Abstract
The aim of this paper is to compare the performance parameters of the Volhard method for chlorides determination obtained applying the bottom-up approach with those experimentally achieved. The method precision, trueness, detection and quantification limits and ruggedness are determined analysing various foods with different chloride contents. Otherwise, the measurement uncertainty is assessed applying the bottom-up approach using only pen and paper. The comparison between the performances established with both methods shows the validity of the metrological approach for volumetric procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The validation checks that a method performs adequately for the purpose throughout the range of analyte concentrations and test materials to which it is applied. Several international organisations and institutions have issued guidelines [1–3] that provide a framework for performing such a validation. In general, the process includes the determination of specificity, linearity, trueness, precision, detection limit (LOD), quantitation limit (LOQ) and ruggedness.
The uncertainty is not included in the list of validation parameters, but the fitness for purpose of an analytical result cannot really be assessed without some estimate of its uncertainty to compare with the confidence required. Therefore, its evaluation is required by accreditation bodies according to the criteria of the International Organization for Standardization [4].
One of the best-known methods for estimating this parameter is the bottom-up (or component-by-component or error budget) approach, developed by metrologists and physicists [5] and applied in analytical chemistry by EURACHEM [6, 7]. This approach estimates the overall uncertainty via the law of propagation of uncertainty, following identification and quantification of uncertainties of individual sources.
The determination of chloride in food takes importance in nutritional aspects and in quality control of processed food technology. Several techniques were developed for its analysis: volumetry, ion chromatography, potentiometry, etc. The Volhard method is a classical way to determine this anion in various food; nevertheless the complete validation data are not available [8]. This procedure consists of the addition of an excess of silver nitrate solution to a sample containing the analyte and, consequently, in the quantitative precipitation of silver chloride. The concentration of chloride is then determined back-titrating the excess silver ions with a thiocyanate solution using the ferric alum as indicator.
The aim of this work is to furnish the overall experimental validation parameters (precision, trueness, detection and quantification limits, ruggedness) for the determination of chlorides in meat and meat products following the Volhard method, evaluating also his fitness for purpose in relation to the method application field. Finally, the comparison between the measurement uncertainty predicted applying the bottom-up approach and that estimated by mean of experimental data are reported.
Experimental
Procedure
About 1.5 g of the sample is accurately weighed into a beaker, then 100 mL of water, 10 mL of halogen-free nitric acid, and 25 mL of silver nitrate 0.1 mol/L are added. The mixture is heated to boiling for about 10 min, stirring, until AgCl precipitation. The beaker is covered with aluminium foil and cooled in a dark place. After cooling, the excess AgNO3 is titrated with 0.1 mol/L ammonium thiocyanate using 5 mL of saturated solution of ferric alum as indicator. The chloride concentration (g(NaCl)/100 g sample) is given by:
where V 1 is the added volume in mL of silver nitrate (25 mL), V 2 is the spent volume in mL of ammonium thiocyanate 0.1 mol/L, M 1 is the silver nitrate molarity (0.1 mol/L), M 2 is the ammonium sulphocyanide molarity (0.1 mol/L) and W is the sample weight (1.5 g).
Validation
The method precision is evaluated in ham and fresh meat samples with different levels of NaCl. For each level, the experiments were carried out over several months by two operators using different silver nitrate and ammonium thiocyanate standard solutions. In this way, a within-laboratory reproducibility is estimated.
The trueness is studied by repetitive analysis of the Certified Reference Material (CRM) SMRD 2000 (pork meat) produced by Swedish Meat R&D.
The limit of detection and quantification were determined using the observed variance of analyses carried out on fresh pork meat containing low levels of chloride.
The ruggedness was investigated using a Youden experimental plan, introducing minor reasonable variations by the laboratory and observing their consequences [9]. Such a design allows the investigation of a maximum of seven factors (variables) with eight experiments. The parameters investigated and the levels chosen are summarised in Table 1. In our case, the selected variables are six. Therefore, in the calculations a dummy variable is inserted.
The effect of a particular variable is estimated by subtracting the mean result with the variable at “high level”, indicated by capital letter, from the mean result achieved with it at “low level”, marked with the corresponding small letter (Table 1). For each variable, the calculated difference is indicated as D i . Using a t-test [10], the influence of each investigated factor can be evaluated. The experimental t is given by:
where n is the number of experiment carried out at each level for each parameter (n = 4) during ruggedness investigation and standard deviation is the estimate of the method precision obtained in the nominal conditions.
For all the six variables, the obtained t value is compared with the two-tailed t critical value for N-1 degrees of freedom (t crit), where N is the number of determination used in the estimation of standard deviation at 95% confidence level. If t is greater than t crit, the investigated variable shows a significant influence, and the method is not sufficiently robust against the chosen modification.
The uncertainty of measurement is estimated following the bottom-up approach. The identification of all major uncertainty sources and the evaluation of their effects on the measurand uncertainty are carried out by drawing a cause and effect diagram (Fig. 1).
Results and discussion
Precision
The validation experiments are shown in Table 2.
All the reported standard deviations are of the same order of magnitude (Bartlett test). Therefore, in the full application field, the method gives the same results dispersion and the precision is estimated as a pooled standard deviation (SD pooled):
Trueness
A certified reference material SMRD 2000 (pork meat) produced by Swedish Meat R&D is analysed. The results are summarized in Table 3.
The method recovery \(\bar R\) is given by:
The recovery uncertainty, \(u(\overline R)\), is evaluated using the results in Table 3 as follows [10]:
To determine whether the recovery is significantly different from 1, a significance t-test is used:
t is compared with the tabulated t=2.57 for 5 degrees of freedom and 95% confidence level. As t<2.57, there is no evidence to suggest that\(\bar R\) is significantly different from 1.
LOD and LOQ
LOD is the lowest concentration of an analyte in a sample that can be detected, but not necessarily quantified under the stated conditions of the test. LOQ is the lowest concentration of an analyte in a sample that can be determined by the method with an acceptable precision and accuracy. Assuming that blank samples and samples with a low level of analyte have the same variance [1], the detection limit is estimated using the results dispersion of seven replicate analysis of fresh swine meat containing 0.11 g (NaCl)/100 g sample (Table 2). The normality distribution of the data has been previously verified (Shapiro-Wilk) and then, multiplying by 3 and 10 times the observed standard deviation, the LOD and the LOQ result equal to 0.09 and 0.30 g/100 g sample of NaCl, respectively.
Ruggedness
Table 4 reports the results of the ruggedness test. The experimental t values are obtained replacing in Eq. (2) the previously estimated method precision in SMRD 2000 (N=6 and SD=0.022 g (NaCl)/100 g sample, reported in Table 3) and are compared with the two-tailed t critical value for 5 degrees of freedom (2.57).
Only the effect of the dark is found significant. This happens because the AgCl is a light-sensitive salt.
Uncertainty
The considered parameters affecting the uncertainty value of measurand are:
-
mass of weight sample (gross and tare)
-
volume of added silver nitrate solution
-
titration volume of ammonium thiocyanate solution
-
concentration of ammonium thiocyanate solution
-
concentration of silver nitrate solution
These sources are also reported in the cause-effect diagram (Fig. 1); other uncertainty components are considered negligible [11]. Furthermore, starting from measurand specification (Eq. (1)), the relative standard combined uncertainty of the final chloride concentration is quantified by the partial differentiation:
The values of W, V 1, V 2 , M 1 and M 2 as well as the respective standard uncertainties u(W), u(V 1), u(V 2), u(M 1) and u(M 2) are given in Table 5. The expanded uncertainty U is calculated applying the coverage factor k = 2 (at ≈ 95% confidence level).
By means of Eq. (3), it is possible to calculate the standard uncertainty at different sodium chloride concentrations. In this equation, all terms are constant except the spent volume of titrant V 2, which progressively increases with the decrease of the analyte content.
In the evaluation of the uncertainty in volumetric operations, only the tolerances and the temperature effects are included [12]. The effect of temperature difference from burettes calibration temperature to the laboratory environment is calculated from an estimated temperature range of ±4°C and the coefficient of volume expansion (0.00021°C−1) [7]. It must be underlined that in the uncertainty estimation of V 2, the effect of temperature also depends on the volume of spent titrant, since the temperature contribution is calculated as follows [7]:
The bias in end-point detection of titration is due to drop size of the burette [13].
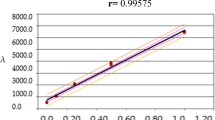
The dependence of u(c NaCl)/c NaCl on the sodium chloride concentration is shown in Fig. 2.
The relative standard deviations reported in Table 2, being obtained in within-laboratory reproducibility conditions, represent a rough estimation of the measurement uncertainty [14]. When compared with the relative standard uncertainty calculated applying Eq. (3), a good agreement is observed (Table 6).
Regarding the detection limit, Wegscheider (1999) observed that when defining the LOD as three times the standard deviation of the blank response, the relative standard measurement uncertainty u(c)/c (RSD in the concentration domain) would be 0.33 at c=LOD [15]. Imposing in the Eq. (3) a relative standard uncertainty equal to 0.33 and resolving respect to NaCl concentration, the calculated value is 0.11 g/100 g sample; therefore the experimental limit of 0.09 g (NaCl)/100 g sample results very close to the predicted one (Table 6). The analogous dependence is also valid for LOQ, which is usually equal to ten times the standard deviation of the blank response. In this case, the relative standard measurement uncertainty is u(c)/c=0.10 at c=LOQ. The calculated value is then 0.37 versus the obtained experimental 0.30 g (NaCl)/100 g sample.
Conclusion
Overall, the validation parameters demonstrate the fitness for purpose of the Volhard method in its application field. Especially, in the observed concentration range the precision is compliant with the international indications [16] and any significant bias has been detected. The limits are adequate for the current chloride contents in foods. Applying the ruggedness test, only the light exposure must be carefully avoided after AgCl precipitation.
Further, comparing the calculated values and the experimental validation data a good agreement is observed for all the investigated method performance parameters. This demonstrates that the metrological bottom-up approach applied using only “pen and paper” can be suitable for a simple volumetric analytical procedure.
References
Thompson M, Ellison SRL, Wood R (2002) Pure Appl Chem 74:835–855
EURACHEM (1998) EURACHEM Guide. The fitness for purpose of analytical methods. A laboratory guide to method validation and related topics, 1st edn. Teddington, UK
Official Journal of the European Communities (2002) L221/8 Commission Decision 2002/657/EC
ISO/IEC (2005) Standard 17025 General Requirements for the Competence of Testing and Calibration Laboratories, ISO, Geneva
ISO (1995) Guide to the Expression of Uncertainty in Measurement, ISO, Geneva, Switzerland
EURACHEM (1995) Quantifying uncertainty in analytical measurement, 1st edn. EURACHEM, Teddington, UK
EURACHEM/CITAC (2000) EURACHEM/CITAC Guide. Quantifying uncertainty in analytical measurement, 2nd edn. Teddington, UK
AOAC (2002) Official Methods of Analysis of AOAC INTERNATIONAL 17th edn, Chapt. 3, Gaithersburg, p 14
Youden J, Steiner EH (1975) Statistical Manual of the AOAC – Association of Official Analytical Chemists, AOAC, Washington, DC
Barwick VJ, Ellison SLR (2000) VAM Project 3.2.1, Development and Harmonisation of Measurement Uncertainty Principles. Part d. Protocol for Uncertainty Evaluation from Validation Data, Report No.: LGC/VAM/1998/088
Kuselman I, Shenhar A (1997) Accred Qual Assur 2:180–185
Kadis R (2004) Talanta 64:167–173
Kuselman I, Sherman F (1999) Accred Qual Assur 4:124–128
NMKL Procedure (1997) Estimation and expression of measurement uncertainty in chemical analysis. Nordic Committee on Food Analysis, Finland
Wegscheider W (1999) EURACHEM. Proceedings of the 2nd EURACHEM Workshop Measurement Uncertainty in Chemical Analysis: Current Practice and Future Directions, Berlin, pp 29–30
Horwitz W, Kamps LR, Boyer KW (1980) J Assoc Off Anal Chem 63:1344–1354
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haouet, M.N., Altissimi, M.S., Framboas, M. et al. Validation of the Volhard method for chloride determination in food. Accred Qual Assur 11, 23–28 (2006). https://doi.org/10.1007/s00769-006-0116-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-006-0116-x