic behavior, and immunochemical properties [16, 17]. Recombinant CRP's pentameric structure was verified via calcium-dependent binding [16]. Kimberly et al. found that various materials, including rhCRP from Oriental Yeast C, showed comparable precision, making them useful for calibrators, quality control, and proficiency tests [19]. Furthermore, a bottom-up proteomic analysis validated CRP's amino acid sequence following the described method [20]. The protein underwent trypsin and glu-c digestion, and the resulting peptides were then subjected to LC-MSMS analysis. This analysis led to the confident identification of the protein, with a false discovery rate (FDR) of 1 %. The PEAKS-10lgP score obtained was 189.32, ensuring high confidence in the identification. The protein coverage reached 99 %, and a total of 47 unique peptides were identified. HR-LC/MS analysis on an Orbitrap Q-Exactive HF-X instrument operating in the High Mass Range Mode accurately determined CRP monomer's molecular weight. BioPharma Finder software processed data, with a deconvolution mass tolerance of 20 ppm. The results of the analysis revealed that the average relative molecular weight of CRP is 23029.60 g. The findings are presented in Fig. 1, which includes two components: (a) the Mass Spectrum of the Candidate CRM, illustrating multiple charges, and (b) the Deconvoluted Mass Spectrum of the Candidate CRM, demonstrating the resolved molecular masses. This result is consistent with the expected molecular mass of human CRP, which is 23029.28 g, accounting for the presence of N-terminal pyroglutamylation and a single disulfide bond. We also employed SEC-UV-HPLC to assess the oligomeric state of the CRP material and confirm its pentameric form. An approximate molecular weight calculation was performed using SEC molecular weight marker proteins. The estimated molecular weight of CRP was approximately 120 kDa. Native CRP consists of five identical subunits that are non-covalently linked, resulting in a total molecular weight of approximately 115 kDa. Therefore, the molecular weight of the tested material as measured by SEC-UV-HPLC closely matched the theoretical molecular weight of the CRP pentameric structure, with no impurities detected. The purity analysis using SEC-UV-HPLC method revealed that CRP displayed a distinct and well-separated single peak with no impurities detected at 280 nm (Fig. 2 a). At 220 nm, a major peak corresponding to the absorption of the CRP was observed, along with a minor peak attributed to the absorption of sodium azide (Fig. 2 b). While SEC-UV-HPLC is indeed a valuable technique for assessing the purity and oligomeric state of proteins, we acknowledge that its sensitivity might be limited in detecting impurities, especially those present at lower concentrations. To address this concern, we applied a complementary method to further evaluate the impurity content of the CRP material. Specifically, we performed RP-LC/MS analysis, which allowed us to deeply analyze the impurities present in the material at a higher level of sensitivity. Figure 3 depicting the HR-LCMS chromatogram, showcasing the absence of minor peaks alongside the main CRP peak exceeding the 0.01 % threshold.
a SEC analysis of the CRP reference material at 220 nm, the CRP reference material exhibited a major peak attributed to the candidate material and a minor peak resulting from the absorption of sodium azide. b At 280 nm revealed a single major peak corresponding to the candidate material, with no minor peaks indicating impurities
The homogeneity study
A homogeneity study is conducted to evaluate if the values determined for CRP are within a certain confidence interval across all units, aiming to represent the entire production. According to ISO Guide 35 [20], the number of units for homogeneity study should be at least 10 units, with the production size being approximately the cube of that number, ensuring representation of the entire material. For the production of UME CRM 1008, a homogeneity study was performed using a stratified random sample selection program called TRaNS produced in TUBITAK UME, including 12 units along with their backups. This program is designed to assist in selecting representative samples from a population for analysis or testing purposes. By utilizing stratified random sampling, the program ensures that the selected samples are proportionally representative of different strata or subgroups within the population. Homogeneity analyses were carried out by measuring three subsamples from each unit under repeatability conditions. The homogeneity analysis was performed using size exclusion chromatography (SEC-UV-HPLC) method. The decision to employ SEC for stability studies was based on its robustness and reliability. Furthermore, the stability of the CRP pentameric structure, a crucial aspect for successful antigen–antibody reactions or enzyme-linked reactions, could be effectively monitored through SEC. The homogeneity study results are shown in Fig. 4. The data obtained for UME CRM 1008 were evaluated, and the relative standard deviation was found to be 1.07 %, indicating that the material possesses sufficient homogeneity suitable for its intended use in the inter-unit homogeneity test. Subsequently, the data obtained from the results were used for further statistical analysis. The Shapiro–Wilk test yielded a p value of 0.056; it was concluded that the data followed a normal distribution. The assessment of within-bottle homogeneity of UME CRM 1008 revealed highly consistent measurements. The highest relative standard deviation (RSD) observed for triplicate analyses of subsamples from the same bottle was 1.5 %. Additionally, the average RSD of triplicate measurements across 12 different units was only 0.4 %, indicating strong homogeneity despite the small 10 µL sample intake. Furthermore, the SEC-UV-HPLC method applied in our study was calibrated using NIMJ 6201.b CRM. The results of the homogeneity study involving 36 analysis from 12 distinct units demonstrated a mean value of 42.8 μmol kg−1 with a standard deviation of 0.4. This average value closely aligns with the certified value of the CRM (43.2 ± 2.2 μmol kg−1) determined through amino acid analysis. This alignment between the SEC-UV-HPLC measurements and the amino acid analysis highlights the method's consistency and its capacity to accurately evaluate the protein's stability and homogeneity over time.
Stability study
Stability studies for UME CRM 1008 were conducted to assess the effects of environmental conditions during the shipment of the reference material (short-term stability-STS) and storage conditions (long-term stability-LTS) by replicating similar conditions in the laboratory. Stability studies were performed using an isochronous design, where measurements were taken simultaneously.
Short term stability testing
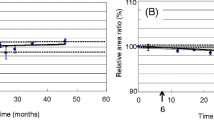
For UME CRM 1008, short-term stability studies were conducted at temperatures of + 4 °C and − 20 °C, with time intervals set at 0, 1, 2, 3 and 4 weeks. The simultaneously conducted measurements for UME CRM 1008 were first grouped according to the same temperature conditions, and evaluation was performed for each time point. After analyzing the data, the standard uncertainty contributions were determined to be 0.09 % at − 20 °C and 0.14 % at + 4 °C after one week. Taking into account the maximum shipping duration of two weeks, the expiration date for short-term storage conditions was determined to be two weeks, considering the uncertainty contributions of 0.18 % at − 20 °C and 0.29 % at + 4 °C after two weeks. In summary, both − 20 °C and + 4 °C storage conditions showed good short-term stability, with no significant degradation observed during the test period. The short-term stability results are also shown in Fig. 5.
Long term stability testing
For UME CRM 1008, long-term stability studies were conducted at temperatures of −20 °C and +4 °C, with time intervals set at 1, 3, 6, 9, and 12 months. The material's stability under storage conditions was tested for 12 months at both −20 °C and +4 °C, and no signs of degradation were observed (Fig. 6). The linear regression analysis conducted for the long-term study showed significant results at a 1 % significance level. No outliers or trends were detected in either of the long-term studies. Additionally, for the long-term study, the standard uncertainty contribution was determined to be 0.37 % at −20 °C and 0.97 % at 4 °C after 12 months. Furthermore, a comparison is made between the variance in the long-term stability test data and the variance from a homogeneity study (Fig. 4). The relative standard deviation (RSD) of homogeneity analyses (n = 36) for subsamples from a 12 units is calculated at 1.28 %, while the average RSD of subsample measurements across 44 distinct units in the long-term stability study is 0.86 %. The variation observed in the stability data is found to fall within the range of within-bottle homogeneity values. Overall, the study demonstrates that the material (UME CRM 1008) maintains stability over a 12-month period at both −20 °C and +4 °C. The statistical analyses and comparisons suggest that the observed variations are within an acceptable range, and the material's stability is consistent with its initial state. Due to the fact that the standard uncertainty value is lower at 20 °C, the chosen storage condition is −20 °C. Consequently, when assigning values and calculating uncertainties for the material, all calculations have been carried out considering the storage conditions at −20 °C.
Value assignment and ID-LC/MS-AAA
For the characterization of UME CRM 1008, the ID-LC/MS-AAA method was employed. AAA provides an absolute quantitative measure of the protein content of a sample based on the content of amino acids comprising the test product, independent of an external protein reference standard. In the AAA method used to measure the absolute concentration of CRP, vapor-phase acid hydrolysis conditions were set at 100 µL, 130 °C, and 24 h, which had been previously optimized by Kato et al. [10]. Five amino acids (Pro, Val, Leu, Phe, and Ala) were selected as analytes, and analysis was performed in duplicate after the hydrolysis process conducted for three days. Calibration curves for amino acid measurements were established using calculated standard mixtures of selected natural and isotopically labeled amino acids, prepared according to the known amino acid sequence of CRP. Additionally, contributions from impurities arising from hydrolysis or derivatization processes of peptide molecules were determined by analyzing peptide/protein-related impurities with the PICAA analysis approach and subtracted from the overall value. The uncertainty evaluations of amino acid analysis used in this study were conducted following the ISO GUIDE Reference Materials-Guidance for characterization and assessment of homogeneity and stability 4th edition [13] and the EURACHEM/CITAC Guide Quantifying Uncertainty in Analytical Measurement [14]. Table 2 presents the total protein concentrations along with the contributions of relative uncertainty.
The validity of the measurements in this study was confirmed through the analysis of NIST SRM 2924 and NIMJ CRM 6201-c CRMs. Table 3 presents the summary statistics of measurements conducted on other commercially available primary reference materials. The CRP level of each CRM was fully evaluated in two separately analyzed vials of the material. Triplicate analysis was performed for each vials. Measured values and fully evaluated 95 % confidence level uncertainties are given in Table 3. The measured value for NIST was 20.4 µmol/L with an uncertainty of ± 1.0 mol/L, while for NIMJ, it was 39.8 µmol/kg with an uncertainty of ± 1.9 µmol/kg. These values were compared to the certified values of the reference materials, which were reported as 20.6 µmol/L with an uncertainty of ± 1.2 µmol/L (k = 2) for NIST, and 40.2 µmol/kg with an uncertainty of ± 1.7 µmol/kg (k = 2) for NIMJ [11]. The results of this comparison showed that the concentration values obtained using the same measurement method had similar values from the certified values of the reference materials. This provides strong evidence for the validity and reliability of the measurement method employed in this study.
Uncertainty evaluation
The combined standard uncertainty (uc) of the property value for the candidate CRM was determined by combining the uncertainty contributions from value assignment (uchar), material inhomogeneity (sbb), uncertainty due to long-term instability (ults), and uncertainty due to short-term instability (usts). The calculation of uc was performed using Eq. 1 [28].
Table 4 presents the relative and combined results of the uncertainty contributions, which were utilized in assigning a value to the candidate reference material. Ultimately, the certified concentration of the reference material was determined as 43.2 µmol/kg ± 2.2 µmol/kg (k = 2).
Conclusion
This study successfully produced a CRM, UME CRM 1008, specifically designed for SI traceable CRP measurement, ensuring traceability in measurement methods in accredited laboratories. The selection of a recombinant CRP solution as the CRM material was based on its exceptional purity, making it a suitable candidate for pure primary reference material. The characterization of the CRM involved the use of advanced analytical techniques such as SEC-UV-HPLC and ID-LC/MS-AAA. The value of the CRM was established through SI traceable amino acid analyses. Through the evaluation of uncertainties originating from factors of characterization, homogeneity, long-term stability, and short-term stability, an uncertainty budget was established to assess the uncertainty associated with the certified CRP value. The certified value of the CRM is 43.2 ± 2.2 μmol/kg with an expanded uncertainty at a coverage factor of k = 2. The ultimate goal for this reference material is to serve as a primary pure substance CRM, accessible to both national and international laboratories, thus enabling the achievement of metrological traceability for CRP measurements. To achieve this goal, the candidate primary reference material resulting from this study will undergo additional investigations to demonstrate its extent-of-equivalence with other certified reference materials (CRMs) addressing the same measurement. Moreover, we will evaluate the measurement of clinical samples using procedures calibrated with our candidate CRM. This evaluation will entail comparing outcomes with results obtained from well-coordinated end-user immunoassays calibrated to ERM DA474/IFCC.
References
Kushner I (2002) C-reactive protein and atherosclerosis. Science 297(5581):520–521. https://doi.org/10.1126/SCIENCE.297.5581.520
Pepys MB, Hirschfield GM (2003) C-reactive protein: a critical update. J Clin Invest 111:1805–1812. https://doi.org/10.1172/JCI200318921
Schultz DR, Arnold PI (1990) Properties of four acute phase proteins: C-reactive protein, serum amyloid a protein, α1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum 20:129–147. https://doi.org/10.1016/0049-0172(90)90055-K
Rifai N, Ridker PM (2001) High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem 47:403–411
Liu J, Zhu W, Sun H, Song D, Xiao P, Xu B, Li H (2021) Development of a primary reference material of natural C-reactive protein: verification of its natural pentameric structure and certification by two isotope dilution mass spectrometry. Anal Methods 13:626–635. https://doi.org/10.1039/D0AY02289F
Miller WG, Panteghini M, Wielgosz R (2023) Implementing metrological traceability of C-reactive protein measurements: consensus summary from the joint committee for traceability in laboratory medicine workshop. Clin Chem Lab Med 61:1558–1560. https://doi.org/10.1515/cclm-2023-0498
Wu C, Zhang S, Liu W, Zeng J, Zhao T, Yue Y, Zhang R, Ma H, Wang Q (2017) Application of commutable ERM-DA474/IFCC for harmonization of C-reactive protein measurement using five analytical assays. Clin Lab 63:1883–1888. https://doi.org/10.7754/CLIN.LAB.2017.170626
Thompson D, Pepys MB, Wood SP (1999) The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 7:169–177. https://doi.org/10.1016/S0969-2126(99)80023-9
Borrillo F, Panteghini M (2023) Current performance of C-reactive protein determination and derivation of quality specifications for its measurement uncertainty. Clin Chem Lab Med 61:1552–1557. https://doi.org/10.1515/CCLM-2023-0069/MACHINEREADABLECITATION/RIS
Kato M, Kinumi T, Yoshioka M, Goto M, Fujii S-I, Takatsu A (2015) Development of C-reactive protein certified reference material NMIJ CRM 6201-b: optimization of a hydrolysis process to improve the accuracy of amino acid analysis. Anal Bioanal Chem 407:3137–3146. https://doi.org/10.1007/s00216-014-8190-0
Tarlov Michael J. SJC (2017) Certificate of Analysis Standard Reference Material ® 2924 C-Reactive Protein Solution
Joint Committee for Traceability in Laboratory Medicine. Policy JCTLM-DBWG-P-04A 20230201 (2023) Demonstrating the extent-of-equivalence between multiple certified reference materials ( CRMs ) for the same measurand. https://www.bipm.org/en/committees/jc/jctlm/wg/jctlm-dbwg/publications
Joint Committee for Traceability in Laboratory Medicine. Policy JCTLM-DBWG-P-04B 20230201. (2023) Demonstrating the extent-of-equivalence between multiple reference measurement methods / procedures ( RMM / Ps ) for the same measurand. https://www.bipm.org/en/committees/jc/jctlm/wg/jctlm-dbwg/publications
Josephs RD, Martos G, Li M, Wu L, Melanson JE, Quaglia M, Beltrão PJ, Prevoo-Franzsen D, Boeuf A, Delatour V, Öztug M, Henrion A, Jeong J-S, Park S-R (2019) Establishment of measurement traceability for peptide and protein quantification through rigorous purity assessment-a review. Metrologia 56:1–30. https://doi.org/10.1088/1681-7575/ab27e5
Josephs RD, Stoppacher N, Westwood S, Wielgosz RI, Li M, Quaglia M, Melanson J, Martos G, Prevoo D, Wu L, Scapin S, Senal MÖ, Wong L, Jeong J-S, Wai K, Chan Y, Arsene CG, Park S-R (2017) Concept paper on SI value assignment of purity model for the classification of peptide protein purity determinations. J Chem Metrol 11:1–8
Tanaka T, Horio T, Matuo Y (2002) Secretory production of recombinant human C-reactive protein in Escherichia coli, capable of binding with phosphorylcholine, and its characterization. Biochem Biophys Res Commun 295:163–166. https://doi.org/10.1016/S0006-291X(02)00622-8
Woo P, Korenberg JR, Whitehead AS (1985) Characterization of genomic and complementary DNA sequence of human C-reactive protein, and comparison with the complementary DNA sequence of serum amyloid P component. J Biol Chem 260:13384–13388. https://doi.org/10.1016/S0021-9258(17)38881-6
Lei KJ, Liu T, Zon G, Soravia E, Liu TY, Goldman ND (1985) Genomic DNA sequence for human C-reactive protein. J Biol Chem 260(24):13377–13383
Kimberly MM, Vesper HW, Caudill SP, Cooper GR, Rifai N, Dati F, Myers GL (2003) Standardization of immunoassays for measurement of high-sensitivity C-reactive protein. Phase I: evaluation of secondary reference materials. Clin Chem 49:611–616. https://doi.org/10.1373/49.4.611
(2017) Reference materials-Guidance for characterization and assessment of homogeneity and stability 4th edition. In: ISO Guid. 352017. https://www.iso.org/standard/60281.html. Accessed 26 Jan 2023
Author information
Authors and Affiliations
Contributions
Merve Oztug contributed to conceptualization, methodology, validation, investigation, resources, data curation, writing—original draft, visualization, project administration. Meltem Asicioglu Kucuk contributed to methodology, validation. Evren Saban contributed to conceptualization, methodology, validation, investigation. Alper Isleyen contributed to conceptualization, validation. Muslum Akgoz contributed to review and editing, project administration
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oztug, M., Saban, E., Asicioglu, M. et al. Development of UME CRM 1008: certified reference material for C-reactive protein. Accred Qual Assur 29, 19–29 (2024). https://doi.org/10.1007/s00769-023-01563-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-023-01563-w